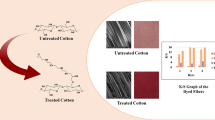
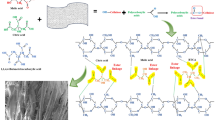
Abstract
An attempt was made to modify the cotton and polyester/cotton (P/C) fabric with a bi-functional cross-linking compound, hexamethylene diisocyanate, to covalently link the cellulosic part of the fabric with the selected disperse dyes. The chemical composition, morphology, and strength of the modified fabric were examined by ATR–FTIR, SEM–EDX, tensile strength testing, water absorbency test, and thermogravimetric analysis (TGA). The results verified the binding of the cross-linker with the cellulose fabric under neutral conditions. The cross-linked fibers were dyed with commercially available disperse dyes bearing amino and/or hydroxyl groups by using the pad–dry–cure dyeing method. The K/S values were measured to analyze the depth of shade on the modified fibers in comparison with the respective untreated dyed samples. The fastness properties of the dyed fabrics were studied by employing ISO standard protocols. It was observed that the modified fabrics have a good overall fastness.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cellulose, a complex hydrocarbon, has been used widely as a clothing material since ancient times. It is a polar molecule having hydroxyl groups in its structure. Fabric made of cellulose fiber is soft, hygroscopic, and breathable, but it also has some drawbacks, such as shrinkage, wrinkling, etc., which limit its use in pure form. Therefore, to add the desired characteristics, it is blended with other fibers, most often polyester. With a global market share of 58.54%, cotton and polyester blend (P/C blend) is the most popular of the blended fabrics worldwide [1]. P/C blend is more absorbent, softer, comfortable, and user-friendly than polyester, while it is more economical, crease resistant, and stronger than 100% cotton [2,3,4]. Despite the distinct attributes, the dyeing of the blend has remained a major challenge for the dyeing industries globally, due to its high cost, and excessive time, energy, and labor requirements. Environmental issues are also associated with its dyeing. In the conventional dyeing method of P/C blend, the polyester portion is colored using disperse dyes, followed by the removal of the unfixed dye by reduction clearing, and finally dyeing the cotton portion with reactive dyes [5]. Scientists all over the world are striving to find a way to dye both the constituents of the blended fabric by using a single type of dye. For this reason, various attempts have been made to modify one of the fibers [3, 6,7,8,9,10,11], but none could find its way to industrial application. New dye molecules, named as disperse reactive dyes, have also been introduced for one-step dyeing of P/C blend using a single dye [12,13,14,15].
Polyisocyanate-based cross linkers are widely used in the textile industry for finishing processes and their ability to bind various compounds to cellulose fabric has been documented in the literature [16,17,18,19,20,21,22,23,24,25,26]. The current study focused on the repurposing of a diisocyanate cross-linker, blocked hexamethylene diisocyanate (HDI), to accomplish one-bath dyeing of the P/C blend with nucleophilic disperse dyes without compromising the performance properties of the fabric such as thermal stability and tensile strength. For this purpose, 100% cellulose as well as 50/50 blend of cellulose with polyester was treated with blocked HDI. The HDI functions as a bridge between the dye and the cellulose fiber by covalently binding to the hydroxyl groups of the cellulose on one side and the amino or hydroxyl group of the disperse dye on the other. Various analytical techniques including tensile strength testing, TGA, ATR–FTIR, SEM–EDX, etc., were used to analyze the strength, morphology, and chemical composition of the modified specimens. The dyeing performance of the modified fibers was examined by measuring their K/S value and comparing with the K/S value of the untreated fabric. The dyed specimens were subjected to fastness testing following ISO standard methods.
2 Experimental
2.1 Materials and Methods
Blocked hexamethylene diisocyanate was purchased from the local market as a milky liquid which was found to contain 40 g/L solid content. Commercial disperse dyes used in this study were provided by the FAV Group of Companies, Karachi, Pakistan. Cotton (129.3 g/m2) and P/C blended fabric 50/50 (116.49 g/m2) were gifted by Bismillah Textile Industries, Karachi, Pakistan.
