Abstract
A biocompatible nanofibrous scaffold incorporated with the antibacterial extract of medicinal plant has a synergic effect on the process of wound healing, which is due to its high surface area to volume ratio and mimicking the natural extracellular matrix (ECM) of nanofibrous structure, in addition to lower side effects and antibiotic resistance. Here, biocompatible nanofibers of (chitosan:gelatin)/poly(vinyl alcohol)(PVA) were prepared through electrospinning with acorn extract as a natural cross-linker and antibacterial agent for wound-healing applications. The morphology of nanofibers containing various amounts of acorn extract [0.5%, 1%, and 1.5% (w/v)] was investigated by SEM images. The chemical composition of nanofibers was investigated by FTIR spectra. The tensile testing demonstrated the enhancement of mechanical properties of nanofibrous webs (up to 118% tensile stress, 117% young modulus, and 77% strain at break) by adding acorn extract. The degradation rate and the acorn release rate of cross-linked nanofibers decreased by increasing acorn concentration acting as a natural cross-linker. The release mechanism of cross-linked nanofibers was non-Fickian diffusion for 0.5% acorn loading and Fickian diffusion for 1% and 1.5% acorn loading. The acorn-loaded nanofibers showed a 90% antibacterial activity against Staphylococcus aureus bacterium by quantitative standard test method (AATCC 100). Furthermore, the MTT assay showed excellent cell viability and cell attachment of nanofibers for mouse fibroblast (L929) cells. The results indicated that (chitosan:gelatin)/PVA nanofibrous scaffold incorporated with acorn extract was a potent candidate for tissue engineering applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nanomaterial is known as a material with at least one dimension ranging from 1 to 100 nm [1, 2]. Three classifications of nanomaterials can be defined based on the number of their nanoscale dimensions including one-dimensional (1D) nano-sized materials such as nanowires and nanorods, two-dimensional (2D) nano-sized materials such as nanofibers, nanoplates, and nanotubes, and three-dimensional (3D) nano-sized materials such as nanoparticles, nanospheres, and quantum dots [3]. Nanomaterial has been used in various fields of applications including biotechnology and agriculture, energy and environment, transportation and aerospace, food and nutrition, textile and clothing, information technology, and healthcare and medicines [3]. The utilization of nanomaterial has been expanded in various parts of medical science such as drug delivery, disease diagnosis, imaging, treatment of cancers, and artificial implants [4]. Nanomaterial in the form of nanofiber which is produced in various profiles (homogeneous nanofiber, coaxial nanofiber, helical and twisted nanofibers, etc.) widely used as an alternative therapeutics for tissue engineering such as bone, cartilage, vascular, cardiac, and skin repairing. [2]. One of the most promising approaches for the engineering of skin tissue scaffolds is using electrospun nanofibers, which provide appropriate biological, mechanical, and physical properties. Some of these properties include absorbing the excess exudate from the wound, the ability to exchange gas and nutrient, acting as a carrier of bioactive materials, and providing protection against microorganism penetration due to the high surface area to volume ratio and the mesh porosities [5,6,7]. Furthermore, the nanofibrous structure mimicking the fibrous component of the natural extracellular matrix (ECM) leads to greater cell compatibility by controlling cellular response including migration, attachment, proliferation, and differentiation, and then more biocompatibility [8, 9]. A wide range of polymers (nature derived and synthetic) have been electrospun alone or in combination for tissue engineering and regenerative medicine [2, 10, 11]. The electrospun nanofibers of chitosan have been widely used in skin tissue engineering [12,13,14,15,16] due to their excellent properties such as biocompatibility, biodegradability, antibacterial, nontoxicity, antifungal, drug loading ability, hemostatic as well as stimulating collagen synthesis, and fibroblast growth factor (FGF) [17,18,19,20,21]. Gelatin is a water-soluble and protein-based polymer derived from acid or alkaline hydrolysis of collagen [20, 22, 23]. It is known to be a promising polymer for tissue engineering and wound-healing applications, because of its biocompatibility, biodegradability, nontoxicity, antigenicity, and high bio-absorptivity [24,25,26]. Many studies have applied electrospinning of chitosan and gelatin in combination with other polymers or bioactive molecules in tissue engineering [22, 27, 28]. Using medicinal plants for wound-healing applications has been a traditional treatment for decades [29,30,31,32]. That is because these plants provide therapeutic effects such as antibacterial, antioxidant, anti-inflammatory, anti-ultraviolet light, antiseptic, anti-wrinkle, and wound-healing stages [29, 33]. Medicinal plant-loaded nanofibrous webs which provide a controlled sustained release within the target site and therefore minimize the side effects as well as mimicking the natural extracellular matrix have received a lot of attention recently [34,35,36]. An overview of medicinal plant extract-incorporated nanofibrous webs for wound-healing application is presented in Table 1.
The fruit of Quercus species, acorn, consists of high content phytochemical compounds, mainly phenolic compounds including phenolic acid, flavonoids, and tannins in addition to carbohydrate, protein, etc. [37]. There are many studies about phytoconstituents and the biological activity of different parts of Quercus species (i.e., seed/acorn, bark, and leaves) such as antibacterial [38], antioxidant, anti-inflammatory [39], antifungal, antiviral, wound healing [40, 41], anti-leishmanial [42], and antidiabetic [37]. In this study, the bead-free (chitosan:gelatin)/PVA nanofibers incorporated with acorn extract as a natural antibacterial and cross-linker agent were electrospun for the first time. The morphology, physicochemical, mechanical, and biological properties of nanofibrous webs were characterized and the results suggested that the obtained nanofibers could potentially be used for tissue engineering applications.
