Abstract
In this study, we prepared a series of polyimide (PI) films based on 4,4ʹ-(hexafluoroisopropylidene) diphthalic anhydride (6FDA) and bis(3-aminophenyl) sulfone (3DDS), incorporating various amounts of terephthaloyl chloride (TPC) as a comonomer, and reported their thermal and optical properties. Fourier transform infrared spectroscopy confirmed the chemical structure of the synthesized PI films and the successful imidization of all PI films through thermal treatment. Differential scanning calorimetry analysis showed that the glass transition temperature (Tg) of pure PI films based on 6FDA and 3DDS was approximately 275 °C, increasing sequentially with an increase in TPC content. For instance, the Tg of PI with 20 mol% TPC increased by about 5 °C to 280.24 °C compared to the Tg of the pure PI film, indicating improved thermal stability. Thermogravimetric analysis revealed a slight decrease in the thermal decomposition temperature with increasing TPC content, which can be attributed to the decomposition of the amide group induced by TPC. The coefficient of thermal expansion (CTE) of pure PI films was evaluated to be 60 ppm/℃, significantly decreasing with increasing TPC content. More specifically, the CTE decreased to 41 ppm/℃ when TPC was introduced at a level of 20 mol%. Based on these results, it is reasonable to conclude that the introduction of TPC improved the thermal dimensional stability of pure PI films based on 6FDA and 3DDS by increasing intermolecular forces induced by hydrogen bonding from the amide group. Overall, these results suggest that the 6FDA and 3DDS-based PI films containing TPC as a comonomer have improved thermal properties and are suitable for high-dimensional stability polymer substrates for display applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With the rapid development of IT technology, portable and mobile display devices are becoming a necessity in modern society. In addition, lightweight and compact design of products are also becoming essential requirements for display materials, and research on more lightweight and flexible displays have been extensively developed as alternatives to conventional glass-based displays [1, 2].
A flexible display, which is created on a light and flexible plastic substrate, refers to a display designed to be bent or rolled without losing its inherent material properties [3, 4]. Optical transparency and shape stability are the most critical material properties of the polymer substrates used in such flexible displays [5]. These polymer substrate materials must not only remain intact during repeated deformation processes such as bending, folding, and stretching but also maintain their various initial properties [6, 7]. Moreover, due to their exposure to high-temperature and cleaning processes involving chemicals, the polymer substrate materials require high heat resistance and chemical resistance [8]. Additionally, it is crucial for the polymer substrate materials to have a coefficient of thermal expansion (CTE) as low as possible to prevent issues like cracking and detachment of the portion bonded to the inorganic layer during the manufacturing process [9, 10].
According to previous studies, it is known that poly(ethylene terephthalate) (PET) [11, 12], polycarbonate (PC) [13], polyethersulfone (PES) [14], polyimide (PI) [15, 16], and polyarylate (PAR) [17] are suitable as polymer substrate materials for flexible displays. Among them, PI polymer exhibits excellent heat resistance, chemical resistance, mechanical properties, electrical properties and dimensional stability due to its rigid chain structure [16]. As results of these excellent properties, PI is widely used in electrical and electronic applications, such as automobiles, aerospace fields, flexible circuit boards, liquid crystal alignment films for LCDs, adhesives and coating materials [18,19,20,21].
However, PI is known to have a relatively high yellow index, which is attributed to the formation of charge transfer complexes (CT complexes) between electron donors and acceptors [22]. To overcome these limitations and achieve colorless and transparent PI, various approaches have been proposed, such as incorporating highly electronegative or bulky structures like trifluoromethyl (CF3–), ether (–O–), and sulfone (–SO2–) into the main chain of PI [23,24,25]. These structural modifications aim to improve the optical properties of the resulting PI by disrupting the CT complex formation, hindering the migration of π electrons, preventing resonance structures, or reducing polymer chain stacking and crystallinity [22]. Nevertheless, it should be noted that introducing these monomers or functional groups into the main chain of PI may compromise its excellent mechanical and thermal properties by reducing interchain interactions and resonance effects. Thus, continuous efforts and research are required to develop polyimides that exhibit outstanding optical properties without compromising their thermal characteristics.
