Abstract
Due to limitations in commercial diagnostic methods, this study aimed to develop a reliable real-time polymerase chain reaction (Rt-PCR) assay for early diagnosis of brucellosis. Optimization of the Rt-PCR method was performed on serum samples spiked by Brucella melitensis with different densities ranging from 101 to 108 colony-forming units (cfu)/mL; each density was prepared in ten samples. The limit of detection was investigated by using Thermo DNA extraction kit with Maxima SYBR Green Rt-PCR and two TaqMan probe–based Rt-PCR protocols performed by QuantiTect and TEMPase multiplex PCR master mixes in two thermal cyclers, which were Rotor-Gene and Bio-Rad. The validation of the optimized protocol was carried on 20 brucellosis-negative samples and 20 samples spiked with B. melitensis by using a combination of Thermo DNA extraction kit with TEMPase PCR master mix. SYBR Green Rt-PCR yielded positive results on all samples having ≥ 104 cfu/mL of B. melitensis in both thermal cyclers. Its limit of detection was 112 DNA copies per reaction. The positivity of both probe-based Rt-PCR protocols was 100% and 80% on the samples having 103 cfu/mL and 102 cfu/mL of B. melitensis, respectively. The limit of detection of probe-based protocols was defined as 4 DNA copies per reaction. The optimized Rt-PCR protocol showed high-level accuracy, precision, specificity, and sensitivity, each having a rate of 100%. The current study indicated that the TaqMan probe–based Rt-PCR protocol optimized and validated with serum samples can be reliably used for early diagnosis of brucellosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brucellosis is a zoonosis caused by Brucella genus, which can be transmitted by consuming unpasteurized milk and milk products or by direct contact with infected materials (Dadar et al. 2019). The exact incidence of Brucella infection in humans is unknown; however, the incidence is reported as < 0.1 per 100,000 populations (ECDC 2018; Majalija et al. 2018). The disease is prevalent in the Mediterranean region, including Turkey. In our country, the morbidity rate of disease is 10/100,000 (Deniz et al. 2015).
Heterogeneous and non-specific clinical symptoms of human brucellosis make the definitive diagnosis of the disease difficult. Culture and serological methods are frequently used in routine diagnostic laboratories. Although isolation of Brucella spp. from blood, bone marrow, or other infected tissues is a gold standard, it takes time and prior use of antibiotics reduces the likelihood of bacterial growth (Christopher et al. 2010; Wang et al. 2014; Barua et al. 2016; Kumar et al. 2019). Serological methods can provide useful information to the clinician in a shorter time than culture. Therefore, they have gained more importance than isolation. However, it is difficult to determine an exact limit in antibody titer for the standard tube agglutination test (STAT) or other serological methods commonly used in routine (Christopher et al. 2010; Wang et al. 2014). Agglutinins may be present at 1:80–1:160 titers in asymptomatic patients coming from endemic areas due to repeated exposure in the past. On the other hand, since the titers may be very low in the early bacteremic period of the disease, the STAT results can be negative or in the dilutions of 1:40–1:80 antibody titers (Christopher et al. 2010; Khan and Zahoor 2018). Additionally, cross-reactions with antibodies to Francisella tularensis, Escherichia coli O157, Salmonella spp., Moraxella phenylpyruvica, and Yersinia enterocolitica might be a problem in serological tests (Christopher et al. 2010; CDC 2017).
Due to shortcomings in routine diagnosis, a rapid, sensitive, and specific molecular diagnostic method has a crucial importance. Recently, many conventional PCR and Rt-PCR tests have been tried with different primers and probes specific for different gene regions such as encodes a 31-kDa cell surface protein (bcsp31), encodes a 26-kDa periplasmic protein (BP26), 16S rRNA, and insertion sequence IS711 (Newby et al. 2003; Sohrabi et al. 2014; Mohamed Zahidi et al. 2015; Moulana et al. 2016; Sanjuan-Jimenez et al. 2017; Dal et al. 2018; Kumar et al. 2019). However, there are big variations on sensitivity and specificity of the homemade protocols (Hasani et al. 2016; Sanjuan-Jimenez et al. 2017; Dal et al. 2019). In this study, we aimed to develop an optimized and validated Rt-PCR protocol for early and reliable diagnosis of brucellosis. We validated the efficacy of Rt-PCR protocol, which was optimized using a combination of Thermo DNA extraction kit with three different PCR amplification mixes in two thermal cycler devices, by determining the performance indicators such as accuracy, precision, sensitivity, and specificity of the method.
