Abstract
Zika virus (ZIKV) is a single-stranded RNA virus in the Flaviviridae family and transmitted to human through infected mosquitos (Aedes aegypti and Aedes albopictus). Virus is closely related with other flaviviruses; dengue virus, yellow fever virus, West Nile virus, and Japanese encephalitis virus phylogenetically. Due to the possible relationship between virus and clinical features including microcephaly, ventricule, and eye deformities, Guillain-Barre syndrome increases the interest on this virus gradually. Along with the vector-borne transmission, exposure via blood transfusion and sexual contact are further concerns. Since December 2015, CDC reported 440.000–1.300.000 possible cases in Brazil and as of 19 January 2016, El Salvador, Venezuela, Colombia, Brazil, Surinam, French Guana, Honduras, Mexico, and Panama are the countries with active epidemic. CDC recommends ZIKV screening for all pregnants including asymptomatic cases those living in the active epidemic areas. Recently, virus is detected in the USA and most European countries including UK, Netherlands, Denmark, Switzerland, and Italy as a travel-associated infection. Owing to the changing world with increased capabilities for transportation globally, this vector-borne infection represents a valuable marker for the ability of spreading of any infection from its original area that it was first seen. In this review, we summarized the up-to-date data and reports in terms of the importance of the ZIKV infection in the public health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zika virus (ZIKV) is a RNA virus belongs to the family Flaviviridae and the genus Flavivirus. Phylogenetically, the virus is closely related with other members of the genus Flavivirus, including dengue virus, West Nile virus, yellow fever virus, and Japanese encephalitis virus. Humans and primates are the main reservoirs, and the virus is transmitted to the human by the infected Aedes mosquito bites, Aedes aegypti and Aedes albopictus, known as the vector for dengue virus and chikungunya virus. Infection is generally asymptomatic and symptoms develop only in the 20 % of the infected individuals. Clinical case is mild and self-limited with symptoms including fever, maculopapullar rash, myalgia, conjunctivitis, and other opthalmological signs lasting for a week (Marcondes and Ximenes 2016; Petersen et al. 2016). Recently, CDC concluded that Zika virus infection during pregnancy could be the cause of microcephaly and other severe fetal brain defects.
The structure of the virus and the pathogenesis
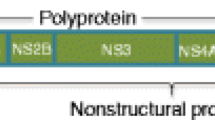
ZIKV is an enveloped, single-stranded, non-segmented, and positive-sense RNA virus with an icosahedral capsid (Marcondes and Ximenes 2016). Two species of the virus, African and Asian origins, were detected. Phylogenetic studies have shown that the virus spread through America was associated with the Asian-strain responsible for the outbreak in French Polynesia in 2013 (Zanluca et al. 2015). Virion is approximately 40 nm in diameter and has protrusions on its surface. The nucleocapsid is 25–30 nm in diameter and surrounded by the host membrane. Genome of the virus is 10,794 bases in length and comprises of two separate regions, 5’NCR and 3’NCR, and encodes three structural and seven non-structural proteins. The envelope includes E and M proteins. E protein covers a large portion of the virion surface and plays role in host cell binding during replication and membrane fusion. ZIKV ORF (open reading frame) region sequence is 5′-C- prM-E-NS1-NS2A-NS2B-NS3-NS4A-NS4B-NS5–3′ and encodes capsid (C), precursor membrane (prM), envelope (E), and polyprotein which turns into non-structural proteins (NS). NS1, N3, and NS45 are large, conservative proteins. However, NS2A, NS2B, NS4A, and NS4B are small and hydrophobic proteins. NCR on the 3′ district consists of 428 nucleotides in the genome and it performs translation, RNA packaging, genome stability, and recognition (Chambers et al. 1990).
Viral replication occurs in the cytoplasm in mammalian cell and nucleus in mosquitoes. The initial phase in replication is binding of protein E to the host cell membrane receptors through receptor-mediated endocytosis and entrance into the host cell with a similar event as in apoptosis. After the binding of viral membrane and endosomal membrane of the host, the virus ssRNA genome is released into the cytoplasm of the host cell. dsRNA is synthesized from genomic ssRNA. Polyproteins are generated from positive-sense genomic ssRNA that will convert to structural and non-structural proteins. dsRNA replicates to generate viral mRNA/new ssRNA genome. Virion is transported by Golgi apparatus. prPM protein leaves Golgi and virion matures, and new virions are secreted with exocytosis (Perera et al. 2008).
