Abstract
The main objective of this work is to impart UV protection properties to cotton and cotton/polyester fabrics and enhance the original chemical and physical properties. In this procedure, the fabrics were coated with nanocomposites based on poly(vinyl alcohol)/plasticized starch (PVA/PLST (80/20%)/Zn NPs. The coated fabrics were then exposed to gamma radiation to induce cross-linked layer structure covering the fibers of the fabrics. The formation of Zn NPs was confirmed by UV/Vis and XRD analysis. In addition, the coated fabrics were characterized by the measurements of water absorption, crease recovery, thermal stability, surface morphology and tensile mechanical measurements. The UV protection of the coated fabrics was determined. The results indicated that the coated fabrics showed highly reducing UV-A, UV-B and ultraviolet protection factor (UPF), particularly with increasing irradiation dose, regardless of fabric kind. It was found that cotton and cotton/PET fabrics treated with PVA/PLST/Zn NPs nanocomposites irradiated to a dose of 30 kGy displayed UPF excellent rating values of 44.31 and 58.23, respectively.
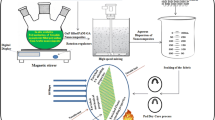
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cotton is widely used as clothing materials due to its special properties such as biodegradation, softness, comfort, warmness, and hygroscopic properties. On the other hand, polyester as synthetic fibers was blended with cotton to enhance the weak mechanical properties. However, both these textile materials lack UV protection. Protection against sun exposure during outdoor activities is one of the practical requirements for safety.
Nanotechnology, a high-tech science and technology rapidly developed in the late 1980s, has been widely used in many fields such as raw material, chemical, textile, medicine, traffic, energy and so on. Compared with ordinary ZnO, nano ZnO, as a new functional material, has some special properties and applications in many fields such as light, electricity, magnetism sensitivity, UV shielding and so on. In this contest, synthesis and characterization of nanosized zinc oxide particles and their application on cotton and wool fabrics for UV shielding were reported [1]. The results showed that the UV-A and UV-B values for cotton by using 5% of zinc NPs were 8.45 and 10.29, respectively. ZnO/carboxymethyl chitosan bionanocomposite was used to impart antibacterial and UV protection for cotton fabric [2]. The results indicated that the UPF factor of UV protection of cotton fabric treated with 2% ZnO–CMCTS bionanocomposite was 7.6 when prepared at 160 °C. The preparation and characterization of UV protection of cotton-based fabrics using nano ZnO and polycarboxylic acids was studied [3]. It was found that by using 5% of zinc NPs, the obtained UPF was 40 and 36 for cotton and cotton/PET, respectively. The characterization of ZnO-coated polyester fabrics for UV protection was investigated [4]. The response of the coated polymer indicates better absorbing UV radiation and dissipating the surface charge, time stability against UV and higher hydrophobic character, without modification of the mechanical properties. The synthesis of ZnO nanoparticles by PNP and its application on the functional finishing of cotton fabrics were investigated [5]. It was found that the treated cotton fabrics exhibited excellent UV protective properties of 58.10, by using 4% of zinc NPs. Cotton fabric with plasma pretreatment and ZnO/carboxymethyl chitosan composite finishing for durable UV resistance and antibacterial property was studied [6]. The results showed that the UV-A (%), UV-B (%) and UPF of the treated cotton with 1% of zinc NPs were 2.58 1.77 and 50, respectively. Durable antibacterial and UV protection of in situ synthesized zinc oxide nanoparticles onto cotton fabrics and hexamethyltriethylene tetramine (HMTETA) was reported [7]. The results showed that the UPF protection value of cotton fabric treated with zinc oxide nanoparticles was 15.2 and rated as good. UV protection and wettability properties of nano zinc oxide (ZnO) with acrylic binder were applied on the cotton fabric using pad–dry–cure method [8]. The results showed that the UPF value of cotton fabrics treated with zinc NPs was 35.52 compared to 7.11 for untreated fabric. The design and functionalization of new composite-based polyester fibers with high anti-UV radiation was investigated [9]. Polyethylene terephthalate fibrous non-woven was coated with titanium dioxide, zinc oxide and silicon oxide. Chitosan and poly(vinylidene fluoride) polymers were used to ensure the good surface compatibility of the resulting composite. The results indicated that the UV-A (%), UV-B (%) and UPF of polyester were 4.88, 1.10, and 80.50, respectively. 4-Aminobenzoic acid ligand (PABA) was used to modify cellulose fabrics as support of zinc oxide nanoparticles for UV protection and antimicrobial activities [10]. It was stated that the ZnO PABA-oxidized cotton fabrics showed excellent UV protection and significant antibacterial efficacy after 20 washing cycles and 100 abrasion cycles, which can be used in advanced protective textiles. Recently, binary chitosan–zinc oxide (CHT–ZnO) nanocomposite was prepared by the precipitation method [11]. Cotton fabrics were treated by obtaining bionanocomposites (BNC) using pad–dry–cure and sol–gel methods. The application of the ternary system changed the UV protection factor (UPF) rating of fabrics from the “very good protection” category to the “excellent protection” category. Biofunctionalized zinc oxide nanoparticles synthesized in the presence of casein were applied to the cotton material to study the sunscreen action of the textiles [12]. The results showed that the UPF value of untreated fabric is 15, whereas the treated textile had good UV protection. The UPF value of extracellular biosynthesized ZnO nanoparticles is 16.4.