In this study, in order to improve the optical properties of PI known so far, a bulky –CF3 and a flexible sulfone (–SO2–) structures, which are known to have high electronegativity, were and introduced into the PI main chain. Specifically, colorless and transparent PI based on 4,4ʹ-(hexafluoroisopropylidene) diphthalic anhydride (6FDA) and bis(3-aminophenyl) sulfone (3DDS) were experimentally synthesized, and the basic thermal and optical properties of the final PI film according to the use of 6FDA and 3DDS monomers were preferentially evaluated. Furthermore, terephthaloyl chloride (TPC) was introduced as a comonomer into the main chain of 6FDA and 3DDS-based PI, the changes in the thermal and optical properties of the PI film according to the introduction of TPC were systematically investigated.
2 Experimental
2.1 Materials
For the PI synthesis in this study, 4,4ʹ-(hexafluoroisopropylidene) diphthalic anhydride (6FDA, > 98.0%), bis(3-aminophenyl) sulfone (3DDS, > 98.0%), and terephthaloyl chloride (TPC, > 99.0%) were purchased from TCI (Tokyo, Japan) and were used. In addition, anhydrous N,Nʹ-dimethylacetamide (DMAc, 99.8%, Sigma-Aldrich Co., USA) was used as the solvent for the PI synthesis. N-Methyl-2-pyrrolidone (NMP) was also used as the solvent for the intrinsic viscosity analysis of the synthesized PI. All monomers used in the experiment were used without further purification after purchase.
2.2 Synthesis of PAA
In general, the PI is prepared through synthesis of PAA, which is a precursor of PI, and subsequent heat treatment [16]. The synthesis method for PAA based on 6FDA and 3DDS used as monomers is shown in Scheme 1, and feed ratios of each monomer are summarized in Table 1. The detailed synthesis process for the PAA, is as follows. Regardless of the introduction of TPC as a comonomer, the synthesis method for all PAA samples is the same. Here, the synthesis of PI_TPC_10, which contains 10 mol% of TPC, will be explained. 2.483 g (0.01 mol) of 3DDS was added to 100 mL three-necked flask, and stirred in DMAc solvent (20.053 g) for 30 min until it was completely dissolved. Then, 3.998 g (0.009 mol) of 6FDA was added in powder form. The mixture was stirred for 1 h to proceed the reaction, and then 0.203 g (0.001 mol) of TPC was added. The final PAA was synthesized by stirring the mixture under a nitrogen atmosphere at 0 ℃ for 12 h until the solid content concentration reached 25 wt%. To compare the effects of TPC incorporation, pure PI (TPC-0) was also synthesized using the same method as above without introducing TPC.
2.3 Preparation PI Film Through Imidization of PAA Film
The colorless and transparent PI film used in this study was manufactured through the heat treatment process of PAA film, and the imidization conditions are summarized in Table 2. The synthesized PAA solution was cast on a clean glass substrate, and the solvent was gradually removed while stabilizing the PAA at 50 ℃ for 1 h in a vacuum oven. After drying at 75 ℃ for 2 h to completely remove the solvent, sequential heat treatment was carried out in a nitrogen atmosphere at 100, 200, and 250 ℃ for 1 h each for thermal imidization reaction. The manufactured film was immersed in distilled water for removal of residual solvent and slowly separated from the glass plate after ultrasound treatment.
2.4 Characterization of PI Films
Fourier transform infrared spectroscopy (FT-IR, Nicolet IS50) was used to analyze the degree of imidization and chemical structure of PAA and PI. FT-IR analysis was performed on PAA and PI films by selecting the average of 32 scans with a resolution of 0.2 cm−1 in the range of 400–4000 cm−1. The intrinsic viscosity was calculated after measuring the reduced viscosity according to the concentration using a Ubbelohde viscometer at a constant temperature of 30 ℃. Differential scanning calorimetry (DSC Q100, TA instrument) was used to analyze the thermal properties of the PI films. DSC analysis was performed in a nitrogen atmosphere with both heating and cooling rates set at 20 ℃/min, and the thermal properties of the PI films were measured in the range of 30– 400 ℃. Thermogravimetric analyzer (TGA Q-50, TA instrument) was used to evaluate the thermal stability of the PI films. The analysis was conducted under a nitrogen flow, and the heating rate was set at 10 ℃/min in the temperature range of 30–800 ℃. Thermomechanical analyzer (TMA 402 F1, Netzsch) was used to observe the thermal expansion characteristics of the PI films. The coefficient of thermal expansion (CTE) was calculated by measuring the linear expansion coefficient from 30 to 250 ℃ with a heating rate of 10 ℃/min and a load of 1N. UV–Vis spectrometer (UV-2450, Shimadzu) was used to analyze the optical properties of the PI films in the range of 200–800 nm. In addition, the yellow index of the PI films was measured using a spectrophotometer (Datacolor 110).
