Abstract
Cystic echinococcosis (CE) is a zoonotic parasitic disease caused by the larval stage (metacestode) of the tapeworm Echinococcus granulosus sensu lato (s.l.). It is one of the most widespread zoonoses of veterinary and public health importance and constitutes a sanitary, economic, and socio-cultural concern globally. It is included in the WHO (World Health Organization) list of the most frequent Neglected Zoonotic Diseases (NZDs) and is a major problem in rural areas where hygienic measures are poor. Prevalence of the disease in humans is often underestimated given the challenges in carrying out studies in resource-poor communities in remote and isolated geographic areas. A prevalence and genotyping study was conducted in the Limpopo National Park (LNP) area, Gaza province, to evaluate effects of this parasitic disease on livestock production, wildlife health, and possible public health risks in this human–wildlife interface conservation area. A total of 204 cattle were inspected in the Massingir slaughterhouse which is the focal point for all animals reared in the LNP and its buffer zone. Inspections detected 25 animals with cystic-like lesions in various organs, of which 22 were microscopically confirmed as E. granulosus s.l., representing a prevalence of 10.8%. Subsequent molecular analysis of the PCR samples and sequencing of the cox1 and nad1 genes confirmed that the samples belonged to strain G5, now reclassified as E. ortleppi, one of the known zoonotic Echinococcus species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cystic echinococcosis (CE) is a parasitic disease known since ancient times, documented by Hippocrates and Aristotle as "a cyst full of water" in human livers and lungs (Sotiraki et al. 2003).
It is present in the WHO (World Health Organization) list of Neglected Zoonotic Diseases (NZDs) (Webster et al. 2015). CE is underreported and current data indicate that is re-emerging as a global public health problem (WHO 2013). As a neglected disease, it represents a major public health problem in rural areas closely linked to the presence of pastoralism and where disease control measures as well as treatment of humans and dogs are nonexistent (Eckert et al.2000; Hotez et al. 2007).
CE is caused by the larval stage of Echinococcus granulosus sensu lato (s.l.) (Cestoda, Taeniidae; Batsch 1786). The biological cycle is indirect and requires two mammal hosts to complete, of which the definitive hosts are domestic or wild canids (Poglayen et al. 2017a). Infected definitive hosts are generally asymptomatic, where the small adult parasite (range from 5 to 7 mm in length depending on the species) is localized in the small intestine. Unlike other taeniids species, it produces no more than six segments which will be divided into a specialized anterior segment called scolex, a body called strobilia and several segments of reproductive units called proglottids in which the eggs are present (Eckert et al. 2001).
Inside the eggs are present the infectious forms of E. granulosus s.l. named oncospheres and are endowed with a high environmental resistance provided by the keratin membrane that surrounds them. Studies carried out in extreme conditions of arid climate have shown that Echinococcus eggs can survive up to 41 months (Thevenet et al. 2005).
The host range of larval stages is quite diverse and includes numerous species of domestic and wild mammals (Maillard et al. 2007). Eggs are ingested from pastures or contaminated water and the enzymatic action of the stomach and the first intestinal tract triggers release of oncospheres. These pass through the intestinal barrier with the help of hooks that help it adhere to the intestinal mucosa and reach the bloodstream and sometimes even the lymphatic vessels and are passively transported toward different organs (Eckert et al. 2001). Statistically, the most affected organs are the liver and lungs, respectively; this is determined by the path followed by the blood vessels that starting from the intestines pass through the portal system; and it may happen that the oncosphere can also pass through the lungs and reach other districts of the body. Here, a slow-growing cystic structure develops with temporal variations determined according to the species of Echinococcus and texture of the occupied organ (McManus and Thompson 2003; Poglayen et al. 2017b).
The cyst is formed by an internal layer (germinal layer) and an external laminated acellular layer. The cyst is filled with transparent liquid in which protoscoleces are suspended in different states of maturation that are generated by the inner membrane (Eckert and Deplazes 2004).
Humans are aberrant intermediate host, and generally are susceptible to infection due to poor sanitation, contaminated food, or water (Trachsel et al. 2007).