ATR–FTIR spectra of the treated and untreated fabric were recorded using a Bruker Tensor II spectrophotometer (Bruker, USA). Color strength values (K/S) of the dyed samples were recorded on a Datacolor SF 650 spectrophotometer (Datacolor International, USA). The dyes 1–5 were applied on the fabric using laboratory-scale padding mangle BVHP 350 and curing was performed on a small tabletop thermo fixation oven (MINI-THERMO, Roaches, UK). Wash fastness was performed on Roaches Washtec-P A2, perspiration and water fastness on Perspirometer from Advance Dyeing Solutions (UK), light fastness on Air Cooled Q-Sun Light Fastness Tester, sublimation fastness on Roaches Contact Heat M1 and rubbing fastness on SDL Atlas CM-5 Crockmeter (Atlas Electric Device Co., USA).
2.2 Application of the Cross-linker
2.2.1 Strategy-I
The cross-linker was applied on scoured and bleached cotton and P/C blended fabric through the pad–dry–cure process. The fabrics were padded with the solutions containing 20, 40, 60, 80, and 120 g/L of the cross-linker at neutral pH to obtain a wet pick-up of 70% using a laboratory-scale padding mangle. The padded fabrics were then dried at 100 °C for 2–3 min and cured in a thermo fixation oven at 160 °C for 2 min. The samples were coded as C-CF (Cotton-Cross-linker Fixed), and PC-CF (P/C-Cross-linker Fixed). Untreated samples were coded as CB (Cotton Blank), and PCB (P/C Blank).
2.2.2 Strategy-II
In an alternative process, the optimized concentration of the cross-linker was applied on the scoured and bleached cotton and P/C blended fibers using laboratory padding mangle at 70% wet pickup. The fibers were dried at 100 °C for 2–3 min and dyed without fixing the cross-linker, thus omitting the curing step. The treated samples were coded as C-CD (Cotton-Cross-linker Dried) and PC-CD (P/C-Cross-linker Dried).
2.3 Dyeing of Treated Fabric
The untreated and treated fabrics were dyed with commercially available disperse dyes (1–5) by the pad–dry–cure method at a concentration of 20 g/L and acidic pH (4.5–5.5), maintained by using acetic acid. Dispersing (2 g/L), wetting (2 g/L), and antimigrating agents (10 g/L) were also added to the dyeing liquor. The prepared dye dispersion was added to the pad bath, and the treated and untreated cotton and P/C fibers were padded with this solution, dried at 100 °C for 2–3 min to avoid migration, followed by curing at 220 °C for 1 min. The dyed specimens were washed first with cold and then with hot water. Finally, soaping was carried out at 60 °C using 2 g/L non-ionic detergent for 30 min.
2.4 Fastness Measurements
Different fastness properties of the dyed fabrics, including wash fastness (International Organization for Standardization, 1997), water fastness (ISO, 1996), perspiration fastness (acidic and basic) (ISO, 1996), light fastness (ISO, 2014), sublimation fastness (ISO, 1993), and rubbing fastness (ISO, 2010) were evaluated using ISO standard methods ISO 105-C06/A1S:1997, ISO 105-E01:1996, ISO 105-E04:1996, ISO 105-B02:2014, ISO 105-P01:1993, ISO 105-X12: 2010, respectively.
2.5 SEM–EDX
A scanning electron microscope (SEM) examination of the treated and untreated cotton samples was carried out by mounting the samples on aluminum stub coated with gold (thickness = 153 Å) in an SC7620 mini sputter coater unit. SEM–EDX analysis was then performed on a Thermo Fisher Scientific Apreo 2 C LoVac instrument (USA).
2.6 Determination of Tensile Strength
Tensile strength of the treated and untreated cotton and P/C fabric specimens was determined using LR5K-Plus tensile strength testing machine (Lloyd Instruments, UK) according to ISO 13934–1:2013 standard method. The measurements were performed for multiple specimens of each sample and average values were reported (ISO, 2013).