3 Results and Discussion
3.1 Structural Analysis
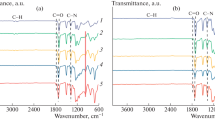
FT-IR analysis was conducted to confirm the chemical structure of the synthesized PAA and the PI films obtained through thermal imidization process of the PAA films. Figure 1A shows the FT-IR spectrum of the PAA films, and the characteristic peaks are summarized in Table 2. All PAA films exhibited broad absorption bands in the range of 2500–3400 cm−1 due to the stretching vibrations of C=O and –CONH–. In addition, peaks corresponding to C=O stretching vibrations of amide and acid groups were observed at 1680 cm−1 and 1717 cm−1, respectively. Figure 1B displays the FT-IR spectrum of the PI films prepared by thermal imidization. As can be seen from the figure, the typical characteristic peaks of the PI films were clearly observed. Specifically, the pure PI film showed a general imide peak at 1720 cm−1 corresponding to C=O stretching vibration and a C–N–C peak at 1380 cm−1 due to the imide ring. Furthermore, the introduction of TPC copolymer resulted in the appearance of amide peaks corresponding to N–H stretching vibration at 3300 cm−1 and C=O stretching vibration at 1650 cm−1. Overall, the presence of peaks corresponding to the PI structures and TPC comonomer confirmed the successful imidization of all PI films used in this study through thermal treatment.
3.2 Thermal Properties
3.2.1 DSC Analysis
The glass transition temperature (Tg) of a polymer is a critical factor in determining its thermal properties. The physical and mechanical properties of a polymer change drastically at the Tg, making it an essential factor in high-dimensional stability polymer substrates for display applications. Typically, the Tg depends on the rigidity of the polymer backbone and free volume, with significant influence from interchain interactions. Figure 2 shows the DSC thermogram (Fig. 1A) of the PI films produced in this study and the changes in Tg with changes in TPC content (Fig. 1B). The Tg of the pure PI film based on 6FDA and 3DDS without TPC comonomer was observed to be in the range of 275 ℃, indicating stable thermal stability despite the introduction of functional groups such as –CF3 and –SO2. Moreover, as depicted in the figure, the Tg of the PI films increased sequentially with an increase in TPC content. For instance, the Tg of the PI film with 20 mol% TPC increased by approximately 5–280.24 ℃ compared to the Tg of the pure PI film. This increase in Tg is thought to be attribute to the positive effect of an increase in intermolecular forces induced by hydrogen bonding from the amide group as TPC comonomer is introduced.
3.3 TGA Analysis
To analyze the thermal stability of the PI films prepared in this study, TGA analysis was performed. To comparatively evaluate thermal stability, the temperature at which 1% weight loss (T1) and 5% weight loss (T5) occurred was measured, and the TGA curves of each PI film (Fig. 3A) and the changes in T1 and T5 with TPC content (Fig. 3B) are shown in Fig. 3. As shown in the figures, the temperature at which 1% weight loss and 5% weight loss occurred was observed to be in the range of 455–505 ℃ and 512–547 ℃, respectively, with a decreasing trend in the decomposition temperature as TPC content increased. Generally, polymer decomposition occurs at the weakest point of a bond, determined by the bond dissociation energy. Therefore, considering that the bond dissociation energy of C–N and C–F is about 300 kJ/mol and 500 kJ/mol, respectively, the decrease in the decomposition temperature of the 6FDA and 3DDS-based PI films due to TPC introduction in this study can be attributed to the decomposition of the amide group induced by TPC comonomer.
3.4 TMA Analysis
All objects expand or increase in volume when heat is applied. Organic polymer materials, including high-performance polymers, which consist of weak covalent bonds, inherently exhibit a high coefficient of thermal expansion (CTE) due to their inherent structural characteristics [26, 27]. Thus, CTE serves as a crucial characteristic for polymer substrate materials that undergo high-temperature processes and is considered a measure of dimensional stability. Figure 4 displays the changes in CTE as TPC content increases. The CTE of pure PI films based on 6FDA and 3DDS without TPC comonomer was determined to be 60 ppm/℃. This high value can be attributed to the presence of bulky and flexible structures, such as -CF3 and –SO2– groups, within the PI backbone. Furthermore, a comparison with DuPont's Kapton® (with a CTE of 30 ppm/℃) highlights the considerably higher CTE of pure PI films. However, as depicted in the figure, the CTE of the PI films notably decreases as the TPC content increases. For instance, when TPC is introduced at a level of 20 mol%, the CTE decreases to 41 ppm/℃. Even a small amount of TPC introduction leads to a rapid decrease in the CTE of the pure PI film. Hence, it is evident that the incorporation of TPC comonomer enhances the thermal dimensional stability of the PI film based on 6FDA and 3DDS, resulting from increased intermolecular forces generated by the amide bonds of TPC units, which replace -CF3.