The variability from a genetic, host range, and morphological point of view has created controversy regarding the taxonomic classification of Echinococcus species (Busi et al. 2007). The use of molecular-based genotyping on the basis of mitochondrial DNA (mtDNA) has led to delineation of 5 species of to Echinococcus granulosus s.l. (Bowles et al. 1992; Laurimäe et al. 2018a): E. granulosus sensu stricto (s.s.) (G1 and G3) (Kinkar et al. 2017; Busi et al. 2007), E. equinus Williams and Sweatman (G4) (Thompson and McManus 2002; Lymbery et al. 2015), E. ortleppi Lopez-Neyra and Soler Planas, 1943 (G5) (Nakao et al. 2006; Bonelli et al. 2020), E. canadensis Webster and Cameron, 1961 (G6–7, G8 and G10) (Nakao et al. 2013; Laurimäe et al. 2018b; Lavikainen et al. 2003), and E. felidis Ortlepp (1937) also defined as ‘lion strain’ (Hüttner et al. 2008).
The information available in the African continent on the prevalence and genotyping of E. granulosus s.l. is limited in humans, wildlife, and livestock. However, there have been reports of infections in animals and humans in southern Africa due to Echinococcus species, such as E. granulosus s.s., E. canadensis, E. ortleppi, E. equinus, and E. felidis (Mogoye et al. 2013; Halajian et al. 2017). In a study conducted in selected districts of Maputo, Gaza, and Inhambane in southern Mozambique on 817 cows and 68 pigs, it was found that the average prevalence was, respectively, 3.9% in cattle and 2.9% in pigs. Furthermore, E. ortleppi was isolated from these animals (Miambo et al. 2022).
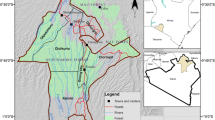
The Limpopo National Park (LNP) area is characterized by the presence of resource-poor rural communities where domesticated animals are raised, including cows, goats, pigs, and dogs. Coexistence of wildlife and human communities in the conservation area facilitates the spread of diseases at the wildlife/livestock/human interface. The purpose of this study was to provide data, through determining data on prevalence and genotypes of Echinococcus spp. in livestock in 21 rural communities (Fig. 1) in the Massingir District of Gaza Province located in LNP and the buffer zone. This research constitutes an appropriate One Health approach, because provides critical data of NZDs in a rural setting that spans a conservation area of high environmental importance.
2 Materials and methods
2.1 Study area
The LNP is part of the Great Limpopo Transfrontier Conservation Area (GLTCA), formally established in November 2000 as an agreement between the governments of South Africa, Mozambique, and Zimbabwe with the name of Gaza–Kruger–Gonarezhou transfrontier conservation area (GKG) (Wolmer 2003). It is bordered by the Limpopo River to the north and east, the Olifans River to the south and with South Africa to the west. It is part of the climatic areas defined as semi-arid, characterized by high temperatures and dry climate (FAO-SAFR 2004). The area covered by the park is about 1,213,315 ha in which there are at least 44 rural communities. In the internal area of the park and the buffer zone, the park hosts a variety of farm animals, with at least 9000 cattle (Massé 2016).
The LNP has an average temperature of 18 °C and average precipitation of around 500 mm of rain per year (Stalmans et al. 2004). All these environmental factors, combined with limited drinking water resources, mean that wild and domestic animals often interact with each other especially at natural water in occupied areas of rural communities. One of the main land-related problems is the home slaughter of livestock, which is mainly practiced in the most remote villages. Our focal point was established in the Massingir village where there is a small slaughterhouse managed by the District Service of Economic Activity (SDAE). All the animals destined for public sale to the market are conveyed to this structure, but they constitute a minority compared to the large quantity of livestock present in the area. The advantage of this focal point is that it was possible to gather a large amount of information and to carry out an adequate post-mortem inspection.
2.2 Ante-mortem inspection
Prior to slaughter, all animals were assessed during ante-mortem inspection according to Mozambican regulations to ensure that they were fit for human consumption and had no public health issues. This is an examination aimed at identifying sick or abnormal animals before they are slaughtered, and all data relating to the individual animal are noted at this stage (Lahti and Soini 2014).
During this phase, an assessment of the body condition score (BCS) of each individual animal is carried out, to evaluate the general status of the animal. Animals were assigned a BCS value based on a five-point scale delineated by fat coverage in the lumbar, thoracic, and pelvic regions. Emaciated animals have a score of 1; lean animals, 2; medium animals, 3; fat animals, 4; and obese animals, 5 (Ferguson et al. 1994).
2.3 Post-mortem inspection
From October 2018 to March 2021, a total of 204 cattle slaughtered at Massingir abattoir were examined for the presence of hydatid cysts in lungs, liver, heart, spleen, and kidneys. Visual inspections and palpations were performed first followed by multiple incisions of the organs to detect hydatid cysts (Eckert et al. 2001). Once cysts were identified, they were carefully removed and put in 15- or 50-ml plastic tubes taking care to divide the aliquots of cystic tissue and hydatid fluid. The latter was sedimented, and then, the excess liquid was removed with the aid of a pipette, leaving only the sediment inside the test tube. The sediment with the protoscoleces was stored in 70% ethanol until processing.