Epidemiology and transmission
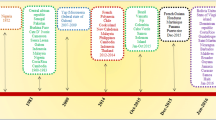
Zika virus is first detected in a monkey in the “Zika” forest in Uganda in 1947. It was isolated from a mosquito, Aedes africanus, in the same region shortly after. The first human case was reported in Uganda in 1952. Spread of the virus increased gradually through years and has been identified in many parts of the Africa continent. The first major outbreak outside Africa was observed at the Yap islands of Micronesia in April–July 2007 and 49 confirmed; 59 probable cases were reported during this epidemic (Lanciotti et al. 2008). No local cases were reported from South America until May 2015, but the first case was reported from Brazil in May. Brazil is the most affected country from the epidemic with 440.000–1.300.000 local cases since December 2015 (Marcondes and Ximenes 2016; ECDC 2015). The most possible route for viral entry to South America could have occured during the 2014 World Cup Soccer Tournament held in Brazil, but this hypothesis has been discredited because of the lack of participation from Pacific countries where ZIKV was previously reported (Zanluca et al. 2015). The virus is expected to enter to South America continent soon after the World Canoeing Championship held in Brazil, 2014, that Pacific countries had participated (Musso et al. 2014).
From February 2, 2016, local cases have been reported from American Samoa, Barbados, Bolivia, Brazil, Colombia, Honduras Costa Rica, Equator, El Salvador, Saint Martin, Fiji, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Jamaica, Maldives, Martinique, Mexico, New Caledonia, Nicaragua, Panama, Paraguay, Puerto Rico, Dominican Republic, Samoa, Suriname, Thailand, Tonga, Vanuatu, Solomon Islands, Venezuela, and the Virgin Islands (Lanciotti et al. 2008; ECDC 2016). Local cases were not reported until recently in Europe, but as of February 2016, imported cases have been reported from 14 EU/EEA countries including Austria, Denmark, Finland, France, Germany, Ireland, Italy, Malta, Netherlands, Portugal, Spain, Sweden, Slovenia, and UK (ECDC 2016).
Although, the most important vector in the transmission is A. aegypti mosquitoes, the virus has been identified in many species such as A. albopictus, A. africanus, Aedes apicoargenteus, Aedes vitattus, Aedes furcifer, Aedes luteocephalus, and Aedes hensilli (ECDC 2015; Hayes 2009). Aedes species of mosquitoes are found in many parts of the world, mostly distributed, A.aegypti in tropical and subtropical regions, and A.albopictus in the Mediterranean countries (Musso et al. 2014; Hayes 2009; Wong et al. 2013). A. aegypti is widely distributed to all America continent except Chile and is generally located and fed from human in city centers, leaving their eggs in stagnant water, flower pot (Wong et al. 2013). A. albopictus is generally located in rural areas and nearby the cities. They feed on many birds and mammals, including human. It has been identified around Southern European countries and northern regions of the USA including New York and Chicago (Wong et al. 2013). A. aegypti and A. albopictus are invasive species as they can arrive at new places by human movements and travels.
Aedes mosquitoes get infected after feeding from the infected human. Virions are inoculated into the skin of the new host during feeding and are replicated in dendritic cells and causes viremia via transmission to the bloodstream and lymph nodes and infection of the dermis and epidermis cells (Hayes 2009). Transmission to a newborn or infant could be observed during all stages of pregnancy and rarely during delivery. Risk for microcephaly is higher in the first 3 months of pregnancy. Although viral RNA has been detected in breast milk, transmission by breastfeeding has not been reported. During the outbreak identified in French Polynesia in 2013–2014, ZIKV positivity by PCR was observed in 3 % of asymptomatic blood donors (ECDC 2015). Sexual transmission and presence of the virus in seminal fluid up to 2 months, indicates the importance of protection in the transmission. Seminal fluid should be considered as a valuable specimen type especially in the diagnosis of symptomatic individuals with travel history (Musso et al. 2015).
Clinical features
Infection is characterized by acute onset of fever, maculopapular rash, myalgia, conjunctivitis, and other ocular findings after an incubation period of 3–12 days. Asymtomatic infection is common and symptoms develop only in 20 % of the infected cases. Moderate or mild symptoms can last for a week and severe illness requiring hospitalization is uncommon (Petersen et al. 2016; ECDC 2015). In some cases, edema in the extremities, retro-orbital pain, iridocyclitis, vertigo, axillary and inguinal lymphadenopathy, and gastrointestinal system disorders have been reported (Zanluca et al. 2015; Fontes Bruno 2016). Clinical signs and medical history is similar to other mosquito-borne diseases such as dengue and chikungunya virus infection, and the presence of the travel history of a patient is a clue to the clinician for the ZIKV infection (Petersen et al. 2016). The virus can spread to the blood-brain and ocular barrier causing clinical ocular symptoms consequently (Fontes Bruno 2016). It is considered that a relationship was observed between congenital anomalies and viral infection. Presence of the virus in the amniotic fluid of a microcephalic fetus is a significant evidence for intrauterine transmission. Moreover, ventriculomegalia, polyhydramnios, cell migration anomalies, central or peripheral nervous system involvement secondary to congenital contractures after maternal infection are reported (ECDC 2015).