3.5 Optical Properties
In this study, the optical properties of PI films based on 6FDA and 3DDS were evaluated through UV transmittance analysis. Figure 5 presents the transmittance graph of the PI films, and Table 3 summarizes the transmittance and cutoff wavelength results of each film in the visible light range. According to Table 3, irrespective of the introduction of TPC as a comonomer, all PI films exhibited a cutoff wavelength (λo) value below 400 nm, indicating their ability to transmit light before the visible light range. Additionally, it is evident that the transmittance of all manufactured PI films is considerably high. For instance, while the transmittance of Kapton® at 500 nm is known to be 30–35%, the PI film manufactured in this study showed transmittance of 84–90% at 500 nm. This result can be attributed to the effective control of the CT-complex formation, an inherent drawback of PI, through the presence of triple fluoromethyl (–CF3–) in the 6FDA monomer and the sulfone (–SO2–) in the 3DDS monomer used in PI synthesis. Furthermore, changes in transmittance were observed with the introduction of TPC as a comonomer. Specifically, transmittance increased by approximately 90% when TPC was introduced up to 10 mol%, but a decreasing trend in transmittance was observed when the TPC content exceeded 10 mol%. This improvement in optical properties can be attributed to the TPC structure introduced to replace the 6FDA monomer, effectively controlling intramolecular and intermolecular charge transfer complex formation inherent to PI. However, the decrease in optical properties observed as the TPC content exceeded 10 mol% may be due to partial crystallization resulting from hydrogen bonding based on amide bonds, requiring further investigation subsequent research. The YI values, representing the degree of yellowing index measured using a colorimeter, are presented in Table 3. The YI value of pure PI film based on 6FDA and 3DDS, which contain strong electron-withdrawing groups like -CF3 and –SO2–, was 12.75, and it was observed that the overall YI value decreased with the introduction of TPC as a comonomer. Specifically, the PI film with 10 mol% TPC exhibited a YI of 4.13, a significantly low value compared to the transmittance of Kapton®. However, similar to the previous UV transmittance results, a turning point was observed in the YI results of the PI film with TPC at the 10 mol% threshold, indicating an increase amide bond formation and intramolecular hydrogen bonding with higher TPC content. Optical photographs of the PI films were provided in Fig. 6 to visually confirm their transparency. As shown in the figure, all PI films manufactured in this study exhibited excellent transparency compared to Kapton®.
4 Conclusion
In this study, we synthesized 6FDA and 3DDS-based PI and systematically observed their thermal and optical properties with the introduction of TPC comonomer. Firstly, FT-IR analysis confirmed the presence of C–N–C and C=O peaks corresponding to the imide ring, as well as the appearance of the amide peak with the introduction of TPC copolymers. Based on this, we concluded that both the synthesized and fabricated PI films were successfully imidized through thermal treatment. The glass transition temperature of the pure PI film based on 6FDA and 3DDS without TPC ranged from 275 ℃, and the Tg of the PI film increased sequentially as the TPC content increased. Additionally, as the TPC content increased, the thermal decomposition temperature of the PI film tended to decrease slightly. However, the thermal expansion coefficient of the PI film decreased significantly with the introduction and increasing content of TPC, which was attributed to the increase in intermolecular forces resulting from the amide bonding of the TPC unit. Moreover, we confirmed that the transmittance of all fabricated PI films was significantly high. Specifically, the transmittance at 500 nm and the YI value of the PI film with 10 mol% TPC were 90% and 4.13, respectively. In summary, the 6FDA and 3DDS-based PI films used in this study exhibited colorless and transparent optical properties due to their structures containing –CF3 and –SO2, groups, and the introduction of a small amount of TPC comonomer resulted in improved thermal stability and optical properties. The enhancement in thermal properties can be attributed to the contribution of hydrogen bonding induced by the amide bonding of the TPC unit. Furthermore, the control of the CTC phenomenon by the 6FDA and 3DDS base monomers was influenced by the introduction of TPC units, leading to greater suppression of linearity and regularity, resulting in improved optical properties. Finally, considering the limited research available on the properties of PI based on 6FDA and 3DDS, the interesting findings of this study regarding the thermal and optical properties of pure PI, as well as the changes observed due to the introduction of TPC comonomer, are expected to be valuable in various academic and industrial fields in the future.