2.4 Laboratory analysis of cysts
The biological material of cystic origin was subjected to microscopic examination to confirm E. granulosus infection and determine the fertility status of the cysts. Fertility was confirmed by the microscopic detection of protoscoleces (larvae) (Fig. 2), where the larvae were vital and in different stages of maturation, clearly visible, with regular margins and showed the presence of flame cells. Based on this description, cysts were classified as fertile, sterile (acephalocysts) in which liquid is present, but protoscolic cannot be visualized or degenerated in which only a hint of membrane and the presence of caseous or calcified material inside can be evidenced (Varcasia et al. 2007).
2.5 Molecular analysis
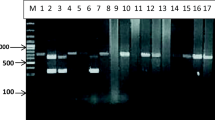
DNA was extracted from a small piece of cyst membrane together with protoscoleces using a commercial kit (QIAamp DNA Mini Kit®) following the manufacturer’s instructions with a modification of the initial incubation time to 5 h. The subunit 1 of the cytochrome c oxidase (cox1) and the subunit 1 of NADH dehydrogenase (nad1) genes were amplified using the primers JB3:5′-TTTTTTGGGCATCCTGAGGTTTAT-3′, JB4:5′ TAAAGAAAGAACATAATGAAAATG-3′′for cox1 and JB11: 5′-AGATTCGTAAGGGGCCTAATA-3′ and JB12: 5′-ACCACTAACTAATTCACTTTC-3′ for nad1 (Bowles et al. 1992; Bowles and McManus 1993), following cycling conditions described by Gasser et al. (1999) with an adjustment of the initial elongation time: initial denaturation of 95 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, elongation at 72 °C for 1 min, and finally an elongation at 72 °C for 5 min. The amplification was performed in 25 μl reaction volume composed by 1 µl of forward and reverse primers (10 µM), 6.5 µl of dH2O, 12.5 µl of Taq master mix (Thermo Fisher Scientific®, USA), and 4 µl of DNA. Visualization of PCR products was made in agarose gel 1.5% stained with Safe green.
Amplicons of samples for cox1 and nad1 were sent to Inqaba Biotechnical Industries Ltd. in Pretoria, South Africa, for sequencing in two directions. The obtained sequences were assembled, manually edited, and aligned with homologous sequences from the GenBank database with Clustal W (Thompson et al. 1997) using the BioEdit program (Hall 1999).
2.6 Statistical analysis
The collected and recorded data were entered into a MS Excel spreadsheet, merged with the microscopical results, and then imported into Stata Statistical Software (Release 15, College Station, TX: StataCorp LLC) for statistical analyses. Animals were considered as positive when harboring cysts referable to Echinococcus granulosus s.l. in liver, lungs or both. BCS, age by year, sex, and locality (inside or outside LNP’s boundaries) were the independent variables obtained at the slaughterhouse. Mean and standard deviation (SD) were calculated for continuous variables. Pearson’s χ2 test was used to compare the proportion of positive cattle and categorical independent variables. Odds ratios (ORs) and their 95% confidence intervals (CIs) were reported. Results were considered significant when P ≤ 0.05.
3 Results
3.1 Demographic data and prevalence of cysts in study animals
A total of 204 animals were included in the study with an average of 4.1 (SD ± 2.5) ranging from 1 to 14 years, and 145 (71.1%) animals were males and 59 (28.9%) were females. On average, male animals were slaughtered younger (mean 3.1 years old ± 1.7) with respect to females (mean 6.5 ± 2.5). One hundred and thirty-seven cattle (67.2%) were from localities outside the park’s boundaries and 67 (32.8%) within the park. The BCS revealed an overall mean of 2.69 (SD ± 0.66), with more than half of the animals having a BCS reported as a value of 3. Overall, 10.8% (22/204) of the slaughtered cattle resulted positive for cysts referred to as E. granulosus s.l. Cysts were recovered in three cattle from liver and in the rest 19 from lung. A total of 25 cysts were collected (Figs. 3 and 4) from the 22 positive animals. Microscopic analysis showed that 22 of the specimens were identified as viable cysts of E. granulosus s.l.. Two of those were purulent-type degenerated (acephalocysts) and one calcified.