Material and method
Optimization of Rt-PCR protocol
Optimization of the Rt-PCR protocol was performed on serum samples spiked with B. melitensis ATCC 23456 standard strain by using a combination of one DNA extraction kit with three different PCR master mixes in two real-time PCR cyclers. The criterion used for the optimization in this study was to develop a Rt-PCR protocol that is able to yield positive results with the lowest number of B. melitensis.
Samples and DNA extraction
Positive serum samples were prepared by using decimal dilutions from 107 to 101 cfu/mL of heat-killed B. melitensis in sera collected from persons who did not meet possible or definitive diagnostic criteria defined by CDC for brucellosis (2017). Each of dilutions was prepared in ten Eppendorf tubes as 10 different samples, totally 70 samples for all dilutions. Negative serum samples were obtained from 20 healthy individuals. DNA extraction was carried out by a Thermo Scientific GeneJet Whole Blood genomic DNA Purification mini kit (Life Technologies Inc., Carlsbad, CA, USA), following the recommendations in kit instruction (https://www.thermofisher.com/order/catalog/product/ K0782). Briefly, 200 μL of serum was mixed with 20 μL of proteinase K and 400 μL of lysis solution. After incubation at 56 °C for 10 min, 200 μL of absolute ethanol was added and mixed by pipetting. Then, this mixture was transferred to a spin column and centrifuged. The column was washed by wash buffer I and wash buffer II. The DNA on the column was eluted with 50 μL of elution buffer.
Limit of detection of Rt-PCR protocol
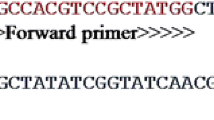
Limit of detection (LOD) of a SYBR Green and TaqMan probe–based protocols was investigated by using the DNA samples with a density of 4 × 104, 4 × 103, 40, 4, 0.4, and 0.04 genome equivalent per μL. LOD was analyzed in ten examples of each density. By using probit analysis, the LOD is defined as the lowest amount of Brucella genome/mL that is distinguishable from a negative control with a 95% level of confidence (Burd 2010). Primers and probes specific to all Brucella species and specific to only B. melitensis that is responsible for 99% of Brucella infections in our country were used (Probet et al. 2004; Dal et al. 2018). Furthermore, the primers and probes specific to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) enzyme gene were tested in order to control for the presence of possible inhibitors (Karataylı et al. 2014).
The first application was carried out using 1X SYBR Green PCR master mix (Thermo Fisher Scientific, USA) with addition of 10 pmol from each forward and reverse primer specific to B. melitensis and 4 μL template DNA in a total reaction volume of 25 μL. Amplification was performed in both Rotor-Gene (Corbett RG 6000, Australia) and Bio-Rad (CFX-96 C1000 Touch Real-Time System) thermal cyclers. The amplification conditions were as follows: initial denaturation at 95 °C for 10 min, followed by 45 cycles including denaturation at 95 °C for 15 s, annealing at 65 °C for 30 s, and elongation at 72 °C for 20 s. Finally, five additional minutes for extension was done at 72 °C. After the amplification, melting curve analysis was performed. When there was no amplification curve, the result was considered negative.
In the probe-based method, two different PCR master mixes, which were multiplex TEMPase 2x PCR master mix (Ampliqon, Copenhagen, Denmark) and QuantiTect Multiplex 2x PCR master mix (Qiagen, Hilden, Germany), were tested by using 10 pmol from each reverse and forward primer, 5 pmol from each probe, and 4 μL target DNA in a total reaction volume of 25 μL. Each reaction tube included primers and probes for Brucella spp., B. melitensis, and GAPDH. The amplification conditions for TEMPase PCR master mix were as follows: the initial denaturation at 95 °C for 10 min, followed by 45 cycles of 95 °C for 20-s denaturation, 60 °C for 50-s annealing, and 72 °C for 50-s extension. The amplification parameters used with QuantiTect PCR master mix included an initial denaturation at 94 °C for 10 min, followed by 45 cycles (denaturation at 94 °C for 15 s, annealing, and elongation at 60 °C for 90 s). Sterilized water was used as a negative control in each experiment.
Validation of the optimized Rt-PCR protocol
Since we obtained the lowest detection limit by using TaqMan probe–based protocols and there was no significant difference between the Rt-PCR reactions performed with multiplex TEMPase PCR master mix and QuantiTect PCR master mix to determine the lowest detection limit, the validation studies were performed by using a Thermo Scientific DNA isolation kit with TEMPase PCR master mix. The TEMPase PCR master mix is cheaper than the QuantiTect master mix, and the Thermo Scientific DNA purification kit allows DNA isolation from all clinical samples.