Data availability
Not applicable.
References
M.C. Choi, Y.K. Kim, C.S. Ha, Prog. Polym. Sci. 33, 581 (2008)
J. Chen, C.T. Liu, IEEE Access 1, 150 (2013)
K. Alzoubi, S. Lu, B. Sammakia, M. Poliks, J. Display Technol. 7, 348 (2011)
Y.H. Yeh, C.C. Cheng, B.C. Lai, C.M. Leu, Y.L. Tseng, J. Soc. Inf. Disp. 21, 34 (2013)
C.J. Ahn, T.Y. Kim, P.H. Hong, S.W. Choi, Y.J. Lee, H. Kwon, H. Jeon, D.W. Ko, I. Park, H.S. Han, S.W. Hong, Adv. Funct. Mater. 32, 2111040 (2022)
M. Hasegawa, Y. Hoshino, N. Katsura, J. Ishii, Polymer 111, 91 (2017)
Y.-Y. Liu, J.-H. Cao, Y. Wang, S.-G. Shen, W.-H. Liang, D.U. Wu, ACS Appl. Polym. Mater. 4, 7664 (2022)
J.H. Souk, W. Lee, J. Soc. Inf. Disp. 18, 258 (2010)
L. Jiao, F. Luo, Z. Du, X. Dai, J. Mu, H. Wang, Z. Dong, X. Qiu, React. Funct. Polym. 181, 105449 (2022)
M. Hasegawa, T. Hishiki, Polymers (Basel) 12, 859 (2020)
M.G. Fara, K. Ibrahim, M.K.M. Al, M.G. Faraj, K. Ibrahim, M.K.M. Ali, Optoelectron. Adv. Mat. 5, 879 (2011)
R.S. Tarighat, A. Goodarzi, S. Mohajerzadeh, B. Arvan, M.R. Gaderi, M. Fathipour, Proc. IEEE 93, 1374 (2005)
T. Ishinabe, A. Sato, H. Fujikake, Phys. Express 7, 111701 (2014)
P.H. Lei, C.M. Hsu, Y.S. Fan, Org. Electron. 14, 236 (2013)
Y. Fang, X. He, J.-C. Kang, L. Wang, T.-M. Ding, X. Lu, S.-Y. Zhang, Q. Lu, Polym. Chem. 13, 5105 (2022)
Y.-Y. Liu, Y.-K. Wang, D.-Y. Wu, J. Appl. Polym. Sci. 139, e52604 (2022)
J. H. Hwang, J. Korean Inst. Electr. Electron. Mater. Eng., 29, 824 (2016).
L.R. Zhou, G.N. Wu, B. Gao, K. Zhou, J. Liu, K.J. Cao, L.J. Zhou, IEEE Trans. Dielectr. Electr. Insul. 16, 1143 (2009)
V.E. Ogbonna, P.I. Popoola, O.M. Popoola, S.O. Adeosun, J. Thermoplast. Compos. Mater. 36, 836 (2023)
I. Gouzman, E. Grossman, R. Verker, N. Atar, A. Bolker, N. Eliaz, Adv. Mater. 31, 1807738 (2019)
Y.S. Park, M.H. Jee, D.H. Baik, Fibers Polym. 23, 360 (2022)
M. Hasegawaa, K. Horie, Prog. Polym. Sci. 26, 259 (2001)
H. Min, B. Kang, Y.S. Shin, B. Kim, S.W. Lee, J.H. Cho, A.C.S. Appl, Mater. Interfaces 12, 18739 (2020)
W. Xu, X. Ma, Y. Su, Y. Song, M. Shang, X. Lu, Q. Lu, J. Appl. Polym. Sci. 137, 48603 (2020)
H.S. Jin, J.H. Chang, J. Appl. Polym. Sci. 107, 109 (2008)
L. Holliday, J. Robinson, J. Mater. Sci. 8, 301 (1973)
R.S. Raghava, Polym. Compos. 9, 1 (1988)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, KE., Jee, M.H. & Baik, D.H. Effect of Terephthaloyl Chloride Comonomer on Thermal and Optical Properties of Colorless and Transparent Polyimide Films Based on 4, 4′-(Hexafluoroisopropylidene) Diphthalic Anhydride and Bis(3-aminophenyl) Sulfone. Fibers Polym 24, 2275–2282 (2023). https://doi.org/10.1007/s12221-023-00211-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12221-023-00211-x