As regard of sex, the proportion of females detected positive resulted significantly larger (22%) compared to males (6.2%) (P = 0.001) showing 4.27 more chances to be detected with cysts compared to male (95% CI 1.71–10.64). Cattle grazed within the LNP had 4.25 higher chance to be diagnosed positive compared to those out from the park (95% CI 1.69–10.75), and the proportion of those diagnosed positive were 20.9% and 5.8% within and outside the boundaries (P = 0.001). As for the age, cattle had around 1.22 more chance to be detected positive for each year increase (95% CI 1.04–1.42). Table 1 summarizes quantitative analysis.
3.2 Molecular results
Based on the cox1 gene, BLAST analysis of sequences of six isolates, all aligned E. ortleppi isolates from the GenBank with homology 100% with published E. ortleppi sequences from Italy (FJ744757), as shown of Fig. 5. Homology of 100% with published E. ortleppi sequences from Italy (FJ744757) was verified. The clade B containing our isolates formed a monophyletic group with isolates from other locations. This clade formed a sister group with E. ortleppi isolate from Mozambique (MZ220594.1) and showed the relationship between E. ortleppi from Poland (MZ322608.1), and Tanzania (MN540101.1) and E. granulosus from India (MH428013.1) and Portugal (HF947565).
BLAST analysis of NAD gene for thirteen (13) sequences also identified all cyst isolates as E. ortleppi as shown in Fig. 6. In the Clade B, there was a homology of 100% of our samples with published E. granulosus sequence from Iran (HM749616) forming a monophyletic group. A paraphyletic group was formed between our samples with isolate from Mozambique (MZ254630) which formed a sister group with E. ortleppi isolates from Sudan (JN637177), Egypt (MK492617), and Poland (MH492788) with homology of 70%.
4 Discussion
To the best of our knowledge, this represents the first description of prevalence and genotyping of Echinococcus ortleppi in the LNP and provides preliminary data on this parasitic disease in fragile conservation areas where rural communities live in close contact with wildlife populations. In fact, the choice of Massingir district was weighted according to its geographical location, as there are rural communities devoted to pastoralism and many problems related to human–wildlife conflict are experienced in various forms. Livestock losses due to predator attacks or food competitions with large herbivores are just some of the more obvious consequences of this problem (Dunham et al. 2010). In this context, the study of zoonoses, and especially neglected ones whose impact on public health or livestock production is not well documented, is crucial.
In a study conducted by Miambo et al. (2022) in neighboring districts of the same of Gaza Province and also in other provinces of Mozambique, it is noted that the average prevalence of CE never exceeded 4.2% of the total number of animals inspected at the slaughterhouse. This contrasts with our study where the prevalence was 10.6%.
The prevalence found in the District of Massingir compared to the others registered in the country still represents a high value, but the interesting data are represented by the greater possibility, about four times higher, of an animal living inside the LNP to be parasitized by Echinoccoccus ortleppi.
This can be attributed to the presence of definitive hosts within the conservation area, which, in addition to having a large number of dogs from rural communities, sees the presence of wild canids and felines, such as lions (Panthera leo), hyenas (Crocuta crocuta), jackals (Lupulella mesomelas), leopards (Pathera pardus), cheetahs (Acinonyx jubatus), and African wilddogs (Lycaon pictus) (Everatt et al. 2014; Andresen et al. 2012; Peace Parks Foundation 2015). Certainly, the role of these definitive hosts in the eco-epidemiology of E. ortleppi is not entirely clear. It has been shown that in an area with small numbers of wild prey available, carnivores can concentrate their attacks on livestock prey, which contributes to a dual biological cycle of the parasite, i.e., domestic and sylvatic (Hüttner and Romig 2009).
The presence of E. equinus and E. felidis in the GLTC area has been demonstrated by several wildlife studies, particularly in the Kruger National Park and adjacent areas (Zaffarano et al 2021; Halajian et al. 2017). Therefore, this study provides additional knowledge that can assist policy makers in making decisions about livestock management in protected areas and in applying surveillance protocols for diseases that impact the public health of communities in the transboundary conservation area.
In animals that tested positive, a higher prevalence of Echinococcus cysts was found in the lungs than in the liver, while no cysts were found in other organs. This result may be related to the fact that lung tissue has a more spongelike and less rigid consistency, characteristic that are is conducive to cyst growth. But also possibly due to the fact that in these ruminants, oncospheres are more easily transported from the thoracic lymphatic duct to the lung level (Negash et al. 2013). This type of distribution of cysts in organs seems to be consistent with reports from other studies done in domestic livestock elsewhere (Himonas et al. 1987; Njoroge et al. 2002).