2.7 Thermogravimetric Analysis
The thermal stability of the treated and untreated fabric samples was studied using an SDT Q600 V8.3 thermogravimetric analyzer and by varying the temperature from 40 °C to 990 °C at a constant rate of 10 °C/min.
2.8 Absorbency Test
The absorbency of the modified and unmodified cotton and P/C samples was analyzed by following the AATCC method 79–1995. The sample was fixed tightly in the embroidery hoop, and a drop of water was dropped onto the fabric from fixed height. The time required for the complete absorption of the drop was measured by using a stopwatch (American Association of Textile Chemists and Colorists, 1995).
3 Results and Discussion
3.1 ATR–FTIR
The fixation of the cross-linker on cotton fabric was verified by recording the ATR–FTIR of the cellulose fabric before and after treatment. Figure 1 displays the FTIR spectrum of the treated cotton fabric (C-CD and C-CF), untreated cotton fabric (CB), and blocked hexamethylene diisocyanate (HDI). The presence of a peak at 1690 cm−1 in the FTIR spectrum of modified cotton fabrics and blocked HDI was attributed to the carbonyl group of carbamate which is absent in the untreated fabric. The stretching vibrations of C–N and C–O of the carbamate functional group were observed at 1341 and 1461 cm−1, respectively. The effective dyeing of the modified cotton using disperse dyes serves as indirect proof of the cross-linker being fixed onto the fiber.
3.2 SEM–EDX
The SEM images of the modified and unmodified cellulose fabric, and their EDX spectra are presented in Figs. 2 and 3. The SEM images demonstrated a distinct change in the surface morphology of the HDI-treated fabric which indicates the binding of the cross-linker with the hydroxyl groups of cellulose. The untreated fabric has a clean surface, while after application of the cross-linker a thin film is formed on the surface of the fabric. Through the semi-quantitative EDX analysis, the elemental composition of treated and untreated samples was examined. Since this technique cannot detect hydrogen atoms, the untreated cotton fabric showed only the presence of carbon and oxygen. On the other hand, the treated samples exhibited the presence of nitrogen along with carbon and oxygen.
3.3 Tensile Strength
The tensile strength of the cotton and P/C fabric before and after the treatment with blocked HDI was examined in both warp and weft directions and the results are shown in Table 1. It is obvious from the results that the strength of the fabric samples is increased considerably in both directions after the application of the cross-linker. This increase in the strength was attributed to the intermolecular hydrogen bonding between the cross-linker and cellulose.
3.4 Thermogravimetric Analysis
The thermal stability of the control and treated fabric samples was assessed to compare the change in thermal behavior of the fabric before and after the treatment. The TGA graphs are presented in Fig. 4. The temperatures for 10% loss (T10%), 50% loss (T50%), and maximum loss (Tmax) are listed in Table 2. The mass loss of 10% was observed at 331.14 °C for CB, 312.05 °C for C-CF, and 306.08 °C for C-CD, which indicated a drop in the temperature of around 20 °C for modified cotton. This drop in temperature was assumed to occur due to the degradation of cross-linker as it is adsorbed on the surface and hence is more accessible to heat. The T50% and Tmax were almost same for all the specimens indicating that the treatment did not alter the thermal properties of the fabric considerably. In the case of the P/C blend, there was only a minor difference between the TGA of treated and untreated samples.
3.5 Absorbency of the Fabric
The effect of the cross-linker on the absorbance of the treated fabric was determined using the AATCC standard test method [19]. The results in Table 3 indicate a decrease in the absorbency of all the treated samples after application of hexamethylene diisocyanate. This reduction in absorbency was due to the engagement of the polar hydroxyl groups and the hydrophobic character of the alkyl chain of HDI. The reduction in absorbency was more in the samples in which the cross-linker was fixed after treatment (C-CF and PC-CF) due to the formation of covalent bond between the hydroxyl group of the fabric and the isocyanate group of the cross-linker. In case of samples treated by following strategy II (C-CD and PC-CD), it was assumed that the cross-linker had only adsorbed on the surface of the fabric, and no permanent interactions existed between them at this stage, and hence a minimum decrease in the absorbency of these fabric samples was observed.