The validation studies of the optimized protocol were carried out by providing the minimum necessary criteria for qualitative nucleic acid detection methods generated within the laboratory (Rabenau et al. 2007; Raymaekers et al. 2009). The accuracy, precision, sensitivity, and specificity of the optimized method were performed with the positive (104 cfu/mL of B. melitensis), low positive (103 cfu/mL of B. melitensis), and negative samples as shown in Table 1.
Ethical statement
This study was approved by the ethics committee of Ankara Yıldırım Beyazıt University, Ankara/Turkey (protocol code 2017-125).
Results
Results of SYBR Green–based Rt-PCR protocol
Rt-PCR protocol performed with Thermo DNA Isolation Kit and Maxima SYBR Green master mix yielded positive results on all tested samples having B. melitensis density ≥ 104 cfu/mL. All samples were analyzed in both Bio-Rad and Rotor-Gene thermal cyclers. In Bio-Rad thermal cycler, the threshold cycle (CT) values of the samples containing 107, 106, 105, and 104 cfu/mL of the B. melitensis were approximately 23, 28, 33, and 37, respectively. These CT values were 27, 33, 37, and 39 in Rotor-Gene thermal cycler, respectively (Fig. 1). The Rt-PCR amplification curves were proved by melting curve analysis. Melting temperature of the all amplicons was recorded as 84 °C in both thermal cycler devices. Amplification curve could not be obtained from most of the samples spiked with B. melitensis less than 104 cfu/mL (103 and 102 cfu/mL). Probit analysis showed that the limit of detection of SYBR Green Rt-PCR protocol was 7 × 103 cfu of B. melitensis per mL of serum samples, equivalent to 112 DNA copies per PCR reaction. No difference on the limit of detection was recorded in two cyclers (Fig. 1).
In order to increase analytical sensitivity and limit of detection, probe-based Rt-PCR protocols were performed using two commercial PCR master mixes: Ampliqon Multiplex TEMPase 2X master mix and QuantiTect Multiplex 2X master mix.
Results of probe-based Rt-PCR protocols
Significant amplification curves were obtained in all samples including B. melitensis ≥ 103 cfu/mL with both QuantiTect PCR master mix and TEMPase PCR master mix. Positive results were obtained in 8 (80%) of the 10 samples having a density of 102 cfu/mL. By using probit analysis, the LOD value of both amplification mixes was found to be 218 cfu/mL (equivalent to ~ 4 DNA copies per amplification reaction). Amplification curves shifted from right to left in the samples having high bacterial density (Fig. 2).
The mean CT values for Brucella spp. and B. melitensis were 34.09 ± 0.49 and 35.07 ± 0.56 on the samples having 104 cfu/mL, respectively. For the density of 103 cfu/mL, the mean CT values for Brucella spp. and B. melitensis were found as 37.42 ± 0.39 and 38.83 ± 0.42, respectively. For the density of 102 cfu/mL, these values were 40.78 ± 0.45 and 41.1 ± 0.32, respectively. The CT values of the GAPDH primers generated with different master mixes ranged from 25.73 to 26.93 (mean 26.16 ± 009) (Fig. 3). The performance of two Rt-PCR cyclers was similar.
Results of validation studies
Amplification was observed in all samples including positive (n = 10) and low positive (n = 10) DNA templates. The analytic sensitivity of the optimized Rt-PCR protocol was estimated as 100% in the samples having B. melitensis DNA ≥ 103 copies/mL. The mean CT values for the samples having 104 and 103 copies DNA/mL were 35.07 and 38.83, respectively. The optimized Rt-PCR protocols yielded no amplification curve with 20 negative control samples indicating 100% specificity (Fig. 4). Both accuracy and precision parameters were found to be 100%. Details of the validation studies were provided in Table 2.
Rt-PCR amplification curves obtained from 10 positive (104 DNA copies/mL) and 10 low positive (103 DNA copies/mL) samples (left) and 20 negative control serums (right). Blue amplification curve represented Brucella spp., green amplification curve represented B. melitensis, and red amplification curve represented GAPDH. RFU relative fluorescence units
Discussion
Due to limitations in conventional laboratory tests used for brucellosis, DNA amplification–based methods have become commonly used approaches to help clinicians for more accurate evaluation of patients with suspected serological results or clinical symptoms. PCR-based approaches are able to give results in a short time; however, there are big variations on their accuracy, sensitivity, specificity, and reproducibility (Queipo-Ortuño et al. 2005a; Wang et al. 2014; Sanjuan-Jimenez et al. 2017). The effectiveness of the PCR protocols has been affected by many internal and external factors such as using different extraction protocols, PCR master mixes, amplification parameters, inhibitors, type of the clinical samples (serum, plasma, etc.), and the used target genes (Garshasbi et al. 2014; Sanjuan-Jimenez et al. 2017; Dal et al. 2018; Hull et al. 2018; Kumar et al. 2019). A Rt-PCR protocol optimized and validated in clinical samples can be a good alternative for making a final decision in the diagnosis and follow-up of human brucellosis.