There is a 22% (OR 1.22) increase in being infected each year of age. This value can be associated with the fact that the increase in cyst size can be 1–5 cm per year, as anticipated in the introduction; in fact, this favors the possibility that cysts will be found on visual and tactile post-mortem inspection, because they are more visible. Older age is also often associated with E. granulosus exposure (Poglayen et al. 2017b), probably due to the likelihood of exposure increases with age. Indeed, these results report a significant higher proportion of female cattle being positive to CE compared to male due to the fact that on average female is slaughtered at an older age with respect to males.
Data on BCS of animals in the geographical reference area are not directly correlated with the presence of pathological or infectious conditions that alter body condition but are instead strongly influenced by various environmental and herd management factors. In particular, the climate of the area, which is characterized by even prolonged periods of drought, and the mode of pastoralism, which is practiced extensively with high energy expenditure caused by the great distances the animals travel daily in search of pasture and water, coupled with poor livestock support, mean that the animals have very different body conditions at different times of the year. In general, therefore, we can say that no direct correlation was found between the presence of E. granulosus and altered body condition. We can say that animals in good body condition or those with debilitating diseases in old age are sold for slaughter. The latter characteristic is still very much influenced by cultural aspects that make the animal as a prestige value for the family, so generally animals in average condition and optimal production are not slaughtered in large numbers (Direito 2021).
The genotype G5 isolated from cattle of LNP area seems to be dominant in Mozambique and neighboring countries in both wild, cattle and humans (Obwaller et al. 2004; Grenouillet et al. 2014; Aschenborn et al. 2022). However, cattle were reported to be the preferred animal host for E. ortleppi (Nakao et al. 2013). For both NAD and COX genes, isolates from cattle of previous study conducted in Mozambique by Miambo et al. (2022) formed a different clade with isolates from our study. This may be due to possible differences in the definitive hosts involved, since cattle from the reported study were from livestock raised together with domestic dogs, and for our study, there may be wild canids involved in the transmission cycles and also due to geographic differences in location. NAD gene homology of 100% between E. ortleppi and E. granulosus sequence from Iran (HM749616) was verified forming monophyletic group. This is not surprising, since E. ortleppi and E. granulosus s.s are both haplotypes of Echinococcus granulosus s.l. (Deplazes et al. 2017).
5 Conclusion
The development of increasingly advanced diagnostic techniques, such as polymerase chain reaction (PCR), has made it possible to identify new genotypes of Echinococcus and to better understand the ecology of this parasite. Neglected characteristics of this parasitic zoonosis are reduced when a multisectoral research effort involving aspects of public health, animal health, and habitat can be implemented. This concept is the basis of the One Health approach. Therefore, the development of further research activities to verify the presence of E. granulosus s.l. in wild species, other domestic animal species, and especially the search for data on human populations living near conservation areas is critical to help understand the epidemiological dynamics of this parasitic disease.
Data availability
The datasets generated and/or analysed during the current study are avaliable from the corresponding author on reasonable request.
References
Andresen L, Everatt KT, Somers MJ, Purchase GK (2012) Evidence for a resident population of cheetah in the Parque Nacional do Limpopo, Mozambique. S Afr J Wildl 42(2):144–146
Aschenborn J, Schneider C, Addy F, Aschenborn O, Kern P, Romig T, Deplazes P, Wassermann M (2022) Cystic echinococcosis of ruminant livestock in Namibia. Vet Parasitol Reg Stud Rep 31:100727. https://doi.org/10.1016/j.vprsr.2022.100727