3.6 Concentration Optimization of Cross-linker
The cotton and polyester/cotton (P/C) blended fabrics were treated with different concentrations of cross-linker using the pad–dry–cure method, followed by dyeing with dye-1. The highest color depth was achieved when the concentration of the cross-linker was 100 g/L, as evident from the K/S values presented in Fig. 5. Further increase in the concentration of the cross-linker did not exhibit considerable change in the K/S value, thus further experiments were carried out at 100 g/L.
3.7 Disperse Dyeing Characteristics of the Treated Cotton and Polyester/Cotton Fabric
Disperse dyes, by virtue of their chemical nature, have no affinity toward cellulosic fibers. However, the dye is fixed to the fabric once the cellulose is modified using any appropriate chemical reagent. Thus, during the current study, blocked hexamethylene diisocyanate was selected for the chemical modification of the cotton fabric. It is assumed that the dye fix on the treated cotton by forming covalent linkage with the remaining isocyanate groups of the HDI. One of the two isocyanate groups reacts with the hydroxyl of the cellulose, while the other reacts with NH2 and/or OH groups in the dye molecule (Scheme 1).
Thus, five commercial disperse dyes 1–5 (Fig. 6), bearing these groups, were selected for the application on treated and untreated fabric samples. All the dyes exhibited reasonable K/S values after modification. The best results were obtained from dye-1 (Disperse Red 13) which gave the highest color depth owing to the presence of more nucleophilic aliphatic hydroxyl group in its structure, as compared to the other dyes in which the amino and/or hydroxyl groups are involved in resonance with the aromatic ring, and thus have less nucleophilic character. The nucleophilic hydroxyl group in dye-1 is also less sterically hindered as compared to other dyes. The K/S values of the dyes on cotton and P/C blend are presented in Fig. 7. The employed strategies have no discernible impact on the dyeing behavior of the treated fibers and it appears that no specific trend has been followed.
3.8 Fastness Studies of the Dyed Specimens
The fastness properties of the treated cotton, P/C, and untreated P/C blend were analyzed by employing ISO standard test methods and are presented in Tables 4 and 5. All the dyes exhibited remarkable wash fastness on C-CF and PC-CF treated fabrics. On treated cotton fabric C-CD, in which cross-linker was only dried after application, the dyes demonstrated a relatively lower resistance toward fading. The analysis of perspiration and water fastness revealed good to excellent resistance of the dyes toward fading on both cotton and polyester/cotton blended fabric treated with the cross-linker. Sublimation fastness results showed that all the dyes (except dye-2 on cotton) have good to excellent fastness on the cotton and P/C fibers, treated by strategy-I (C-CF and PC-CF), while on the cotton fabric modified by using strategy-II (C-CD) only two dyes, 1 and 3, exhibited good resistance toward fading. The reason for the improved sublimation properties of the used disperse dyes on C-CF and PC-CF is the polarity of the dye molecules, which increases the hydrogen bonding between fiber and the dye, thus increasing sublimation fastness [27, 28]. Rubbing and light fastness of all the dyes were also good to excellent on both fibers, except on C-CD.
It has been noted that the drop in the fastness ratings for the samples treated employing strategy-II (C-CD and PC-CD) was higher compared to the specimens treated by strategy-I. Since it was assumed that in strategy-II, the cross-linker only adsorbed on the surface of the fiber and no permanent interactions existed between them at this point, there is a chance that when the fabric is dyed with nucleophilic disperse dyes, the dye will occupy both ends of the cross-linkers, and the resulting specie remaining on the surface of the fabric is removed when the fabric is put through fastness assessments. On the other hand, low fastness of the same dyes on treated cotton fabric as compared to P/C fabric may be due to the different interactions of the dyes with both the fibers.