In the current study, LOD of Rt-PCR protocols was investigated using a SYBR Green and two TaqMan probe–based master mixes in two real-time PCR thermal cyclers. The LOD of SYBR Green Rt-PCR was 112 DNA copies per reaction. In contrast to our result, the LOD of SYBR Green I–based Rt-PCR was reported as low as 5 fg (a genome equivalent) in a previous study (Queipo-Ortuño et al. 2005a). In that previous study, DNA was extracted by boiling method and Rt-PCR was performed with the primers specific for the gene encoding an immunogenic membrane protein of 31 kDa (bcsp31) of B. abortus in LightCycler instrument (Roche Diagnostic, Mannheim, Germany). In the current study, we used the primers targeting to bcsp31 for Brucella spp. and primers targeting to the insertion of an IS711 element downstream of BMEI1162 gene for B. melitensis on the DNA samples extracted by a commercial DNA isolation kit. Garshasbi et al. (2014) also showed that PCR efficacy was affected by the primers used in the PCR protocol. They obtained higher positivity with the primers specific for bcsp31 gene than those specific for the IS711 gene. Moreover, Thakur et al. (2018) used the primers specific for bcsp31 gene in SYBR Green Rt-PCR, and they found positive results on all samples having 10 or more DNA copies. By contrast, Bounaadja et al. (2009) compared the detection limit of Rt-PCR method performed with the primers and probes targeting the insertion sequence IS711, bcsp31, and per genes for the detection of Brucella at genus level, and the lowest detection limit (2 fg DNA) was reported with the primers targeting the IS711 gene.
Although SYBR Green–based PCR protocol is relatively cost-effective and easy to use, its low specificity because of any non-specific binding of SYBR Green to DNA is a problem (Tajadini et al. 2014). In order to increase the analytic sensitivity, specificity, and reproducibility of the Rt-PCR protocol, probe-based PCR protocols have become in use. In the current study, the TaqMan probe–based Rt-PCR protocols increased analytic sensitivity at least 28-fold; its LOD was defined as approximately 4 genome copies per reaction (218 cfu/mL) while it was 112 copies for SYBR Green–based PCR. Similar to our experimental design, two previous studies performed on the serum samples spiked with B. abortus and B. melitensis cells found analytical sensitivity of the TaqMan probe–based Rt-PCR as 2 genome copies (Queipo-Ortuño et al. 2008; Sohrabi et al. 2014). In another study, TaqMan probe–based Rt-PCR was performed on B. melitensis isolated from biopsy material and its LOD was reported as 57 copies/mg of tissue (Navarro-Martinez et al. 2008). Newby et al. (2003) used SYBR Green I, TaqMan, and hybridization probes (fluorescence resonance energy transfer [FRET]) to evaluate Rt-PCR detection of B. abortus. They found the lowest LOD for all three approaches as 2 genome copies; however, the higher efficiency was reported for probe-based Rt-PCR protocols (Table 3).
The limit of detection of PCR protocols have been directly affected by the quality and purity of the extracted DNA (Queipo-Ortuño et al. 2007; Olson and Morrow 2012; Ali et al. 2017; Dilhari et al. 2017; Hull et al. 2018). The DNA extraction protocols should be able to provide good-quality DNA and to remove the existing inhibitors in clinical samples. Recently, improved DNA isolation methods are available to remove inhibitors and to recover a maximum amount of DNA, which is acceptable for PCR amplification (Ali et al. 2017). The Thermo Scientific GeneJet Whole Blood genomic DNA purification mini kit, which is an enzymatic lysis and colon purification–based protocol, was used in the current study. We always obtained positive results on internal control DNAs, indicating successfully removing the inhibitors. This is in agreement with a previous study that tested the performance of this extraction protocol in whole blood samples (Dal et al. 2018).