Bonelli P, Dei Giudici S, Peruzzu A, Piseddu T, Santucciu C, Masu G, Masala G (2020) Genetic diversity of Echinococcus granulosus sensu stricto in Sardinia (Italy). Parasitol Int 77:102120. https://doi.org/10.1016/j.parint.2020.102120
Bowles J, McManus DP (1993) NADH dehydrogenase 1 gene sequences compared for species and strains of the genus Echinococcus. Int J Parasitol 23(7):969–972. https://doi.org/10.1016/0020-7519(93)90065-7
Bowles J, Blair D, McManus DP (1992) Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol Biochem Parasitol 54(2):165–173. https://doi.org/10.1016/0166-6851(92)90109-W
Busi M, Šnábel V, Varcasia A, Garippa G, Perrone V, De Liberato C, D’Amelio S (2007) Genetic variation within and between G1 and G3 genotypes of Echinococcus granulosus in Italy revealed by multilocus DNA sequencing. Vet Parasitol 150(1–2):75–83. https://doi.org/10.1016/j.vetpar.2007.09.003
Deplazes P, Rinaldi L, Rojas CA, Torgerson PR, Harandi MF, Romig T, Antolova D, Schurer JM, Lahmar S, Cringoli G, Magambo J, Thompson RCA, Jenkins EJ (2017) Global distribution of alveolar and cystic echinococcosis. Adv Parasitol 95:315–493. https://doi.org/10.1016/bs.apar.2016.11.001
Direito B (2021) Livestock and veterinary health in southern Mozambique in the beginning of the twentieth century: the case of the fight against East Coast fever. História, Ciências, Saúde-Manguinhos 28:939–959. https://doi.org/10.1590/S0104-59702021000400003
Dunham KM, Ghiurghi A, Cumbi R, Urbano F (2010) Human–wildlife conflict in Mozambique: a national perspective, with emphasis on wildlife attacks on humans. Oryx 44(2):185–193. https://doi.org/10.1017/S003060530999086X
Eckert J, Deplazes P (2004) Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev 17(1):107–135
Eckert J, Conraths FJ, Tackmann K (2000) Echinococcosis: an emerging or re-emerging zoonosis? Int J Parasitol 30(12–13):1283–1294. https://doi.org/10.1016/S0020-7519(00)00130-2
Eckert J, Gemmel MA, Meslin FX, Pawlowski ZS (2001) WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health, Paris. https://apps.who.int/iris/bitstream/handle/10665/42427/929044522X.pdf
Everatt KT, Andresen L, Somers MJ (2014) Trophic scaling and occupancy analysis reveals a lion population limited by top-down anthropogenic pressure in the Limpopo National Park, Mozambique. PLoS One 9(6):e99389. https://doi.org/10.1371/journal.pone.0099389
FAO-SAFR (2004) Drought impact mitigation and prevention in the Limpopo River basin: a situation analysis. Sub-Regional Office for Southern Africa/Harare, Rome. https://www.fao.org/3/y5744e/y5744e04.htm
Ferguson JD, Galligan DT, Thomsen N (1994) Principal descriptors of body condition score in Holstein cows. J Dairy Sci 77(9):2695–2703. https://doi.org/10.3168/jds.S0022-0302(94)77212-X
Gasser RB, Zhu X, McManus DP (1999) NADH dehydrogenase subunit 1 and cytochrome c oxidase subunit I sequences compared for members of the genus Taenia (Cestoda). Int J Parasitol 29(12):1965–1970. https://doi.org/10.1016/S0020-7519(99)00153-8
Grenouillet F, Umhang G, Arbez-Gindre F, Mantion G, Delabrousse E, Millon L, Boué F (2014) Echinococcus ortleppi infections in humans and cattle, France. Emerg Infect Dis 20(12):2100. https://doi.org/10.3201/eid2012.140641
Halajian A, Luus-Powell WJ, Roux F, Nakao M, Sasaki M, Lavikainen A (2017) Echinococcus felidis in hippopotamus, South Africa. Vet Parasitol 243:24–28. https://doi.org/10.1016/j.vetpar.2017.06.001
Hall T (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In: Nucleic Acids Symp. Ser., vol 41, p 95–98
Himonas C, Frydas S, Antoniadol-Sotiriadou K (1987) The fertility of hydatid cysts in food animals in Greece. Helminth zoonoses. Springer, Dordrecht, pp 12–21. https://doi.org/10.1007/978-94-009-3341-5_2
Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, Savioli L (2007) Control of neglected tropical diseases. N Engl J Med 357(10):1018–1027. https://doi.org/10.1056/NEJMra064142
Hüttner M, Romig T (2009) Echinococcus species in African wildlife. Parasitology 136(10):1089–1095. https://doi.org/10.1017/S0031182009990461
Hüttner M, Nakao M, Wassermann T, Siefert L, Boomker JD, Dinkel A, Sako Y, Mackenstedt U, Romig T, Ito A (2008) Genetic characterization and phylogenetic position of Echinococcus felidis (Cestoda: Taeniidae) from the African lion. Int J Parasitol 38(7):861–868. https://doi.org/10.1016/j.ijpara.2007.10.013