2 Experimental
2.1 Materials
Chitosan (medium molecular weight with 75–85% degree of deacetylation (DD)), gelatin type B from bovine skin, glutaraldehyde (GTA, 25% aqueous solution), and 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich Co., USA. Poly(vinyl alcohol) (PVA) (Mw = 72 kDa) and acetic acid were obtained from Merck Co., Germany. Acorn extract powder (ethanolic extract) with the recovery of solvent was prepared from Jahad Daneshgahi, Tehran, Iran. Mouse fibroblast cells (L929, Cell bank number C161) were obtained from the National Cell Bank, Pasteur Institute, Tehran, Iran.
2.2 Solution Preparation
Initially, chitosan (3 wt%) and gelatin (20 wt%) solutions were separately prepared in acetic acid 60% (v/v) by stirring for 24 h and 5 h, respectively, at room temperature. PVA solution (10 wt%) was prepared in distilled water by stirring for 10 h at 50 °C. To prepare (chitosan:gelatin)/PVA solution, the chitosan and gelatin solutions were mixed homogeneously in 50:50 volume ratios and then blended with PVA solution in a 60/40 volume ratio, stirring for 5 h at room temperature. Various amounts of acorn powder [0.5%, 1%, and 1.5% (w/v)] were added to (chitosan:gelatin)/PVA solution and stirred for an additional 12 h to prepare acorn extract-loaded solutions which coded as CGP.0.5, CGP.1 and CGP.1.5, respectively. The (chitosan:gelatin)/PVA solution without acorn extract was also coded as CGP.0.
2.3 Electrospinning
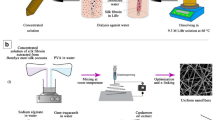
The prepared polymer solutions were fed into a 1 ml syringe with a metal capillary needle (23 G) and electrospun on a rotating collector (wrapped with an aluminum foil with a rotating speed of 60 rpm) with the following conditions: 0.2 mL/h feeding rate, 20 kV applied voltage and 14 cm distance between the needle and collector at 25ºC temperature and 30% humidity as shown in Fig. 1. The nanofibrous membranes were cross-linked by GTA vapor (25%) to improve the structural stability and mechanical properties. The samples were placed in the presence of GTA:hydrochloric acid (10:1 v/v) in a sealed glass container heating in an oven for 3 h at 40 °C. To remove any unreacted aldehyde groups from the cross-linked nanofibers, they were washed with glycine (0.2 M) and phosphate-buffered saline (PBS) three times and then dried for 10 h at 30ºC.
2.4 Total Phenolic Content
The concentration of total phenols of acorn extract was estimated with Folin–Ciocalteau reagent [54]. Suitable aliquots of acorn extract solution or standard tannic acid (12.5, 25, 50, 100, and 200 µg/ml in distilled water) were added to separate flasks. After that, 5 ml of distilled water and 0.5 ml of Folin–Ciocalteu reagent were added and mixed by shaking. After 3 min, 1 ml of sodium carbonate (20%) was added to each flask and diluted with distilled water to 10 ml volume. After 1 h, the absorbance of each sample (in triplicate) was determined against reagent blank at 725 nm using UV–visible spectrophotometer (Human crop, Xma-2000). The amount of total phenolic content was calculated from the calibration curve of tannic acid and expressed as mg of tannic acid equivalent /g of dry acorn extract.
2.5 Total Flavonoid Content
The total flavonoid content of acorn extract was measured by the colorimetric method with aluminum chloride [55]. One milliliter of the standard solution of catechine (75, 150, 300, 600, and 1200 µg/ml in methanol) or suitable aliquots of acorn extract solution was added to separate flasks containing 4 ml of distilled water, followed by the addition of 0.3 ml of sodium nitrite (5% w/v). After 5 min, 0.3 ml of aluminum chloride (10% w/v) was added and after 6 min, 2 ml of sodium hydroxide (1 M) was added to each flask and diluted with distilled water to 10 ml volume. The absorbance of the pink color mixture was determined against the water blank at 510 nm. The total flavonoid content was expressed as mg catechin equivalents/g of dry acorn extract. The analysis of each sample was done in triplicate.
2.6 Scanning Electron Microscopy (SEM)
The surface morphology of nanofibrous scaffolds was studied with scanning electron microscopy (SEM: Philips XL30 Netherland). The samples were sputter-coated with a thin layer of gold/palladium for 160 s (Sputter coater: Emitech SC7620) to prevent the charging effect. Image analysis software, Image-J (NH, USA) was used to determine the mean diameter and size distribution of nanofibers by measuring the diameter of two individual points of 50 randomly selected nanofibers in each SEM image.
2.7 Mechanical Testing
The mechanical properties, including the ultimate tensile strength, elongation at break, and Young’s modulus of samples were measured by tensile strength instrument (Instron-5566 universal testing machine) at room temperature. Each nanofibrous web (seven replicates) was cut into a rectangle shape (5 × 20 mm2) and fixed in the middle of the square-shaped paper holder. The holder was clamped and cut at both sides before applying the tensile loading and then exposed to a 50 N load cell in a 5 mm/min extension rate and 20 mm gauge length.
2.8 Fourier Transform Infrared Spectroscopy
The Fourier transform infrared (FTIR) spectrometer (Thermo Nicolet, Nexus670, USA) was used to investigate the chemical structure of nanofibrous webs in the spectral range of 500–4000 cm−1 (4.0 resolution) at room temperature. The OMNIC software was used to take spectra and collect data from molecular structure and chemical bonds of the samples.