In previous studies, we were interested in using ionizing radiation to modify the chemical and physical properties of textile fabrics for protective applications. In this regard, a novel coating formulation to impart ultraviolet (UV) protection to cotton, PET and cotton/PET fabrics utilizing gamma radiation for surface curing was studied [13]. In this regard, naturally occurring aluminum potassium sulfate (Alum) in binary coating with zinc oxide (ZnO) was used to induce UV blocking. The finishing of textiles by ionizing radiation designed for protective properties was also reported [14]. The present work aimed at imparting UV protection to cotton and cotton/polyester fabrics via surface coating with nanocomposites based on poly(vinyl alcohol) (PVA), plasticized starch (PLST) and ZnO NPs under the effect of gamma irradiation. PVA was used in this formulation for two reasons: firstly, because it is a radiation cross-linkable polymer and, sceondly, it is similar in composition to cotton cellulose. PLST was used because it is also similar in composition to the fabrics. Thus, in this way, there is a compatibly between the fabrics and polymer blends. ZnO NPs were used due to their UV absorbance activity. The finished fabrics were characterized in terms of water absorption, crease recovery, thermal stability, and mechanical and surface morphology. The UV protection properties of the fabrics were investigated by the ultraviolet transmittance spectra and the ultraviolet protection factor was calculated (UPF).
2 Experimental
2.1 Materials
The cotton and cotton/polyester (50/50%) blend fabrics used in this study were desized, washed, bleached and subjected to no other finishing process produced by Misr for Spinning and Weaving, El-Mehallah El-Kobra, Egypt. Poly(vinyl alcohol) (PVA) laboratory grade was obtained from Backer Chemical Co., USA, in the form of powder, fully hydrolyzed and with an average molecular weight of 125,000 g/mol. Zinc acetate, sodium hydroxide and soluble starch were laboratory chemicals and purchased from Backer Chemical Co., USA. Zn(CH3COO)2(H2O)2, and sodium hydroxide, laboratory reagent, was purchased from ADVENT CHEMBIO PVT Ltd., India.
2.2 Preparation of Plasticized Starch (PLST)
The PLST was prepared according to a reported method, by dissolving the cornstarch in water and the heating the solution to 95 °C for 1 h to obtain a fully gelatinized solution. Glycerol (20% by weight of the starch weight) was added with continuous stirring and heating up to > 100 °C for 1 h to complete the gelatinized solution.
2.3 Preparation of ZnO Nanoparticles
Zinc oxide (ZnO) nanoparticles were prepared by a wet chemical process using zinc nitrate and sodium hydroxide as precursors and soluble starch as a stabilizing agent [15]. Starch (1%) was dissolved in 500 ml of distilled water and zinc nitrate (0.1 mol) was added with stirring to completely dissolve the zinc nitrate. A 0.2 mol sodium hydroxide solution was added dropwise with constant stirring for 2 h. The solution was allowed to settle overnight and the supernatant solution was then carefully removed. The remaining solution was centrifuged at 10,000×g for 10 min and the supernatant discarded. Thus, the obtained nanoparticles were washed three times with distilled water to remove the by-products and the excessive starch bound to the nanoparticles. After washing, the nanoparticles were dried overnight at 80 °C. During drying, the complete conversion of Zn(OH)2 into ZnO nanoparticles takes place.