Kinkar L, Laurimäe T, Sharbatkhori M, Mirhendi H, Kia EB, Ponce-Gordo F et al (2017) New mitogenome and nuclear evidence on the phylogeny and taxonomy of the highly zoonotic tapeworm Echinococcus granulosus sensu stricto. Infect Genet Evol 52:52–58. https://doi.org/10.1016/j.meegid.2017.04.023
Lahti P, Soini J (2014) Ante‐mortem inspection. In: Meat inspection and control in the slaughterhouse, p 19–28. https://doi.org/10.1002/9781118525821.ch3
Laurimäe T, Kinkar L, Romig T, Omer RA, Casulli A, Umhang G, Gasser RB et al (2018a) The benefits of analysing complete mitochondrial genomes: deep insights into the phylogeny and population structure of Echinococcus granulosus sensu lato genotypes G6 and G7. Infect Genet Evol 64:85–94. https://doi.org/10.1016/j.meegid.2018.06.016
Laurimäe T, Kinkar L, Moks E, Romig T, Omer RA, Casulli A, Umhang G, Bagrade G et al (2018b) Molecular phylogeny based on six nuclear genes suggests that Echinococcus granulosus sensu lato genotypes G6/G7 and G8/G10 can be regarded as two distinct species. Parasitology 145(14):1929–1937. https://doi.org/10.1017/S0031182018000719
Lavikainen A, Lehtinen MJ, Meri T, Hirvelä-Koski V, Meri S (2003) Molecular genetic characterization of the Fennoscandian cervid strain, a new genotypic group (G10) of Echinococcus granulosus. Parasitology 127(3):207–215. https://doi.org/10.1017/S0031182003003780
Lymbery AJ, Jenkins EJ, Schurer JM, Thompson RA (2015) Echinococcus canadensis, E. borealis, and E. intermedius. What’s in a name? Trends Parasitol 31(1):23–29. https://doi.org/10.1016/j.pt.2014.11.003
Maillard S, Benchikh-Elfegoun MC, Knapp J, Bart JM, Koskei P, Gottstein B, Piarroux R (2007) Taxonomic position and geographical distribution of the common sheep G1 and camel G6 strains of Echinococcus granulosus in three African countries. Parasitol Res 100(3):495–503. https://doi.org/10.1007/s00436-006-0286-9
Massé F (2016) The political ecology of human-wildlife conflict: producing wilderness, insecurity, and displacement in the Limpopo National Park. Conserv Soc 14(2):100–111. https://doi.org/10.4103/0972-4923.186331
McManus DP, Thompson RCA (2003) Molecular epidemiology of cystic echinococcosis. Parasitology 127(S1):S37–S51. https://doi.org/10.1017/S0031182003003524
Miambo RD, Afonso SMS, Noormahomed EV, Malatji MP, Mukaratirwa S (2022) Prevalence and molecular characterization of cystic hydatidosis in livestock slaughtered in southern Mozambique. J Parasit Dis 46(1):186–195. https://doi.org/10.1007/s12639-021-01434-6
Mogoye BK, Menezes CN, Wong ML, Stacey S, von Delft D, Wahlers KJ et al (2013) First insights into species and genotypes of Echinococcus in South Africa. Vet Parasitol 196(3–4):427–432. https://doi.org/10.1016/j.vetpar.2013.03.033
Nakao M, McManus DP, Schantz PM, Craig PS, Ito A (2006) A molecular phylogeny of the genus Echinococcus inferred from complete mitochondrial genomes. Parasitology 134(5):713–722. https://doi.org/10.1017/S0031182006001934
Nakao M, Lavikainen A, Yanagida T, Ito A (2013) Phylogenetic systematics of the genus Echinococcus (Cestoda: Taeniidae). Int J Parasitol 43(12–13):1017–1029. https://doi.org/10.1016/j.ijpara.2013.06.002
Negash K, Beyene D, Kumsa B (2013) Cystic echinococcosis in cattle slaughtered at Shashemanne Municipal Abattoir, south central Oromia, Ethiopia: prevalence, cyst distribution and fertility. Trans R Soc Trop Med Hyg 107(4):229–234. https://doi.org/10.1093/trstmh/trt003
Njoroge EM, Mbithi PMF, Gathuma JM, Wachira TM, Gathura PB, Magambo JK, Zeyhle E (2002) A study of cystic echinococcosis in slaughter animals in three selected areas of northern Turkana, Kenya. Vet Parasitol 104(1):85–91. https://doi.org/10.1016/S0304-4017(01)00614-8
Obwaller A, Schneider R, Walochnik J, Gollackner B, Deutz A, Janitschke K, Aspöck H, Auer H (2004) Echinococcus granulosus strain differentiation based on sequence heterogeneity in mitochondrial genes of cytochrome c oxidase-1 and NADH dehydrogenase-1. Parasitology 128(5):569–575. https://doi.org/10.1017/S0031182004004871
Peace Parks Foundation (2015) Wildlife diversity in Limpopo National Park. https://www.peaceparks.org/wildlife-diversity-in-limpopo-national-park/