2.9 Degradation Analysis
To investigate the stability and physical integrity of nanofibrous scaffolds, their weight loss was measured during the degradation process as follows. The dry samples were carefully weighted (Wi) and then individually immersed in phosphate- buffered saline (PBS, PH 7.4) and incubated at 37 °C for 1, 3, 5, 7, 14, 21, 28, and 35 days. The residual of nanofibrous webs was withdrawn and washed in distilled water two times and then dried and carefully weighted (Wf). The calculated weight loss percentage was reported as an index of their stability according to Eq. (1) [43].
2.9.1 In Vitro Release of Acorn Extract
To study the release profile of acorn extract from nanofibrous scaffolds, 5 × 5 cm2 of each sample was carefully weighed and incubated at 37 °C in 6 ml of PBS, as a release media. A certain volume of acorn extract release media was taken out to study the release kinetics and replaced with fresh PBS at certain time intervals. The absorbance of release media was determined by UV–Vis spectrophotometry at each time point (in triplicate) against a blank sample (the release media of nanofibrous web without acorn extract content). The corresponded acorn concentration (C) was calculated using the standard graph, according to Eq. (2) which was obtained as follows:
To plot the standard curve of the acorn extract, the absorbance of five concentrations of acorn extract solution (in PBS) was characterized against a PBS blank by UV–Vis spectrophotometry (Photonix Ar 2015, Iran) at wavelengths from 200 to 800 nm as shown in Fig. 2. The wavelength of 272 nm was chosen as the characteristic peak of the acorn extract and the standard graph (absorbance vs concentration) of the acorn extract was plotted.
The cumulative release percentage of acorn extract from nanofibrous webs (Er) was calculated according to Eq. (3).
where V0 and Vr are the initial volume of release media and replace media, respectively, Cn is the acorn extract concentration in each media and mtotal is the acorn extract contained in each nanofibrous web [43]. To investigate the release kinetics of acorn extract from cross-linked nanofibrous webs, the Korsmeyer–Peppas model was used according to Eq. (4):
where Mt/M is the release friction at time t, Kkp is the release velocity constant corresponding to the geometrical and structural characterization of the polymeric system and n is the release exponent in a function of time indicating the release mechanism for Mt/M∞ < 0.6 as follows. If n \(\le \) 0.45, the release mechanism is Fickian diffusion, for 0.45 < n < 1, it is related to anomalous (non-Fickian) diffusion, and for n = 1 and n > 1, it is related to the case II transport (zero-order release) and super case II transport mechanism release, respectively [11, 56, 57].
2.10 Antibacterial Activity Test
The antibacterial activity of samples was evaluated against Gram-negative bacteria, Escherichia coli (E. coli: ATCC 25,922), and Gram-positive bacteria, Staphylococcus aureus (S. aureus: ATCC 6538), through quantitative standard test method (AATCC 100). The E. coli and S. aureus bacteria were grown separately, in nutrient broth (37 °C for 18 h). Bacterial cells were collected by centrifugation (3000×g for 15 min), washed with PBS three times, and then resuspended in PBS and diluted to an approximate bacterial concentration of 1 × 105 CFU/ml. Each sample (in triplicate) was cut in a circular shape (5 cm diameter), placed in distinct Petri dishes, and sterilized with UV radiation (30 min each side). A certain volume of bacterial suspension was added to each sample and then incubated at 37 °C overnight. For recovering viable bacterial cells, 20 ml of sterile PBS was added to each sample and vortexed vigorously for 5 min. Serial dilutions of 1:10 ratio were prepared from each test in duplicate. A volume of 0.1 ml of dilutions was spread onto nutrient and incubated at 37 °C for 16–18 h. The bacterial count for each sample was calculated, and the antibacterial activity was calculated according to Eq. (5) [18].
where R is the bacterial reduction percentage, and A and B are the initial number and recovered count of bacteria, respectively.
2.11 Cytotoxicity and Cell Growth
To investigate the viability, cell growth, and proliferation of the nanofibrous webs, the quantitative determination method, 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (MTT), was done according to standard ISO 10993-5 (indirect contact) using the mouse fibroblast (L929) cells as follows. Firstly, the L929 (NCBI C161) were defreezed and then put in Roswell Park Memorial Institute (RPMI) medium with 10% (v/v) fetal bovine serum (FBS) and incubated in air containing 90% humidity and 5% CO2 at 37 °C, replacing the culture medium every 3–4 days. The samples (1.5 cm2) with four replicates were washed with ethanol 70% (v/v) for 5 min and then sterilized with UV radiation (1 h each side). The sterile samples were placed in a 12-well plate and then cells at a density of 5 × 103 L929 cells (with 100 µl culture medium) were added to each well and incubated (37 °C) for 4 h. After ensuring cell adherence, a certain amount of culture medium was added to each well and thrown completely away after 1, 3, and 7 days. 400 µl of MTT formazan solution was added to each and incubated for 4 h. The absorbance of each sample was detected by spectrophotometer (BioTek ELx808, USA) at 570 nm as optical density (OD). The viability percentage of each sample normalized by the control group (nanofibrous web without acorn extract) was calculated according to Eq. (6).
2.12 Visualization of Cell Attachment
The morphology of cells cultured on the surface of nanofibrous scaffolds was observed by SEM after 24 h cell seeding. The sterile samples were placed in a 24-well plate and then cells at a density of 3 × 104 L929 (with 100 µl of culture medium) were added and incubated for 5 h. A certain amount of culture medium with FBS 10% was added to each well and removed after 24 h by washing with PBS for 30 s. The samples were treated with GTA 3.5% to fix the cells and washed with deionized water and a series of ethanol solutions (50%, 60%, 70%, 80%, and 96%). Finally, the dried samples were imaged with SEM.
2.13 Statistical Analysis
All of the experimental data were collected in triplicate and the results were presented as average ± standard deviation (SD). Statistical analysis as an analyzing way to evaluate variance and independent samples were used with one-way ANOVA at p < 0.05 statistical significance.