Case classification
Possible case; Presence of one or more of the following symptoms; rash or unexplained other medical reasons with fever >37.2 °C, arthralgia/myalgia, or non-purulent conjunctivitis, conjunctival hyperemia, headache or fatigue.
Confirmed case is defined as positive test results for ZIKV specific tests (PAHO 2015).
Laboratory diagnosis
The diagnosis of infection is difficult because of atypical clinical and laboratory findings, but leukopenia and thrombocytopenia have been reported in most cases (Zanluca et al. 2015). Clinical information, destination of travel should be evaluated along with the activities carried out and laboratory findings. Similar with other flaviviruses, serological tests, viral culture, and nucleic acid amplification techniques are used in diagnosis. Viral genome was isolated and development of point of care testing is ongoing (ECDC 2015).
Direct detection and serological diagnostic tests
Antibody diagnostic tests (IgM and plaque reduction neutralization test-PRNT) and virus isolation can be performed in patient sera and cerebrospinal fluid. As virus isolation requires biosecurity level 3 laboratory design, which takes long time and is labor intensive, serological methods are in routine use similar with other arboviral diseases. Although specific IgM response and neutralizing antibodies develop towards the end of the first week of the disease, antibody detection by ELISA at the third day was also reported. It should be considered that IgM is not specific for ZIKV, and positivity could be the result of (i) cross-reaction with dengue, JEV, WNV, YFV; (ii) false positivity due to the yellow fever vaccine; and (iii) acute infection of flaviviruses including JEV, Murray Valley, and ZIKV (Hayes 2009). Therefore, PRNT is required for the detection of virus-specific neutralizing antibodies. However, neutralizing antibodies could be seen in recent flavivirus infections and cross-reaction could be observed due to yellow fewer and JEV vaccinations (Hayes 2009). In such cases, ZIKV infection is confirmed by the presence of neutralizing antibody titer four or more times higher than dengue virus titers. Values less than four times are considered as borderline test result and repeated tests with a new sample is suggested after 2 weeks. Co-infection of ZIKV and dengue virus should be taken into account as well.
Molecular tests
CDC suggests viral RNA detection in the serum after the first week of symptoms. Viremia generally lasts for a few days but sometimes can prolong up to 10 days (Hayes 2009). As detection of virus by RT-PCR in blood is difficult due to the short duration of viremia, urine is a valuable specimen for virus detection by RT-PCR with the advantages of virus excretion up to 3 weeks after the development of the symptoms (Gourinat et al. 2015). It is reported that virus could be detected for longer periods in seminal fluid compared to blood, and also there were cases in which ZIKV positivity were identified only by seminal fluid at the 27th and 62nd day after symptoms’ onset (Musso et al. 2015). In near future, ZIKV should be defined as sexually transmitted disease considering the fact that virus can be detected in seminal fluid (Musso et al. 2015). Urine specimen should have priority over seminal fluid in the diagnosis if the specimen sampling was performed simultaneously. Viral nucleic acid was also isolated from saliva. In such cases, dry cotton swab should be used for sampling and transported in viral transport medium.
Diagnosis of congenital infection
Virus can be detected from amniotic fluid, fetal cerebrospinal fluid, cord blood, and infant blood at the second day of birth and placenta by RT-PCR (Calvet et al. 2016). Virus would not be detected by RT-PCR if viremia period is over for the newborn who is exposed to virus during pregnancy. Therefore, it is recommended to perform ZIKV IgM screening with ELISA in infant blood and cerebrospinal fluid samples and maternal blood sample. Dengue virus IgM antibodies should be also screened in ZIKV-suspected cases since false positive results can be obtained due to cross-reaction with other flaviviruses (Table 1). Plaque reduction neutralization test should be applied in confirmation of positive serological tests and determination of specific neutralizing antibodies against ZIKV. However, it should be considered that cross-reactions could be observed because of the maternal antibodies. Viral RNA was also detected in amniotic fluid by RT-PCR (Wong et al. 2013).
It is reported that virus was not detected in maternal blood and urine samples of microcephalic fetuses, but strain displaying 97–100 % genomic similarity with the strain of 2013 French Polynesia outbreak was identified in the amniotic fluid (Calvet et al. 2016). Leptospirosis, malaria, rickettsioses, rubella, parvovirus, enterovirus, adenovirus, and alphavirus infections (Mayaro, chikungunya, Barmah Forest, Ross River, Sindbis viruses) should also be considered in differential diagnosis.