Poglayen G, Gori F, Morandi B, Galuppi R, Fabbri E, Caniglia R, Milanesi P, Galaverni M, Randi E, Marchesi B, Deplazes P (2017a) Italian wolves (Canis lupus italicus Altobello, 1921) and molecular detection of taeniids in the Foreste Casentinesi National Park, Northern Italian Apennines. Int J Parasitol Parasit Wildl 6(1):1–7. https://doi.org/10.1016/j.ijppaw.2017.01.001
Poglayen G, Varcasia A, Pipia AP, Tamponi C, Parigi M, Marchesi B, Morandi B, Benfenati V, Scala A (2017b) Retrospective study on cystic echinococcosis in cattle of Italy. J Infect Dev Ctries 11(09):719–726. https://doi.org/10.3855/jidc.9433
Sotiraki S, Himonas C, Korkoliakou P (2003) Hydatidosis–echinococcosis in Greece. Acta Trop 85(2):197–201. https://doi.org/10.1016/S0001-706X(02)00273-5
Stalmans M, Gertenbach WPD, Carvalho-Serfontein F (2004) Plant communities and landscapes of the Parque Nacional do Limpopo, Moçambique. Koedoe 47(2):61–81. https://doi.org/10.4102/koedoe.v47i2.83
Thevenet PS, Jensen O, Drut R, Cerrone GE, Grenóvero MS, Alvarez HM, Targovnik HM, Basualdo JA (2005) Viability and infectiousness of eggs of Echinococcus granulosus aged under natural conditions of inferior arid climate. Vet Parasitol 133(1):71–77. https://doi.org/10.1016/j.vetpar.2005.05.048
Thompson RA, McManus DP (2002) Towards a taxonomic revision of the genus Echinococcus. Trends Parasitol 18(10):452–457. https://doi.org/10.1016/S1471-4922(02)02358-9
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25(24):4876–4882. https://doi.org/10.1093/nar/25.24.4876
Trachsel D, Deplazes P, Mathis A (2007) Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology 134(6):911–920. https://doi.org/10.1017/S0031182007002235
Varcasia A, Canu S, Kogkos A, Pipia AP, Scala A, Garippa G, Seimenis A (2007) Molecular characterization of Echinococcus granulosus in sheep and goats of Peloponnesus, Greece. Parasitol Res 101(4):1135–1139. https://doi.org/10.1007/s00436-007-0568-x
Webster JP, Gower CM, Knowles SC, Molyneux DH, Fenton A (2015) One health—an ecological and evolutionary framework for tackling Neglected Zoonotic Diseases. Evol Appl 9(2):313–333. https://doi.org/10.1111/eva.12341
WHO (2013) World Health Assembly resolution WHA66.12: neglected tropical diseases: prevention, control, elimination and eradication. World Health Organization, Geneva. https://apps.who.int/gb/ebwha/pdf_files/WHA66-REC1/A66_REC1-en.pdf
Wolmer W (2003) Transboundary conservation: the politics of ecological integrity in the Great Limpopo Transfrontier Park. J S Afr Stud 29(1):261–278. https://doi.org/10.1080/0305707032000060449
Zaffarano GP, de Klerk-Lorist LM, Junker K, Mitchell E, Bhoora RV, Poglayen G, Govender D (2021) First report of cystic echinococcosis in rhinos: a fertile infection of Echinococcus equinus in a Southern white rhinoceros (Ceratotherium simum simum) of Kruger National Park, South Africa. Int J Parasit Parasit Wildl 14:260–266. https://doi.org/10.1016/j.ijppaw.2021.02.007
Acknowledgements
The present study is part of the research activity of the SECOSUD II Project “Conservation and equitable use of biodiversity in Southern African Development Community” and is founded by the Italian Agency for Development Cooperation in Southern African Development Community (SADC). The contents of this publication are the exclusive responsibility of the authors and do not necessarily represent the point of view of the Agency. The authors would like to thank the Center of Biotechnology and the Faculty of Medicine of the Eduardo Mondlane University for their support in the implementation of the molecular biology protocol.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to a Topical Collection originated from a long scientific collaboration between Mozambican and Italian universities promoted by the Italian Agency for Development Cooperation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zaffarano, G.P., Miambo, R.D., Ussivane, É.E. et al. Cystic echinococcosis in cattle (Bos taurus) from rural communities of Limpopo National Park, Gaza province, Mozambique: a One Health perspective. Rend. Fis. Acc. Lincei 34, 59–68 (2023). https://doi.org/10.1007/s12210-022-01119-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-022-01119-z