3 Results and Discussion
3.1 Morphology of Nanofibrous Webs
The SEM images, mean diameter, and diameter distribution histograms of (Cs:Ge)/PVA nanofibers incorporated with various amounts of acorn extract are shown in Fig. 3. The mean diameter of (chitosan:gelatin)/PVA nanofiber increased from 120.53 to 142.97 nm, 185.81 nm, and 215.24 nm by adding 0.5%, 1%, and 1.5% of acorn extract, respectively. Adding acorn extract led to an increase in the molecular entanglements between chitosan, gelatin, and PVA polymers and acorn extract, as a natural cross-linker. It caused an increase in the viscosity and repulsive force to stretch the solution droplet in the Taylor cone phase and, consequently, an increase in the mean diameter of nanofibers. Zhang et al. indicated the C–N interactions created between the natural phenolic compound and the amino group in the gelatin structure [58].
3.2 Total Phenolics and Flavonoids
The results revealed that the acorn extract consists of a high content of total phenol (805.50 ± 76.90 mg of tannic acid equivalents/g dry acorn extract) and total flavonoids (189.07 ± 5.39 mg of catechin equivalents/g dry acorn extract). Many studies investigating the phytochemical compounds of acorn indicated that the main and ubiquitous compounds of acorn were phenolic acid, flavonoid, and tannins [37, 59].
3.3 FTIR Spectroscopy
The FTIR spectra of chitosan, gelatin, PVA, and acorn extract powders, in addition to the FTIR spectra of CGP.0 and CGP.1 nanofibrous webs, before and after cross-linking with GTA vapor, are illustrated in Fig. 4. The FTIR spectrum of gelatin powder exhibits a peak at 3414 cm−1 attributed to stretching of the OH and NH groups (amide A). The characteristic peaks at 1635 cm−1, 1560 cm−1, and 1240 cm−1 are associated with amide I (C=O), amide II (N–H and C–H), and amide III (C–N stretch and N–H in the phase bending), respectively [25, 60]. The FTIR spectrum of chitosan powder displays a characteristic peak at 3427 cm−1 related to N–H and O–H stretching and intermolecular hydrogen bonding of the chitosan backbone [18]. The absorbance peaks at 1656 cm−1, 1590 cm−1, and 1074 cm−1 are attributed to amide I (C=O), amide II (NH2 bending), and C–O stretching, respectively [25, 61]. Other peaks at 1431 cm−1 and 1319 cm−1 are associated with CH3 symmetrical deformation modes [24]. Moreover, the peaks at around 900 cm−1 to 1153 cm−1 are assigned to the saccharine structure of chitosan [24]. The FTIR spectrum of PVA powder shows the characteristic peaks at 3424 cm−1, 2924 cm−1, 1435 cm−1, and 1098 cm−1 assigned to O–H stretching vibration, CH2, CHOH, and CO groups, respectively [25, 35, 62]. The FTIR spectrum of acorn extract displays an absorbance peak at 3401 cm−1 related to phenolic groups and a large amount of intermolecular and intramolecular hydrogen bonding in its structure. The peaks at 1611 cm−1, 1522 cm−1, and 1444 cm−1 are related to stretching C=C vibration from the aromatic rings [63]. Furthermore, the peaks at 1706 cm−1, 1200 cm−1, 1330 cm−1, and 1030 cm−1 are assigned to C=O, C–OH symmetrical and asymmetrical C–O vibrations, respectively. The peaks around 680–860 cm−1 are attributed to the CH bond vibration in the benzene rings [25, 60]. Furthermore, the FTIR spectrum of the CGP.0 nanofibers (before cross-linking with GTA) exhibits an absorbance peak at 3347 cm−1 associated with NH and OH stretching vibration and intermolecular and intramolecular hydrogen bondings. Furthermore, the obvious sharp peaks at 2938 cm−1, 1654 cm−1, and 1540 cm−1 are related to C–H bonding, amide I (C=O), and amide II (N–H), respectively [18, 25]. Therefore, the CGP.0 nanofibrous web is composed of all three polymers, including chitosan, gelatin, and PVA [18]. The peak at 3347 cm−1 from the FTIR spectrum of CGP.0 nanofibers (before cross-linking with GTA) is shifted gradually to higher wavenumber 3365 cm−1 for CGP.1 (before cross-linking with GTA), which indicates the increase of intermolecular hydrogen bonding between the polymers and phenolic groups of acorn extract content, as a natural cross-linker [64,65,66]. Furthermore, the relative intensity of peaks around 680–860 cm−1 ascribed to the CH bond of aromatic groups is higher in CGP.1 nanofibers (before cross-linking with GTA), compared to CGP.0 nanofibers (before cross-linking with GTA), which could exhibit the existence of acorn extract in its structure [60]. Moreover, the FTIR spectra from CGP.0 and CGP.1 nanofibers, which are cross-linked with GTA, exhibit broader and more intense peaks at 3415 cm−1 and 3426 cm−1 wavenumbers, respectively, in comparison with non-cross-linked ones. It could correspond to hydrogen bonding between GTA and nanofibers contents [65]. Moreover, there are two new peaks at 960 cm−1 and 870 cm−1 in cross-linked nanofibrous scaffold corresponding to symmetric and asymmetric vibration of COCOC groups [61].