The validation studies of the optimized Rt-PCR protocol indicate a complete accuracy and intra- and inter-assay reproducibility. Both intra- and inter-assay variations of our assay were lower than those of previous studies that used a similar approach (Queipo-Ortuño et al. 2005b, 2008). The CT values of repeated experiments on the samples spiked with equal numbers of B. melitensis were found to be close to each other. For instance, the mean CT values of 20 different Rt-PCR reactions for Brucella spp. and B. melitensis primers/probes were 34.09 ± 0.49 and 35.07 ± 0.56 on the samples having 104 cfu/mL, respectively. Such a limited variation in mean CT values was also recorded for samples having Brucella ≤ 103 cfu/mL. These results also provided additional data to support the reproducibility and reliability of the results obtained from the optimized Rt-PCR method in the current study.
TaqMan-based Rt-PCR protocol optimized and validated in the current study had a 90% sensitivity on the samples having ≥ 102 cfu/mL of B. melitensis and 100% specificity on negative control samples. As it was expected, while the sensitivity was 100% on the samples having ≥ 103 cfu/mL of B. melitensis (equal to 4 DNA copies per reaction), it decreased to 80% for the samples having 102 cfu/mL (equivalent to 1.6 DNA copies per reaction). In parallel to our study, a recent study, which used TaqMan probe–based Rt-PCR protocols on the samples spiked by B. melitensis standard strain, showed that the sensitivity was 100% at 6.25 genomes per reaction; however, it decreased to 80% at 3125 genomes per reaction (Kaden et al. 2017). Queipo-Ortuño et al. (2008) reported 93.5% sensitivity of TaqMan real-time PCR in the samples having 5 × 103 DNA copies/mL with a specificity of 98.4%. Sensitivity and specificity of PCR methods also showed variation based on conventional or Rt-PCR applications and reference tests used to evaluate the results of PCR protocols. The sensitivity of TaqMan Rt-PCR among the samples from brucellosis patients whose blood cultures were positive and patients suspected of having brucellosis was reported as 100% and 72%, respectively (Sohrabi et al. 2014). In a recently published study comparing TaqMan Rt-PCR with conventional diagnostic tests, 97.2%, 77.3%, and 83% sensitivities were reported on the culture, STAT, and Coombs’ positive samples, respectively (Dal et al. 2019). Mukherjee et al. (2015) optimized a TaqMan Rt-PCR having a sensitivity of 77.8% and specificity of 100%. Li et al. (2018) used a non-probe-based Rt-PCR for detection of brucellar spondylitis on formalin-fixed paraffin-embedded tissues, and they reported sensitivity of 93.5% and specificity of 100%. In another study performed on serum samples, the sensitivity and specificity of the SYBR Green Rt-PCR were estimated as 93.3% and 91.9%, respectively (Queipo-Ortuño et al. 2005a). Hinić et al. (2009) compared the performance of TaqMan Rt-PCR with serological tests on blood and tissue samples of wild boars, and they reported higher Rt-PCR positivities (11% for blood and 26% for tissues samples) than those of serological tests (16% for Rose-Bengal agglutination test and 7% for ELISA). In their study, there was no comparison of Rt-PCR with seropositive or negative samples. Das et al. (2018) compared the sensitivities of SYBR Green Rt-PCR, polymerase spiral reaction, and conventional PCR protocols on the stomach contents of fetal abortion animals; the sensitivities of these protocols were found as 44.6%, 44.6%, and 35.7%, respectively. In another study, the sensitivity and specificity of the conventional PCR protocols were reported to be 88–96% and 80.7%, respectively, when the standard tube agglutination test was used as a reference method (Garshasbi et al. 2014). Hajia et al. (2016) used conventional multiplex PCR, and they reported sensitivity as 37% in ELISA-positive and specificity as 6% in ELISA-negative serum samples (Table 3).
A variety of PCR protocols have been developed to detect Brucella spp. in blood or serum samples spiked with various number of Brucella spp., as well as in clinical samples. However, there are only few studies optimized and validated Rt-PCR to detect a low number of B. melitensis in clinical samples such as our study. The results of the current study showed that TaqMan-based Rt-PCR protocol was able to give reliable and reproducible results with a 90% sensitivity on samples having Brucella spp. ≥ 102 cfu/mL, which was much lower than that of the defined cutoff value of 5 × 103 copies/mL for active brucellosis (Queipo-Ortuño et al. 2008). Detection and identification of such a low number of B. melitensis in clinical samples are important because of the predominance of this species in Turkey and many other countries (Probet et al. 2004; Hajia et al. 2016; Mohamed Zahidi et al. 2015; Li et al. 2018; Dal et al. 2019).
In conclusion, we suggest that this sensitive and specific Rt-PCR protocol can be used as an alternative approach to overcome the drawbacks in the conventional tests for the diagnosis of brucellosis in patients with clinical signs compatible with brucellosis or in the case having fever with unknown origin in endemic area. Despite the clinical findings, this method may contribute to treatment in immunosuppressive individuals with seronegative results and change prognosis by shortening the time required for diagnosis.