4 Conclusions
The current study details the use of blocked hexamethylene diisocyanate for the modification of cotton fabric to make it disperse dyeable. The interaction of the HDI with the cellulose fabric was validated by the ATR–FTIR and SEM–EDX analysis. After optimizing the parameters on cotton, the cross-linker was applied on the P/C blended fabric as well. Tensile strength analysis displayed an increase in the strength of both the fibers after treatment, while absorbency test revealed a decrease in the water absorption properties of the modified fabrics. The dyeing of both the fabrics with commercial disperse dyes displayed a significant improvement in the substantivity of the disperse dyes on cellulose after the modification. The dyed fibers passed many ISO standard fastness tests with a high degree of resistance to fading. Two strategies were employed to apply the cross-linker on the fabric. The fastness results indicated that strategy-I, in which the cross-linker was fixed on the fabric before dyeing, was a better option, as it gave better fastness compared to the fabric treated by strategy-II.
Data availability
Not applicable.
References
F. Sarker, R.K. Prasad, M.R. Howlader, N. Abir, R. Akter. Nurunnabi, IOSR J. Polym. Text. Eng. 2(3), 12 (2015). https://doi.org/10.9790/019X-0231216
G.K. Bana, G.G. Gelebo, G.K. Janka, Preprints 2021, 2021040540 (2021) https://doi.org/10.20944/preprints202104.0540.v1
N.A. Ibrahim, W.E. Zairy, B.M. Eid, Carbohydr. Polym. 79, 839 (2010). https://doi.org/10.1016/j.carbpol.2009.10.008
N.A. Ibrahim, H.M. Fahmy, M. Awad, L.E. El-Badawy, Polym. Plast. Technol. Eng. 44, 133 (2005). https://doi.org/10.1081/PTE-200046105
B. Muralidharan, S. Laya, Int. Sch. Res. Notices. 2011, 907493 (2011). https://doi.org/10.5402/2011/907493
P.J. Broadbent, D.M. Lewis, Dyes Pigm. 45, 35 (2000). https://doi.org/10.1016/S0143-7208(99)00095-9
P. Venkidusamy, L. Ammayappan, P.A. Subramanian, K.R. Karthikauraja, S. Muthumani, Man-Made Text. India 45, 65 (2002)
N. El-Shemy, N. El-Hawary, H. El-Sayed, J. Chem. Eng. Process Technol. 7, 1000271 (2016). https://doi.org/10.4172/2157-7048.1000271
T. Kim, Y. Son, Dyes Pigm. 66, 27 (2005). https://doi.org/10.1016/j.dyepig.2004.08.010
M. Kim, S. Yoon, T. Kim, J.S. Bae, N. Yoon, Fibers Polym. 7, 352 (2006). https://doi.org/10.1007/BF02875766
T. Kim, Y. Son, Dyes Pigm. 65, 261 (2005). https://doi.org/10.1016/j.dyepig.2004.07.023
D. Gao, H.S. Cui, T.T. Huang, D.F. Yang, J.X. Lin, J. Supercrit. Fluids 86, 108 (2014). https://doi.org/10.1016/j.supflu.2013.12.006
J.J. Long, G.D. Xiao, H.M. Xu, L. Wang, C.L. Cui, J. Liu, M.-Y. Yang, K. Wang, C. Chen, Y.-M. Ren, T. Luan, Z.-F. Ding, J. Supercrit. Fluids 69, 13 (2012). https://doi.org/10.1016/j.supflu.2012.05.002
S. Maeda, K. Kunitou, T. Hihara, K. Mishima, Text. Res. J. 74, 989 (2004). https://doi.org/10.1177/004051750407401109
A. Schmidt, E. Bach, E. Schollmeyer, Dyes Pigm. 56, 27 (2003). https://doi.org/10.1016/S0143-7208(02)00108-0
K. Zimmerman, S. Fu, M.J. Farrell, (CN111527257A). China national intellectual property administration. https://patents.google.com/patent/CN111527257A/en#citedBy. Accessed 5 July 2023