3.4 Mechanical Properties
One of the critical factors for preparing a skin wound dress is achieving appropriate mechanical properties to withstand handling, stretching, and physiological forces during the wound-healing processes. The values of ultimate tensile stress (UTS), elongation to break (EB), and Young’s modulus (EM) of non-cross-linked and cross-linked CGP.0, CGP.0.5, CGP.1, and CGP.1.5 nanofibrous webs are indicated in Table 2. The UTS and EM of cross-linked nanofibers increased and their EB decreased by introducing new bondings within cross-linked nanofibers, which caused to reduce the slippage of nanofibers and improve the mechanical properties of the nanofibrous web [25, 67]. Reui-Yi Tsai et al. revealed that cross-linking of chitosan/gelatin/PVA nanofibers by GTA vapor enhanced the UTS and decreased the EB of nanofibrous webs because of polymer chain restrictions [22]. The UTS of CGP.0.5, CGP.1 and CGP.1.5 nanofibers increase by 87%, 96%, and 118%, respectively, as well as their EM increase by 43%, 50%, and 117%, respectively. These were attributed to introduction of intermolecular interactions between the phenolic compounds of acorn extract (acting as a cross-linker) and polymeric structure of nanofibrous webs, such as C–N covalent bonds and hydrogen bonding, which caused an increase in stretch resistance and tensile strength [58]. The role of tannic acid as an effective cross-linker of gelatin film on the increase of tensile strength was proved [60, 66]. Adding acorn extract led to an increase in the diameter of the nanofibers and then reduced the number of contact points and frictional cohesion forces between nanofibers, which resulted in the enhancement of the EB of CGP.0.5 and CGP.1 nanofibrous webs to 50% and 77%, respectively [68]. However, by adding more acorn extract (1.5%), the hydrogen bond force between polymer and acorn extract overcomes the lower number of contact points (because of higher nanofiber diameter) between nanofibers, which resulted in greater resistance to elongation and higher rigidity.
3.5 Biodegradation Analysis
One of the critical factors for a biocompatible scaffold is the proportion between scaffold biodegradation and tissue repair rate [69]. To investigate the physical stability of cross-linked nanofibrous webs, the weight loss percentages of cross-linked CGP.0, CGP.0.5, CGP.1, and CGP.1.5 nanofibrous scaffolds were obtained at 1, 3, 5, 7, 14, 21, 28, and 35 days (Fig. 5). The weight loss of all samples increased suddenly and then continued with a gradual and constant slope as shown in Fig. 5. The highest weight loss rate was attributed to CGP.0 with 38% mass loss, which decreased by 34%, 14%, and 12% by adding 0.5%, 1%, and 1.5% of the acorn extract, respectively. It might be due to the effect of the acorn extract as a cross-linking agent, which caused intermolecular reactions between its phenolic compounds and polymers [22, 58, 60, 65]. Bitao Lu et al. indicated that the interactions between the phenolic hydroxyls and the hydrogen bond of gelatin acted as a cross-linking agent for chitosan/gelatin sponges [66]. Moreover, the mean diameter of nanofibrous webs increased by adding acorn extract content, leading to the decrease in the surface area to volume ratio and then decrease in their connection with the surrounding aquatic medium and, consequently, structural degradation [43].
3.6 In Vitro Release Study of Acorn Extract
The cumulative release percentages of cross-linked CGP.0.5, CGP.1, and CGP.1.5 nanofibrous webs (in triplicate) incubated (37 °C) in PBS solution for 1, 3, 5, 7, 14, 21, and 28 days are plotted in Fig. 6. There was a sudden release on day 1 of about 19%, 15%, and 7%, attributed to acorn extract release from CGP.0.5, CGP.1, and CGP.1.5, respectively. The release rate increased gradually until day 7 and then continued with a relatively constant rate untile day 28, as shown in Fig. 6. The release from the biodegradable polymeric system was governed by two main factors, including desorption/diffusion of acorn extract and dissolution/erosion of the polymeric system [70]. The sudden release was concerned with the amount of acorn extract content in the nanofibrous structure located on the surface of nanofibers, which were in more contact with an aqueous environment [43, 70]. Moreover, filling the existing pores in the nanofibrous structure and its swelling led to passing the dissolved acorn extract through the pores and entering the surrounding media. This was followed by the gradual increase in the decomposition of the polymeric system and as a result the release of the trapped acorn extract in the nanofibrous structure [70]. The final release rate of acorn extract from CGP.0.5, CGP.1, and CGP.1.5 reached 82%, 37%, and 26% respectively, which reduced despite the increase of the acorn extract content. It might be due to the higher structural stability of nanofibrous webs in the presence of acorn extract as a cross-linker agent by increasing hydrogen bonding between them as shown in Fig. 5 [58, 60, 64]. Moreover, the mean diameter of nanofibrous webs arose by increasing the acorn extract content, which led to the lower surface area to volume ratio and then reduction in the surface contact with the surrounding environment [71]. The Korsmeyer–Peppas kinetic model was used to investigate the mechanism of acorn extract release from nanofibrous webs. The n value, as release exponent, indicates the release mechanisms for Mt/M∞ < 0.6. The release velocity constant (Kkp value) and regression coefficient (R2) were calculated for CGP.0.5, CGP.1, and CGP.1.5, which are summarized in Table 3. The n values of CGP.1 and CGP.1.5 (for Mt/M∞ < 0.6, within 28 days) were equal to 0.27 and 0.39 < 0.45, respectively, located in the Fickian diffusion category, and n value equal to 0.57 > 0.45 for CGP.0.5 (for Mt/M∞ = 0.57 < 0.6 within 7 days) corresponded to non-Fickian diffusion, which related to drug diffusion and polymer swelling together [56, 57].