References
Ali N, Rampazzo RCP, Costa ADT, Krieger MA (2017) Current nucleic acid extraction methods and their implications to point-of-care diagnostics. Biomed Res Int 2017:1–13. https://doi.org/10.1155/2017/9306564
Barua A, Kumar A, Thavaselvam D, Mangalgi S, Prakash A, Tiwari S, Arora S, Sathyaseelan K (2016) Isolation and characterization of Brucella melitensis isolated from patients suspected for human brucellosis in India. Indian J Med Res 143:652–658. https://doi.org/10.4103/0971-5916.187115
Bounaadja L, Albert D, Chénais B, Hénault S, Zygmunt MS, Poliak S, Garin-Bastuji B (2009) Real-time PCR for identification of Brucella spp.: a comparative study of IS711, bcsp31 and per target genes. Vet Microbiol 137(1–2):156–164. https://doi.org/10.1016/j.vetmic.2008.12.023
Burd EM (2010) Validation of laboratory-developed molecular assays for infectious diseases. Clin Microbiol Rev 23(3):550–576. https://doi.org/10.1128/CMR.00074-09
Centers for Disease, Control and Prevention (2017) Brucellosis reference guide: exposure, testing and prevention National center for Emerging and Zoonotic Infectious Diseases. www.cdc.gov/brucellosis/pdf/brucellosi-reference-guide.pdf. Accessed 21 June 2019
Christopher S, Umapathy BL, Ravikumar KL (2010) Brucellosis: review on the recent trends in pathogenicity and laboratory diagnosis. J Lab Physicians 2(2):55–60. https://doi.org/10.4103/0974-2727.72149
Dadar M, Shahali Y, Whatmore AM (2019) Human brucellosis caused by raw dairy products: a review on the occurrence, major risk factors and prevention. Int J Food Microbiol 292:39–47. https://doi.org/10.1016/j.ijfoodmicro.2018.12.009
Dal T, Açıkgöz ZC, Başyiğit T, Zeybek H, Durmaz R (2018) Comparison of two commercial DNA extraction kits and PCR master mixes for the detection of Brucella from blood samples and blood culture bottles. Mikrobiol Bul 52(2):135–146. https://doi.org/10.5578/mb.66742
Dal T, Kara SS, Cikman A, Balkan CE, Acıkgoz ZC, Zeybek H, Uslu H, Durmaz R (2019) Comparison of multiplex real-time polymerase chain reaction with serological tests and culture for diagnosing human brucellosis. J Infect Public Health 12(3):337–342. https://doi.org/10.1016/j.jiph.2018.11.008
Das A, Kumar B, Chakravarti S, Prakash C, Singh RP, Gupta V, Singh PK, Agrawal RK, Chaturvedi VK, Abhishek SG (2018) Rapid visual isothermal nucleic acid-based detection assay of Brucella species by polymerase spiral reaction. J Appl Microbiol 125(3):646–654. https://doi.org/10.1111/jam.13882
Deniz S, Baykam N, Celikbas A, Yilmaz SM, Guzel TC, Dokuzoguz B, Ergonul O (2015) Screening household members of acute brucellosis cases in endemic areas and risk factors for brucellosis. Vector-Borne Zoonotic Dis 15(8):468–472. https://doi.org/10.1089/vbz.2014.1723
Dilhari A, Sampath A, Gunasekara C, Fernando N, Weerasekara D, Sissons C, McBain A, Weerasekera M (2017) Evaluation of the impact of six different DNA extraction methods for the representation of the microbial community associated with human chronic wound infections using a gel-based DNA profiling method. AMB Express 7(1):179. https://doi.org/10.1186/s13568-017-0477-z
European Centre for Disease Prevention and Control (2018) Brucellosis in ECDC annual epidemiological report for 2016. Stockholm. https://www.ecdc.europa.eu/sites/portal/files/documents/AER_for_2016-brucellosis.pdf. Accessed 21 June 2019
Garshasbi M, Ramazani A, Sorouri R, Javani S, Moradi S (2014) Molecular detection of Brucella species in patients suspicious of brucellosis from Zanjan, Iran. Braz J Microbiol 45(2):533–538. https://doi.org/10.1590/S1517-83822014005000048
Hajia M, Fallah F, Angoti G, Karimi A, Rahbar M, Gachkar L, Mokhtari B, Sanaei A, Lari AR (2016) Comparison of methods for diagnosing brucellosis. Lab Medicine 44:29–33. https://doi.org/10.1309/LM4J9MWOBIPA6RBN