2.4 Coating of Cotton and Cotton/Polyester Fabrics with [(PVA/PLST)/ZnO NPs] Nanocomposites under the Effect of Gamma Radiation
A blend mixture containing PVA/PLST (80/20 wt %) was prepared. ZnO NPs at a concentration of 5%, suspended in deionized water, was then added to the PVA/PLST blend solution. Cotton and cotton/polyester fabrics were padded in the nanocomposites for 5 min, followed by squeezing to 100% wet pickup. The treated wet fabrics were then exposed to gamma irradiation to various doses. Radiation was performed in the cobalt-60 gamma cell unit (Russia) at the National Center for Radiation Research and Technology (NCRRT) in Cairo at a dose rate of 4.8 kGy/h.
3 Characterization
3.1 Formation of ZnO Nanoparticles
The formation of ZnO nanoparticles was confirmed by UV/Vis absorbance and XRD schematic measurements. UV/Vis absorption capacity was measured with a double beam UV/Vis spectrophotometer from Unicam, Cambridge (UK) in the wavelength range of 190–1000 nm. X-ray diffraction (XRD) samples were studied with an X-ray diffractometer (Shimadzu-XRD-6000, Tokyo, Japan). XRD samples at 2° intervals between 4° and 90° were obtained using a diffractometer with a Cu Kα radiation source, a generator voltage of 40 kV, a generator current of 40 mA and a wavelength of 2°/speed scan/min in a room temperature of 0.1546 nm.
3.2 Measurement of the Chemical and Physical Properties
The IR spectra of the treated substances were recorded using a Vertex 70 FT-IR attenuated total reflection (ATR-FT-IR) spectrometer HYPERION ™ series (Bruker Optik GmbH, Ettlingen, Germany) in the range of 4000–400 cm−1 with a resolution of 4 cm −1. The software OPUS 6.0 (Bruker) was used for data processing.
The water absorption (%) of the treated fabric was determined by weighing the dry weight (W1) of samples and immersing them in distilled water. Samples were removed at regular intervals and plotted with filter paper to remove excess water, then weighed (W2). The water absorption (%) was determined by the following equation:
The recorded data of water absorption (%) were the average of three measurements.
The crease recovery of dry treated fabric was determined according to ASTM D-1295-67 using an ff-07 (Hungary) type (METEFEM-METINPEX). 1 kg load was added for 5 min, at room temperature. The tested sample was rectangle shaped and the recorded recovery angle was the average of five measurements.
The mechanical tests were performed for 2 cm × 5 cm samples. The used device is Mecmesin, type multi Test 25-I (UK).
The surface morphology was examined by scanning electron microscopy (SEM). Micrographs were made on a JSM-5400 instrument (Joel, Japan). A spray coater was used to precoat the fracture surfaces with conductive gold at 30 kV.
A thermogravimetric analysis (TGA) was carried out on a TGA-50 instrument from Shimadzu (Japan) at a heating rate of 10 °C/min under a nitrogen gas atmosphere from room temperature to 500 °C, to evaluate the thermal stability of the treated tissues.
3.3 UV Protection Evaluation
The UV protection of the fabrics was assessed by the UV protection factor (UPF), evaluated by Unicam Dual Beam UV/Vis spectrophotometer, Cambridge (UK), according to the AATCC Test Method 183-2004, Transmittance or Blocking of Erythemally Weighted Ultraviolet Radiation Through Fabrics AATCC Technical Manual, vol. 85, 2010, pp. 318–321 [16]. In the ultraviolet range of 280–400 nm, at 2 nm intervals, UPF was conducted as in the following equation:

where Eλ, Sλ, Tλ and Δλ are the relative erythemal spectral effectiveness, solar spectral irradiance in W/cm2/nm−1, spectral transmittance of sample and wavelength step in nm, respectively. The numerator of the above equation describes the quantity of the UV radiation, which reaches the skin. The denominator describes the quantity of the UV radiation reaching the skin protected by a garment. UV-A (400–315 nm) and UV-B (315–280 nm) were calculated according to the following equations:


4 Results and Discussion
4.1 Confirmation of ZnO Nanoparticles
The formation of Zn nanomaterials was confirmed by measurements of UV absorption and XRD samples as shown in Fig. 1. Peaks 32, 34.8, 36.6, 47.8, 56.8, 63.2 and 68.2° (100), (002), (101), (102), (110), (103) can be represented graphically as shown in the upper curve and (200), a crystalline plane of ZnO with a hexagonal structure of wurcite, calculated accordingly [17]. The widening of the tip is mainly due to the nano-effect. However, the wide reflection at 15° is related to the low crystallinity of soluble starch, confirming the presence of ZnO in the synthesized material [18]. The aqueous synthesis of ZnO nanoparticles results in an initial precipitate of Zn (OH)2, then into ZnO nanoparticles by subsequent modification. The possible chemical reaction is [19]:
As shown in Fig. 1, the maximum absorption peak of the formed ZnO NPs occurred at wavelength 320 nm. This observation is in agreement with other reports, indicating that the characterization peak of ZnO NPs is positioned around a wavelength of 320–390 nm [20].