3.7 Antibacterial Activity Test
The results of antibacterial activity (AATCC 100 standard test method) of CGP.1 and CGP.0 nanofibrous scaffold (after cross-linking with GTA), against Gram-negative bacteria, E. coli, and Gram-positive bacteria, S. aureus, are illustrated in Fig. 7. The antibacterial percentage of (chitosan:gelatin)/PVA nanofibrous scaffolds without acorn extract was 40% (6 × 104 CFU/ml1) against S. aureus. The antibacterial activity of chitosan has been investigated by many authors and has been attributed to some mechanisms, including the interactions between the negative charge of the bacterial cell membrane and positive charge of polycationic chitosan molecules, which cause cell membrane disruption of bacteria and leaking intracellular contents [14, 16]. Moreover, the antibacterial activity of acorn extract has been reported in many studies, because of bioactive compounds such as flavonoids, tannins, polyphenols, and alkaloids [37]. The mechanism of phenolic toxicity to microorganisms was assigned to the ability of hydroxyl groups or phenolic compounds, which founded in acorn extract to complex with protein and bacterial cell wall polypeptides, which caused decomposite of the mitochondrial and cell membrane of bacteria, and therefore change its permeability and death of bacterial cell [30, 72, 73]. It could be the reason of high antibacterial activity of acorn-loaded nanofibers (CGP.1) against S. aureus (90% reduction in bacterial concentration, 1 × 104 CFU.ml−1). Moreover, many studies indicate that the multilayer structure of Gram-negative bacterium cell, enveloped with an outer membrane containing lipopolysaccharide, leads to more resistance against antimicrobial agents rather than Gram-positive bacteria [63, 74]. This could be the reason for the less than 5% (9.5 × 104 CFU/ml) and 10% (9 × 104 CFU/ml) antibacterial activity of CGP.0 and CGP.1 against gram-negative bacteria, E. coli.
3.8 Cytotoxicity and Cell Growth
The biocompatibility of nbanofibers with various amounts of acorn extract was evaluated with MTT assay in vitro. This method is based on changing yellow tetrazolium dye powder to insoluble dark purple formazan crystals, which only occur in living cells. The intensity of optical density has a direct relationship with formazan concentration and the number of living cells as a consequence. The viability and optical density of CGP.0, CGP.0.5, CGP.1, and CGP.1.5 nanofibrous scaffolds after 1, 3, and 7 days of culturing time are illustrated in Fig. 8. The viability of the nanofibers grew by adding acorn extract and keeping for 1, 3, and 7 days, which was confirmed by the gradual enhancement of their optical density [37] as shown in Fig. 8. It is consistent with the result of Bitao Lu et al., which revealed that the incorporation of chitosan/gelatin sponge with tannin as a cross-linker had no toxicity against L929 fibroblast cell and promoted wound healing in vivo [40, 66]. Yin et al. have shown that the phenolic compounds isolated from Quercus mongolica Fisch. ex Ledeb had a significant effect on inhibition of inflammatory cytokines and chemokines as well as expression of immune factors, thereby having potential for inflammatory skin diseases reduction [75, 76].
3.9 Morphology of the Cells
The SEM images of the cell morphology of CGP.0, CGP.0.5, CGP.1, and CGP.1.5 after 24 h of culturing are illustrated (three magnifications) in Fig. 9. The cell attachment, anchoring to nanofibrous scaffolds, spreading, and interconnections between L929 cells were observed for all the samples which increased by adding acorn extract. It could confirm the biocompatibility of the nanofibrous scaffold, not only because of their natural compositions and the efficient role of acorn extract combined with chitosan and gelatin, but also because of their nanofibrous structure with high surface area to volume ratio mimicking an ECM structure [37, 77, 78]. Xu et al. revealed that the ternary composite nanofiber of tannic acid/chitosan/pullulan favored the growth and attachment of fibroblast cells [79].
4 Conclusion
The nanofibrous composite of (chitosan:gelatin)/PVA incorporated with acorn extract was successfully electrospun for wound-healing applications with lower side effects like antibiotic resistance. Cross-linking of nanofibrous scaffolds with GTA vapor led to improving their mechanical properties. The mechanical properties and physical stability of nanofibrous webs are dramatically enhanced by increasing acorn concentration while increasing the nanofiber diameter. The acorn release rate of nanofibers decreased despite the increase in acorn content. This release followed two mechanisms including non-Fickian diffusion for CGP.0.5 and Fickian diffusion for CGP.1 and CGP.1.5. The acorn extract-loaded nanofibrous webs showed excellent antibacterial activity (90%) against S. aureus, according to AATCC 100 standard. The results showed that acorn-loaded nanofibers had no toxicity against L929 fibroblast cells. In conclusion, acorn extract-loaded (chitosan:gelatin)/PVA nanofiber showed excellent biocompatibility for skin tissue engineering, not only because of its natural biocompatible composition, but also because of its nanofibrous structure with high surface area to volume ratio and mimicking the natural ECM.
Data availability
Data will be available upon contacting the corresponding author.