A.I. Ayten, M.A. Taşdelen, B. Ekici, Ceram Int 46, 26724 (2020). https://doi.org/10.1016/j.ceramint.2020.07.147
J. Charbonnet, J. Lawrence, L. Rubin, New approaches in cotton crosslinking (Greener Solutions, 2013). https://bcgc.berkeley.edu/sites/default/files/greenfinalreport-levi-crosslink-2013.pdf. Accessed 5 July 2023
I.A. Misbah, K.M. Bhatti, H.N. Zia, M.S. Bhatti, J. Ind. Text. 50, 1625 (2021). https://doi.org/10.1177/1528083719867445
D.A. Wicks, Z.W. Wicks Jr., Prog. Org. Coat. 41(1–3), 1 (2001). https://doi.org/10.1016/S0300-9440(00)00164-8
L. Zhang, L. Tian, M. Wu, Fibers Polym. 21, 300 (2020). https://doi.org/10.1007/s12221-020-9514-7
Y. Guan, Y.H. Mao, Q.K. Zheng, G.H. Zheng, T. Tian, Fibers Polym. 10, 488 (2009). https://doi.org/10.1007/s12221-009-0488-8
J. Girones, M.T.B. Pimenta, F. Vilaseca, A.J.F. Carvalho, P. Mutjé, A.A.S. Curvelo, Carbohydr. Polym. 74, 106 (2008). https://doi.org/10.1016/j.carbpol.2008.01.026
W.D. Schindler, P.J. Hauser, Chemical Finishing of Textiles (Elsevier, 2004), https://books.google.com.pk/books?hl=en&lr=&id=S42kAgAAQBAJ&oi=fnd&pg=PP1&ots=u2M4IokY5C&sig=iSQV0WcDB0rD4I3Nh6-igYK-dYk&redir_esc=y#v=onepage&q&f=false
F. Audenaert, H. Lens, D. Rolly, P.V. Elst, J. Text. Inst. 90, 76 (1999). https://doi.org/10.1080/00405009908659480
X.D. Zhou, R.Z. Cai, Y.P. Shi, Adv. Mat. Res. 175, 729 (2011). https://doi.org/10.4028/www.scientific.net/AMR.175-176.729
J. Koh, in Textile Dyeing, ed. by P. Hauser (IntechOpen, 2011), pp. 195–220. https://admin.umt.edu.pk/Media/Site/STD1/FileManager/OsamaArticle/articleNov24/8.pdf. Accessed 13 Mar 2023
F.I. Ovi, S. Mahmud, Int. J. Text. Sci. 10(2), 27 (2021). https://doi.org/10.5923/j.textile.20211002.02
Acknowledgements
The authors are grateful to Farid Ahmed Vawda (FAV) Group of Companies (Karachi, Pakistan) for providing commercial disperse dyes used in the study along with their C. I. numbers and giving access to the Tensile Strength Testing instrument. The authors are also thankful to Warsi Chemicals (Karachi, Pakistan) for providing wetting agent and antimigrating agent.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Saadia Riaz: conceptualization, methodology, validation, investigation, formal analysis, software, visualization, writing—original draft, writing—editing. Abdul Jabbar: conceptualization, resources, supervision, validation, writing—review and editing. Hina Siddiqui: conceptualization, resources, supervision, validation, writing—review and editing. Ambreen: investigation, methodology, formal analysis, writing—editing. Idrees Bashir: formal analysis, software. Muhammad Iqbal Choudhary: supervision, resources, validation, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Riaz, S., Jabbar, A., Siddiqui, H. et al. Chemical Modification of Cellulose and Polyester/Cellulose Blended Fabric to Make It Disperse Dyeable: A Sustainable Approach to Achieve Dyeing of P/C Blend with Disperse Dyes. Fibers Polym 25, 2845–2854 (2024). https://doi.org/10.1007/s12221-024-00627-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12221-024-00627-z