4.2 Coating of Fabrics Under the Effect of Gamma Irradiation
In this work, gamma radiation was used to form hybrid structure based on fabric treated by cross-linked coatings involving the primary and secondary hydroxyl groups on cotton cellulose backbone and the hydroxyl groups on PLST and PVA. PVA as a vinyl polymer is a radiation cross-linkable polymer which undergoes cross-linking under the effect of gamma irradiation [21]. In addition, in water radiolysis, the transfer of radicals to the polymer increases the concentration of radicals, which increases the rate of cross-linking and gelation.

The spontaneous formation of hybrid structure of fabrics and PVA/PLST/ZnO NPs may be explained as follows. In the first step, under the effect of gamma irradiation, an interpenetrating network structure (IPN) of hydrogels composed of cross-linked PVA and non-cross-linked PLST is produced. On the other hand, a chemical bonding between ZnO NPs and the hydroxyl groups of PVA and PLST through the formed free radicals could form. In the second step, because of gamma irradiation of polymers and the radiolysis of water, free radicals are formed on the primary and secondary hydroxyl groups of cotton cellulose as well as on PVA and PLST. These free radicals will enhance the formation of chemical bonding between PVA/PLST/ZnO NPs nanocomposites and those on the backbone of fabrics, in which a fabric hybrid structure is produced as a coatings covering the fibers of fabrics.
4.3 FT-IR Analysis
Figures 2 and 3 show the FT-IR spectra of cotton fabrics and cotton/PET blends before and after coating with a PVA/PLST mixture containing zinc oxide nanoparticles (e.g., 5%) affected by gamma rays at different doses. As with almost all organic compounds, the FT-IR spectrum of cotton fabric has a wide peak in the center at 3413 cm−1 and a peak at 2919 cm−1 corresponding to the O–H stretching and due to the C-H stretching of cellulose, respectively.
As shown in Fig. 3, the IR spectrum of untreated cotton/PET showed an absorption band of 2950 cm−1 due to C–H elongation. In addition, an absorption band can be seen at 1730 cm−1 due to the elongation of C = O of the PET ester group. In addition, the series of absorption bands 2850 cm −1 and 1622–1420 cm−1 indicate the presence of CH–H and benzene aromatic rings, respectively. As with almost all organic compounds, the FT-IR spectrum of raw cotton/PET fabric has an average wide peak at 3413 cm−1 and a peak at 2919 cm−1, corresponding to the O–H stretching for the C–H stretching of cellulose, respectively.
4.4 Water Absorption
Figure 4 shows the water absorption of cotton and cotton/polyester fabrics coated with PVA/PLST (80/20%)/zinc NPs (5%) nanocomposites as a function of gamma irradiation. It can be seen that the rate of absorption increases sharply after about 1 h regardless of the type of tissue or irradiation dose, then gradually increases to 5 h, reaching a steady state of up to 48 h. However, the cotton fabric showed higher water absorption (%) than the cotton fabric/PET fabric due to the presence of synthetic components. It is known that water absorption is inversely proportional to gel formation due to the dense structure formed because of cross-linking. As shown, the increased irradiation dose decreases the absorption of water, which indicates the formation of interconnected PVA/PLST in the tissues after closure, where the dense chains close the cavity for the diffusion of water molecules [6, 22]. In this regard, the reduction in the equilibrium absorption rate of the purified cotton fabric was 6.76, 11.77 and 15.16% when the radiation dose was increased by 10, 20 and 30 kGy, respectively, compared to the untreated fabric. At the same time, the reduction in equilibrium absorption of cotton/PET fabric was 4.66, 6.06 and 8.23%, respectively, compared to untreated by increasing the radiation dose by 10, 20 and 30 kGy.