References
N.A. Singh, Environ. Chem. Lett 15, 185–191 (2017)
A.D. Juncos-Bombin, N.J. Dunne, H.O. McCarthy, Mater. Sci. Eng. C 114, 110994 (2020)
T. Hassan, A. Salam, A. Khan, S.U. Khan, H. Khanzada, M. Wasim, M.Q. Khan, I.S. Kim, J. Polym. Res 28, 1–22 (2021)
G. Nikaeen, S. Abbaszadeh, S. Yousefinejad, Nanomedicine 15, 1501–1512 (2020)
S. Homaeigohar, A.R. Boccaccini, Acta Biomater. 107, 25–49 (2020)
M. Mirjalili, S. Zohoori, J. Nanostruct. Chem 6, 207–213 (2016)
D.M. Ibrahim, A. Kakarougkas, N.K. Allam, Mater. Today Chem 5, 11–23 (2017)
S. Hinderer, S.L. Layland, K. Schenke-Layland, Adv. Drug Deliv. Rev 97, 260–269 (2016)
S. Du, N. Zhou, Y. Gao, G. Xie, H. Du, H. Jiang, L. Zhang, J. Tao, J. Zhu, Nano Res. 13, 2525–2533 (2020)
D. Sundaramurthi, U.M. Krishnan, S. Sethuraman, Polym. Rev. 54, 348–376 (2014)
R.S. Ambekar, B. Kandasubramanian, Eur. Polym. J 117, 304–336 (2019)
I. Bano, M. Arshad, T. Yasin, M.A. Ghauri, M. Younus, Int. J. Biol. Macromol. 102, 380–383 (2017)
R.A.A. Muzzarelli, Carbohydr. Polym 76, 167–182 (2009)
F. Croisier, C. Jérôme, Eur. Polym. J 49, 780–792 (2013)
M.Z. Elsabee, H.F. Naguib, R.E. Morsi, Mater. Sci. Eng. C 32, 1711–1726 (2012)
T. Dai, M. Tanaka, Y.Y. Huang, M.R. Hamblin, Expert Rev. Anti. Infect. Ther. 9, 857–879 (2011)
L. Tan, J. Hu, H. Huang, J. Han, H. Hu, Int. J. Biol. Macromol 79, 469–476 (2015)
E. Hadipour-Goudarzi, M. Montazer, M. Latifi, A.A.G. Aghaji, Carbohydr. Polym. 113, 231–239 (2014)
A. Moeini, P. Pedram, P. Makvandi, M. Malinconico, G. Gomez d’Ayala, Carbohydr. Polym. 233, 115839 (2020)
J. Jacob, J.T. Haponiuk, S. Thomas, S. Gopi, Mater. Today Chem. 9, 43–55 (2018)
L. Behbood, S. Karimi, E. Mirzaei, G. Mohammadi, M. Azami, E. Arkan, Fibers Polym. 19, 1454–1462 (2018)
R.Y. Tsai, S.C. Hung, J.Y. Lai, D.M. Wang, H.J. Hsieh, J. Taiwan Inst. Chem. Eng. 45, 1975–1981 (2014)
X. Han, Z. Xing, S. Si, Y. Yao, Q. Zhang, Fibers Polym. 15, 2572–2580 (2014)
H.H. Sokker, A.M. Abdel Ghaffar, Y.H. Gad, A.S. Aly, Carbohydr. Polym. 75, 222–229 (2009)
N.T. Ardekani, M. Khorram, K. Zomorodian, S. Yazdanpanah, H. Veisi, H. Veisi, Int. J. Biol. Macromol. 125, 743–750 (2019)
E. Mohamed, A. Fitzgerald, T. Tsuzuki, Mater. Today NANO 16, 100137 (2021)
J. Jafari, S.H. Emami, A. Samadikuchaksaraei, M.A. Bahar, F. Gorjipour, Biomed. Mater. Eng. 21, 99–112 (2011)
M. Pezeshki-Modaress, M. Zandi, S. Rajabi, Prog. Biomater. 7, 207–218 (2018)
U. Das, S.S. Behera, S. Singh, S.I. Rizvi, A.K. Singh, Phyther. Res. 30, 1895–1904 (2016)
M.M. Cowan, Clin. Microbiol. Rev. 12, 564–582 (1999)
R. Zhang, M. Lei, B. Hu, H. Tu, M. Hu, Fibers Polym. 0, 1–9 (2021)
B.P. Nagori, R. Solanki, Res. J. Med. Plant 5, 392–405 (2011)
Y.P. Talekar, K.G. Apte, S.V. Paygude, P.R. Tondare, P.B. Parab, J. Ayurveda Integr. Med. 8, 73–81 (2017)
A. Zarghami, M. Irani, A. Mostafazadeh, M. Golpour, A. Heidarinasab, I. Haririan, Fibers Polym 16, 1201–1212 (2015)
K. Lee, S. Lee, Fibers Polym. 21, 999–1012 (2020)
G. Jin, M.P. Prabhakaran, D. Kai, S.K. Annamalai, K.D. Arunachalam, S. Ramakrishna, Biomaterials 34, 724–734 (2013)
E. Burlacu, A. Nisca, C. Tanase, Forests 11, 1–24 (2020)
D.F. Basri, S.H. Fan, Indian J. Pharmacol. 37, 26–29 (2005)
B. George, N. Bhatia, T.V. Suchithra, Eur. Polym. J. 157, 110640 (2021)
J. Chokpaisarn, S. Chusri, T. Amnuaikit, W. Udomuksorn, S.P. Voravuthikunchai, PeerJ 2017, 1–16 (2017)
R. Thakur, N. Jain, R. Pathak, S.S. Sandhu, Evid.-Based Complement. Altern. Med 2011, 1–17 (2011)
A. Farghi-Yamchi, M. Dabirzadeh, Y. Maroufi, Med. Lab. J 12, 23–28 (2018)
M. Shokrollahi, S.H. Bahrami, M.H. Nazarpak, A. Solouk, Int. J. Biol. Macromol. 147, 547–559 (2020)
M. Emin, S. Cesur, T. Taskin, G. Erdemir, D. Serap, Y. Muge, L. Kabasakal, O. Gunduz, Eur. Polym. J. 120, 109239 (2019)
Z. Guleken, J. Depciuch, H. Ege, G. İlbay, C. Kalkandelen, D. Ozbeyli, H. Bulut, G. Sener, N. Tarhan, S. Erdem Kuruca, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 254, 119639 (2021)