Treatment and prevention
There is no specific antiviral agent to treat infection. Symptomatic therapy (antipyretics, antihistamines) is adequate in most cases. Nonsteroidal anti-inflammatory drugs and aspirin are not recommended as they increase the risk of bleeding (ECDC 2015). Although clinical cases often progress mildly, patients should also be monitored against the risk of coagulopathy and development of multiple organ failure. There is no vaccine available for protection against infection. CDC and ECDC warn travelers to take personal protective measures against mosquitoes, postpone travel plans to risky areas for pregnant women and/or women with pregnancy plans as well as screening for ZIKV for pregnant women who had visited affected regions even if they were asymptomatic. Pregnants diagnosed with ZIKV should be advised to be consulted by an obstetrician and perinatologist for a series of ultrasonography in 3–4 weeks intervals.
Mosquito repellents should be used throughout the day to prevent exposure, and it is also recommended to wear long-sleeved shirts and pants especially during periods of most intense mosquito activity. N-N diethyl-meta-toluamide (DEET) based mosquito repellents are not recommended for infants younger than 3 months, but it has been reported that it can be used in pregnant women up to 50 % concentration (ECDC 2015). People who have traveled to active ZIKV and dengue virus infection areas should not be accepted for blood and tissue donors. Viral RNA screening is also recommended for all blood donated in affected areas (Petersen et al. 2016).
Conclusion
Besides chikungunya and dengue viruses, ZIKV should also be investigated in the presence of clinical signs such as high fever, maculopapular rash, myalgia, and weakness for people who visited areas where infection is endemic. The most important difference of ZIKV from other arboviral infection is its capability of affecting the fetus through placenta in pregnant women. The main reason for gaining global importance for ongoing epidemic is relation with microcephaly. The development and introduction of specific and rapid diagnostic tests will be useful for detecting microcephaly, GBS, and other risks associated with ZIKV.
References
Calvet G, Aguiar RS, Melo AS, Sampaio SA, de Filippis I, Fabri A, Araujo ES, de Sequeira PC, de Mendonça MC, de Oliveira L, Tschoeke DA, Schrago CG, Thompson FL, Brasil P, Dos Santos FB, Nogueira RM, Tanuri A, de Filippis AM (2016) Detection and sequencing of zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis doi. doi:10.1016/S1473-3099(16)00095-5
Chambers T, Hahn C, Galler R, Rice C (1990) Flavivirus genome organization, expression, and replication. Ann Rev Microbiol 44:649–688
European Centre for Disease Prevention and Control (2015) Rapid risk assessment. Zika virus epidemic in the Americas: potential association with microcephaly and Guillain-Barré syndrome. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association-with-microcephaly-rapid-risk-assessment.pdf
ECDC (2016) Communicable disesases threat report week 6. http://ecdc.europa.eu/en/publications/Publications/communicable-disease-threats-report--13-feb-2016.pdf
Fontes Bruno M (2016) Zika virus-related hypertensive iridocyclitis. Arq Bras Oftalmol 79(1):63
Gourinat A-C, O’Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M (2015) Detection of zika virus in urine. Emerg Infect Dis 21(1):84–86
Hayes EB (2009) Zika virus outside Africa. Emerg Infect Dis 15:1347–1350
Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ (2008) Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14:1232–1239
Marcondes CB, Ximenes MF (2016) Zika virus in Brazil and the danger of infestation by Aedes (Stegomyia) mosquitoes. Rev Soc Bras Med Trop 49(1):4–10
Musso D, Nilles EJ, Cao-Lormeau V-M (2014) Rapid spread of emerging zika virus in the Pacific area. Clin Microbiol Infect 20:595–596
Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM (2015) Potential sexual transmission of Zika virus. Emerg Infect Dis 21:359–361
Pan American Health Organisation (PAHO) (2015) ZIKV surveillance in the Americas: Interim guidance for laboratory detection and diagnosis. http://iris.paho.org/xmlui/handle/123456789/18602. Accessed 26 Jul 2016
Perera R, Khaliq M, Kuhn RJ (2008) Closing the door for flaviviruses: entry as a target for antiviral drug design. Antivir Res 80(1):11–22
Petersen E, Wilson ME, Touch S, McCloskey B, Mwaba P, Bates M, Dar O, Mattes F, Kidd M, Ippolito G, Azhar EI, Zumla A (2016) Rapid spread of zika virus in the Americas- implications for public health preparedness for mass gatherings at the 2016 Brazil Olympic games. Int J Infect Dis 44:11–15
Zanluca C, de Melo VCA, Mosimann ALP, dos Santos GIV, dos Santos CND, Luz K (2015) First report of autochthonous transmission of zika virus in Brazil. Mem Inst Oswaldo Cruz 110:569–572
Wong PS-J, Li MY, Chong CS, Ng LC, Tan CH (2013) Aedes (Stegomyia) albopictus (Skuse): a potential vector of ZIKV in Singapore. PLoS Negl Trop Dis 7:e2348
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Demir, T., Kilic, S. Zika virus: a new arboviral public health problem. Folia Microbiol 61, 523–527 (2016). https://doi.org/10.1007/s12223-016-0467-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-016-0467-6