4.5 Crease Recovery
Crease recovery is the ability of a fabric to resist and recover the deformation after releasing any load to the initial wrinkle-free surface and is measured by the angle between the folded halves that is termed as the crease recovery angle (CRA). Figure 5 shows the crease recovery angle (CRA) of cotton and cotton/polyester blend fabrics treated with nanocomposites based on PVA/PLST (80/20%)/zinc NPs (5%) under the effect of different doses of gamma irradiation. Based on the data in Fig. 5, few points may be concluded:
-
(1)
The untreated cotton fabric had a CRA of 78° and the untreated cotton/PET blend had a CRA of 95,0° due to the presence of the synthetic polyester component.
-
(2)
The treated [(PVA/PLST)/Zn NPs/coated cotton/polyester blend had a higher CRA than cotton fabric processed with the same nanocomposite, regardless of radiation dose.
-
(3)
CRA of cotton or cotton/PET fabrics increased when the irradiation dose was increased to 20 kGy and decreased at a relatively high dose. This increase in CRA can be explained by the formation of cross-links formed by the reaction of hydroxyl groups of PVA with cotton cellulose and the formation of hydrogen bonds between the PLST components involved in cross-linking. However, the reduction in large capacity is explained by the appearance of the oxidative decomposition of the cotton pulp.
4.6 Tensile Mechanical Properties
Mechanical properties are considered as one of the most important physical properties of macromolecules in most applications. Many structural factors would influence the mechanical behavior. In addition to the chemical structure, molecular weight, cross-linking, copolymerization, molecular orientation and filling are structural factors that influence the mechanical properties. Stress–strain test is the most commonly used method of all mechanical tests. Figure 6 shows the tensile strengths of cotton and cotton/PET fabrics treated with nanocomposites based on PVA/PLST (80/20%)/ZnO nanoparticles (5%), before and after exposure to different doses of gamma irradiation. Based on the data on this figure, two points can be addressed:
-
(1)
In general, cotton/PET fabrics have a higher tensile strength than cotton fabrics due to the presence of synthetic components. The tensile strength of cotton and cotton/PET was highly dependent on the radiation dose, and the tensile strength of the untreated fabric decreased with increasing radiation dose. This can be explained by the formation of oxidative decomposition of cellulosic components under the influence of gamma rays.
-
(2)
Treated cotton and cotton/PET fabrics displayed higher tensile strength than untreated fabrics, regardless of irradiation dose. This could be attributed to the stress resistance of the treatment of the fabrics with PLST/PVA/Zn NPs nanocomposites. However, the tensile strength of treated cotton or cotton/PET fabrics has been found to increase with increasing irradiation dose to 30 kGy.
4.7 Surface Morphology by Scanning Electron Microscopy (SEM)
Figure 7 shows SEM micrographs of cotton and cotton/PET fabrics coated with nanocomposites based on PVA/PLST (80/20%)/zinc NPs (5%) exposed to different doses of gamma irradiation. Based on these SEM micrographs, several points can be indicated.
-
(1)
In the SEM micrograph of the untreated cotton and cotton/PET fabric, the surface of the fibers constituting the fabric structure appears smooth, stacked together and free of additives.
-
(2)
SEM micrographs of the treated fabric showed that the surface of the fibers constituting the fabric structure still stacked together; however, the surface of the fabrics became relatively rough and tiny white particles can be observed on the surface of the strings constituting the fabrics due the treatments. These tiny white particles were found to increase with increasing irradiation dose. This finding can be explained based on the increase in cross-linking density associated with increasing the irradiation dose.
-
(3)
In general, the SEM micrograph of the treated fabrics showed that the amount of finishes on the surface of the cotton/PET fabric is relatively higher than that on surface of the cotton fabric.