J. Yin, L. Xu, Int. J. Biol. Macromol. 160, 352–363 (2020)
S. Ahmadi, A. Hivechi, S.H. Bahrami, P.B. Milan, S.S. Ashraf, Int. J. Biol. Macromol. 173, 580–590 (2021)
N. Barnthip, A. Muakngam, J. Bionanosci. 8, 313–318 (2014)
B. Dhurai, N. Saraswathy, R. Maheswaran, P. Sethupathi, P. Vanitha, S. Vigneshwaran, V. Rameshbabu, Front. Mater. Sci 7, 350–361 (2013)
Z. Hadisi, J. Nourmohammadi, S.M. Nassiri, Int. J. Biol. Macromol 107, 2008–2019 (2018)
M. Sadri, S. Arab-Sorkhi, H. Vatani, A. Bagheri-Pebdeni, Fibers Polym. 16, 1742–1750 (2015)
I.A. Isoglu, N. Koc, Fibers Polym. 21, 1453–1465 (2020)
Y.H. Shan, L.H. Peng, X. Liu, X. Chen, J. Xiong, J.Q. Gao, Int. J. Pharm. 479, 291–301 (2015)
S. Kamtekar, V. Keer, V. Patil, J. Appl. Pharm. Sci 4, 61–65 (2014)
K.M. Yoo, C.H. Lee, H. Lee, B.K. Moon, C.Y. Lee, Food Chem. 106, 929–936 (2008)
R.W. Korsmeyer, R. Gurny, E. Doelker, P. Buri, N.A. Peppas, Int. J. Pharm. 15, 25–35 (1983)
S. Dash, P.N. Murthy, L. Nath, P. Chowdhury, Acta Pol. Pharm. Drug Res. 67, 217–223 (2010)
X. Zhang, M.D. Do, P. Casey, A. Sulistio, G.G. Qiao, L. Lundin, P. Lillford, S. Kosaraju, Biomacromol 11, 1125–1132 (2010)
A.F. Vinha, J.C.M. Barreira, I.C.F.R. Ferreira, M.B.P.P. Oliveira, Ref. Ser. Phytochem, 273–287 (2020)
M.L.L.R. do Menezes, N.R. da Pires, P.L.R. da Cunha, M. de Freitas-Rosa, B.W.S. de Souza, J.P.A. de Feitosa, M.S.M. de Souza-Filho, Food Packag. Shelf Life 19, 7–15 (2019)
S. Gomes, G. Rodrigues, G. Martins, C. Henriques, J.C. Silva, Int. J. Biol. Macromol. 102, 1174–1185 (2017)
L. Fan, H. Yang, J. Yang, M. Peng, J. Hu, Carbohydr. Polym. 146, 427–434 (2016)
Z. Kharat, M. Sadri, M. Kabiri, Fibers Polym. 22, 989–999 (2021)
N. Cai, C. Li, C. Han, X. Luo, L. Shen, Y. Xue, F. Yu, Appl. Surf. Sci. 369, 492–500 (2016)
Y.N. Chen, C. Jiao, Y. Zhao, J. Zhang, H. Wang, ACS Omega 3, 11788–11795 (2018)
B. Lu, T. Wang, Z. Li, F. Dai, L. Lv, F. Tang, K. Yu, J. Liu, G. Lan, Int. J. Biol. Macromol. 82, 884–891 (2016)
K. Jalaja, D. Naskar, S.C. Kundu, N.R. James, Carbohydr. Polym. 136, 1098–1107 (2016)
M. ElMessiry, N. Fadel, Alex. Eng. J 58, 885–890 (2019)
T. Agarwal, R. Narayan, S. Maji, S. Behera, S. Kulanthaivel, T.K. Maiti, I. Banerjee, K. Pal, S. Giri, Int. J. Biol. Macromol. 93, 1499–1506 (2016)
M. Sadri, A. Mohammadi, H. Hosseini, Nanomed. Res. J 1, 112–121 (2016)
A.M. Moydeen, M.S. Ali-Padusha, E.F. Aboelfetoh, S.S. Al-Deyab, M.H. El-Newehy, Int. J. Biol. Macromol. 116, 1250–1259 (2018)
E. Aleebrahim-Dehkordy, M. Rafieian-kopaei, H. Amini-Khoei, S. Abbasi, J. Diet. Suppl. 16, 408–416 (2019)
I.J. Sagbo, A.J. Afolayan, G. Bradley, Asian Pac. J Trop. Biomed. 7, 817–825 (2017)
T.J. Silhavy, D. Kahne, S. Walker, Cold Spring Harb. Perspect. Biol. 2, a000414 (2010)
S. Ghuman, B. Ncube, J.F. Finnie, L.J. McGaw, E. Mfotie Njoya, R.M. Coopoosamy, J. Van Staden, South Afr. J. Bot. 126, 232–240 (2019)
J. Yin, H.H. Kim, I.H. Hwang, D.H. Kim, M.W. Lee, Molecules 24, 3094 (2019)
S. Ranganathan, K. Balagangadharan, N. Selvamurugan, Int. J. Biol. Macromol. 133, 354–364 (2019)
X. Wang, B. Ding, B. Li, Mater. Today 16, 229–241 (2013)
F. Xu, B. Weng, R. Gilkerson, L.A. Materon, K. Lozano, Carbohydr. Polym. 115, 16–24 (2015)
Acknowledgements
The authors appreciate the National Cell Bank of Iran, Pasteur Institute of Iran, for cytocompatibility tests as well as the Microbiology and Biotechnology Lab, University of Tehran, Iran, for the antibacterial test.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hadipour-Goudarzi, E., hemmatinejad, N. & Shokrgozar, M.A. Fabrication of Acorn-Loaded Chitosan/Gelatin Nanofibrous Web to Increase Antibacterial Activity for Wound-Healing Applications. Fibers Polym 24, 893–905 (2023). https://doi.org/10.1007/s12221-023-00043-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12221-023-00043-9