Hasani SM, Mirnejad R, Piranfar V, Amani J, Vafadar MJ (2016) Comparing rapid and specific detection of Brucella in clinical samples by PCR-ELISA and multiplex-PCR method. Iran J Pathol 11(2):144–150
Hinić V, Brodard I, Thomann A, Holub M, Miserez R, Abril C (2009) IS711-based real-time PCR assay as a tool for detection of Brucella spp. in wild boars and comparison with bacterial isolation and serology. BMC Vet Res 5:22. https://doi.org/10.1186/1746-6148-5-22
Hull N, Miller J, Berry D, Laegreid W, Smith A, Klinghagen C, Schumaker B (2018) Coptimization of Brucella abortus protocols for downstream molecular applications. J Clin Microbiol 56(4). https://doi.org/10.1128/JCM.01894-17
Kaden R, Ferrari S, Alm E, Wahab T (2017) A novel real-time PCR assay for specific detection of Brucella melitensis. BMC Infect Dis 17(1):230. https://doi.org/10.1186/s12879-017-2327-7
Karataylı E, Çelik Altunoglu Y, Karataylı SC, Alagoz KSG, Çınar K, Yalçın K, İdilman R, Yurdaydın C, Bozdayı AM (2014) A one step real time PCR method for the quantification of hepatitis delta virus RNA using an external armored RNA standard and intrinsic internal control. J Clin Virol 60(1):11–15. https://doi.org/10.1016/j.jcv.2014.01.021
Khan MZ, Zahoor M (2018) An overview of brucellosis in cattle and humans, and its serological and molecular diagnosis in control strategies. Rev Trop Med Infect Dis 3(2):65. https://doi.org/10.3390/tropicalmed3020065
Kumar V, Bansal N, Nanda T, Kumar A, Kumari R, Maan S (2019) PCR based molecular diagnostic assays for brucellosis: a review. Int J Curr Microbiol App Sci 8(2):2666–2681. https://doi.org/10.20546/ijcmas.2019.802.312
Li M, Zhou X, Li J, Sun L, Chen X, Wang P (2018) Real-time PCR assays for diagnosing brucellar spondylitis using formalin-fixed paraffin-embedded tissues. Medicine (Baltimore) 97(9):e0062. https://doi.org/10.1097/MD.0000000000010062
Majalija S, Luyombo P, Tumwine G (2018) Sero-prevalence and associated risk factors of brucellosis among malaria negative febrile out-patients in Wakiso district, Central Uganda. BMC Res Notes 11:803. https://doi.org/10.1186/s13104-018-3907-3
Mohamed Zahidi J, Bee Yong T, Hashim R, Mohd Noor A, Hamzah SH, Ahmad N (2015) Identification of Brucella spp. isolated from human brucellosis in Malaysia using high-resolution melt (HRM) analysis. Diagn Microbiol Infect Dis 81(4):227–233. https://doi.org/10.1016/j.diagmicrobio.2014.12.012
Moulana Z, Roushan MR, Marashi SM (2016) Evaluation of different primers for detection of Brucella by using PCR method. Electron Physician 8(11):3222–3227. https://doi.org/10.19082/3222”10.19082/3222
Mukherjee F, Nagmani K, Surendra KSNL, Subramanian BM, Bahekar VS, Prasad A, Rana SK, Muthappa PN, Sharma GK, Srinivasan VA (2015) Optimization and validation of a diagnostic real-time PCR for bovine brucellosis. Adv Anim Vet Sci 3(11):577–587. https://doi.org/10.14737/journal.aavs/2015/3.11.577.587
Navarro-Martinez A, Navarro E, Castano MJ, Solera J (2008) Rapid diagnosis of human brucellosis by quantitative real-time PCR: a case report of brucellar spondylitis. J Clin Microbiol 46:385–387. https://doi.org/10.1128/JCM.01303-07
Newby DT, Hadfield TL, Roberto FF (2003) Real-time PCR detection of Brucella abortus: a comparative study of SYBR green I, 5′-exonuclease, and hybridization probe assays. Appl Environ Microbiol 69(8):4753–4759. https://doi.org/10.1128/aem.69.8.4753-4759.2003
Olson ND, Morrow JB (2012) DNA extract characterization process for microbial detection methods development and validation. BMC Res Notes 5:668. https://doi.org/10.1186/1756-0500-5-668
Probet WS, Schrader KN, Khuong NY, Bystrom SL, Graves MH (2004) Real-time multiplex PCR assay for detection of Brucella spp., B. abortus, and B. melitensis. J Clin Microbiol 42(3):1290–1293. https://doi.org/10.1128/jcm.42.3.1290-1293.2004