4.8 Thermogravimetric Analysis (TGA)
Figure 8 shows the TGA thermograms and the corresponding rate of thermal decomposition reaction of cotton and cotton/PET blend fabrics treated with nanocomposites based on PVA/PLST (80/20%)/zinc NPs (5%), before and after exposure to different doses of gamma irradiation. Meanwhile, the weight loss (%) at different heating temperatures and the temperatures of maximum rate of thermal decomposition reaction (Tmax) of cotton and cotton/PET fabrics under the same conditions are shown in Table 1. It can be seen that the TGA thermograms of untreated cotton and cotton/PET fabrics displayed three stages of decomposition. In the first stage, up to 200 °C, the weight loss is the result of physical damage that normally occurs in the amorphous region of cellulose [23]. Major weight loss occurs in the second stage (200–400 °C), due to the formation of glucose with various combustible gases. In the third stage, the process continues through decomposition and the release of carbon dioxide. Based on the data in Fig. 8 and Table 1, several points can be addressed:
-
(1)
The coated fabrics showed a relatively higher thermal stability than untreated fabrics in the temperature range of ~ 200–400 °C, as shown in Table 1.
-
(2)
The rate of the thermal decomposition curve showed a similar behavior; however, the temperatures of the maximum reaction rate (Tmax) differs from one substrate to another. In this regard, the untreated and treated cotton/PET fabrics showed two Tmax temperatures due to the presence of two fiber components, regardless of the irradiation dose. However, the Tmax was found to increase with increasing irradiation dose for treated cotton and cotton/PET fabrics.
-
(3)
The temperatures at different weight loss (%) or (Tmax) indicates that the untreated and treated cotton/PET fabrics are thermally stable than untreated or treated cotton fabrics. The higher thermal stability of the treated fabrics compared to the untreated fabrics is due to the occurrence of cross-linking resulting from the treatment under gamma irradiation.
4.9 UV Protection Characters
The sun emits ultraviolet light in the wavelength range of 100–400 nm, which is divided into UV-C (100–280 nm), UV-B (280–320 nm) and UV-A (320–400 nm). The atmosphere absorbs some of this radiation, and UV rays reach mainly the Earth's surface as UV and UV-A. Figures 9 and 10 show the UV transmittance, in the region of 280–400 nm, of cotton fabrics treated with nanocomposite based on PVA/PLST (80/20%)/zinc NPs (5%), before and after exposure to different doses of gamma irradiation. The blocking in the UV-A (315–400 nm) and UV-B (280–315 nm) regions and the UV protection factor (UPF) were calculated using the AATCC test method and the results are summarized in Table 2. It is clear that all treated fabrics showed sufficient UPF blocking properties. In this context, the UPF rating for cotton fabrics was found to increase from 3.18 to 23.10, 28.12 and 44.31 by increasing the radiation dose from 10, 20 and 30 kGy, respectively. On the other hand, the UPF rating for cotton/PET fabrics was found to increase from 5.80 to 28.70, 32.96 and 58.23 by increasing the radiation dose from 10, 20 and 30 kGy, respectively. Therefore, it is clear that increasing the irradiation dose from 10 to 30 kGy results in a significant reduction in UV transmission through the fabric samples. These results can be explained based on a formed cross-linking layer covering the fibers of the fabrics, in which as the irradiation dose increases, the thickness of the cross-linking layer increases. As a result, the Zn NPs will be homogeneously distributed on the coating and no Zn NPs will be lost when washing the samples after treatment. As shown in Table 2, cotton/PET blend fabric had higher UPF, UV-A and UV-B values than the cotton fabric. This can be attributed to the presence of the aromatic polyester component, which improves UV protection, by absorption.
According to the UPF Classification System based on the ASTM standard, the UPF ranges 15–24, 25–40 and 40–50+ were classified as good protection, very good protection and excellent protection, respectively [24]. Thus, based on the data on Table 2, the UV protection factor of cotton and cotton/PET fabrics treated with nanocomposite based on PVA/PLST (80/20%)/zinc NPs (5%), exposed to a dose of 30 kGy, can be classified as excellent protection.
These data are consistent with previous reports on UV protection of cotton and cotton/PET fabrics as shown in Table 3.
5 Conclusion
UV protection of fabrics is one of the practical requirements for safety and maintenance conditions, such as prolonged exposure to sunlight during outdoor activities. In this study, cotton and cotton/polyester fabrics are coated with nanocomposite based on a polymer blend containing poly(vinyl alcohol) and PLST plasticized starch as cross-radiation compatible polymer components and subjected to irradiation In addition, the main properties of the treated fabrics were not much affected.
Data Availability
The data that support the findings of this study are available from the corresponding author, [N.M.Ali], upon request.
References
A. Becheri, M. Durrr, P.L. Nostro, P. Baglioni, J Nanoparticles Res 10, 679–689 (2008)
A. El Shafei, A. Abou-Okeil, Carbohyd. Polym. (2011). https://doi.org/10.1016/j.carbpol.2010.08.083
MM Abd El-Hady, A Farouk, S Sharaf, Carbohydrate Polymers 92, 400- 406 (2013).
G. Borcia, N. Dumitrascu, N. Vrinceanu, Appl. Surf. Sci. 279, 272–278 (2013)
D. Zhang, G. Zhang, L. Chen, Y. Liao, Y. Chen, Lin, H., H. Morikawa, Fibers and Polymers 15 (2014) 1842-1849
M. Wang, L. Xu, M. Zhai, J. Peng, J. Li, G. Wei, Carbohyd. Polym. 74, 498–503 (2008)
Th.I. Shaheen, M.E. El-Naggar, A.M. Abdelgawad, A. Hebeish, Int. J. Biol. Macromol. 83, 426–432 (2016)
N. Becenen, Int’l Journal of Advances in Chemical Engg., & Biological Sciences (IJACEBS) 4 (2017) ISSN 2349–1507 EISSN 2349–1515.
N. Bouazizi, A. Abed, S. Giraud, A. El-Achari, C. Campagne, M.N. Morshed, O. Thoumire, R. El-Moznine, O. Cherkaoui, J. Vieillard, F.L. Derf, Physica E 118, 113905 (2020)
S.A. Noorian, N. Hemmatinejad, J.A.R. Navarro, Int. J. Biol. Macromol. 154, 1215–1226 (2020)
M. Türemen, A. Aslı Demir, Y. Gokce, Materials Chemistry and Physics 268, 124736 (2021).
M. Joseph, V.P.N. Nampoori, M. Kailasnath, Materials Today: Proceedings 68, 363–366 (2022)
M.H. Zohdy, M. El Hossamy, A.M. El-Naggar, A.I. Fathalla, N.M. Ali, Eur. Polym. J. 45, 2926–2934 (2009)
M.H. Zohdy, H. Abdel Kareem, A.M. El-Naggar, M.S. Hassan, J. Appl. Polym. Sci. 89, 2604–2612 (2013).
A. Yadav, V. Prasad, A.A. Kathe, S. Raj, D. Yadav, C. Sundaramoorthy, N. Vigneshwaran, Bull. Mater. Sci. 29, 641–645 (2006)
H.E. Emam, T. Bechtold, Appl. Surf. Sci. 357, 1878–1889 (2015)
N. Abidi, E.F. Hequet, G. Abdalah, cotton fabric and UV-Protection, the Proceedings of the Beltwide Cotton Conference, 2:1301–1303 (2001), National Cotton Council, Memphis TN, USA.
N. Vigneshwaran, S. Kumar, A.A. Kathe, P.V. Varadarajan, V. Prasad, Nanocomposites 17, 5087–5095 (2006)
A. Chapiro, Radiation Chemistry of Polymeric Systems (Interscience, New York, 1962), pp.22–81
A. Fouda, S.E. Hassan, S.S. Salem, T. Shaheen, Microb. Pathog. 125, 252–261 (2018)
T.R.R. Singh, P.A. Mc Carron, A.D. Woolfson, R.F. Donnelly, Eur. Polym. J. 45, 1239–49 (2009).
M/P. Gashti, A. Almasian, Composites: Part B, 45, 282–289 (2013).
Z. Bilimis, (2011), "Measuring the UV protection factor (UPF) of fabrics and clothing" Aglient Technologies, Inc, Australia, SI-A-1148.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
No potential conflict of interest was reported by the authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ali, N.M., Saleh, S.N., Ahmed, M. et al. Characterization and UV Protection of Cotton and Cotton/Polyester Blend Fabrics Coated With PVA/PLST/ZnO NPs Nanocomposites Under the Effect of Gamma Irradiation. Fibers Polym 24, 3901–3912 (2023). https://doi.org/10.1007/s12221-023-00318-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12221-023-00318-1