Queipo-Ortuño MI, Colmenero JD, Baeza G, Morata P (2005a) Comparison between lightCycler real-time polymerase chain reaction (PCR) assay with serum and PCR-enzyme-linked immunosorbent assay with whole blood samples for the diagnosis of human brucellosis. Clin Infect Dis 40(2):260–264. https://doi.org/10.1086/426818
Queipo-Ortuño MI, Colmenero JD, Reguera JM, Garcia-Ordonez MA, Pachon ME, Gonzalez M, Morata P (2005b) Rapid diagnosis of human brucellosis by SYBR Green I-based real-time PCR assay and melting curve analysis in serum samples. Clin Microbiol Infect 11(9):713–718. https://doi.org/10.1111/j.1469-0691.2005.01202.x
Queipo-Ortuño MI, Colmenero JD, Macias M, Bravo MJ, Morata P (2007) Preparation of bacterial DNA template by boiling and effect of immunoglobulin G as an inhibitor in real-time PCR for serum samples from patients with brucellosis. Clin Vaccine Immunol 15(2):293–296. https://doi.org/10.1128/CVI.00270-07
Queipo-Ortuño MI, Colmenero JD, Bravo MJ, Garcıa-Ordonez MA, Morata P (2008) Usefulness of a quantitative real-time PCR assay using serum samples to discriminate between inactive, serologically positive and active human brucellosis. Clin Microbiol Infect 14:1128–1134. https://doi.org/10.1111/j.1469-0691.2008.02095.x
Rabenau HF, Kessler HH, Kortenbusch M, Steinhorst A, Raggam RB, Berger A (2007) Verification and validation of diagnostic laboratory tests in clinical virology. J Clin Virolog 40(2):93–98. https://doi.org/10.1016/j.jcv.2007.07.009
Raymaekers M, Smets R, Maes B, Cartuyvels R (2009) Checklist for optimization and validation of real-time PCR assays. J Clin Lab Anal 23(3):145–151. https://doi.org/10.1002/jcla.20307
Sanjuan-Jimenez R, Colmenero JD, Morata P (2017) Lessons learned with molecular methods targeting the BCSP-31 membrane protein for diagnosis of human brucellosis. Clin Chim Acta 469:1–9. https://doi.org/10.1016/j.cca.2017.03.014
Sohrabi M, Mobarez M, Khoramabadi N, Doust RH, Behmanesh M (2014) Efficient diagnosis and treatment follow-up of human brucellosis by a novel quantitative TaqMan real-time PCR assay: a human clinical survey. J Clin Microbiol 52(12):4239–4243. https://doi.org/10.1128/JCM.01819-14
Tajadini M, Panjehpour M, Javanmard SH (2014) Comparison of SYBR Green and TaqMan methods in quantitative real-time polymerase chain reaction analysis of four adenosine receptor subtypes. Adv Biomed Res 3:85. https://doi.org/10.4103/2277-9175.127998
Thakur S, Bedi JS, Singh R, Gill JPS, Arora AK, Kashyap N (2018) Quantitative polymerase chain reaction based quantification of Brucella DNA in serum of pre- and post-therapeutic occupationally exposed infected human population. J Infect Public Health 11(4):514–520. https://doi.org/10.1016/j.jiph.2017.10.004
Wang Y, Wang Z, Zhang Y, Bai L, Zhao Y, Liu C, Ma A, Yu H (2014) Polymerase chain reaction-based assays for the diagnosis of human brucellosis. Ann Clin Microbiol Antimicrob 13:31. https://doi.org/10.1186/s12941-014-0031-7
Authors and contributors
RD: Management and coordination responsibility for the research activity planning and execution. Preparation of the published work, specifically critical review of the manuscript. Formulation and evaluation of research goals and aims.
HZ: Conducting a research and investigation process, specifically performing the experiments and data collection. Writing the initial draft and evaluation of the experimental data.
ZCA: Performing data analysis and review of the manuscript.
TD: Writing the initial draft, evaluation of the experimental data, and analysis of the raw data.
Funding
This study was supported by Ankara Yıldırım Beyazıt University, Research Foundation (Grant No. 4759).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeybek, H., Acikgoz, Z.C., Dal, T. et al. Optimization and validation of a real-time polymerase chain reaction protocol for the diagnosis of human brucellosis. Folia Microbiol 65, 353–361 (2020). https://doi.org/10.1007/s12223-019-00731-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-019-00731-1