Abstract
We explored whether leaf detritus of an exotic species, Eucalyptus camaldulensis, affects the structural and functional processes of macrozoobenthic assemblages in four increased salt stress conditions of a transitional aquatic ecosystem (central Italy). We compared the dynamics of the weight loss of leaves of E. camaldulensis by leaching and feeding activities on palatable fraction, measuring the relative breakdown rates, with the weight loss of native Phragmites australis detritus. Highest values of abundance (481 and 245 individuals) were observed on native and exotic resources in 52.64 ± 6.01 psu due to Hydrobia complex acuta on the detritus leaves of Phragmites and Eucalyptus. While the two leaf detritus resources showed low breakdown rates by leaching, the donor-controlled community responded in terms of palatable fraction consumption better on native resources than on exotic plant detritus. Comparing the responses of macrozoobenthic assemblages to the different salt stress conditions and resources, we obtained two complex patterns, one denoting changes in structuring metrics (abundance and biomass) and another denoting a change in donor functionality (reduction of palatable fraction, increase of recalcitrant/leaching fractions). The macrozoobenthic assemblages responded simplifying their structure in stressed conditions of high salinity. Our results indicate that, when estimating the impact of exotic plant detritus on structuring processes of macrozoobenthic assemblages, we should include the breakdown rates of their trophic leaf resources, as well as the relevance of the relative fraction types (leaching, recalcitrant and palatable) influencing those processes. Eucalyptus trees, considered a foreign element in the Mediterranean landscapes, can play a paradoxically role in the detritus food webs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Plant detritus differs from living matter because neither reproduces nor its own dynamic is directly affected by consumers (Moore et al. 2004). The relationship between detritus and consumers is formalized by the so-called donor-controlled models, stemming from the classical prey-predator models (Moore et al. 2004). Donor-control means that the transfer of matter and energy between species is entirely under the control of the donating species and not controlled by the recipient species (Pastor 2008).
The biodiversity of aquatic macroinvertebrate assemblages is assumed to be dependent on the autochthonous and allochthonous detritus inputs, which constitute important trophic components of donor-controlled food webs, as well as on fresh plant materials for the “green” food webs (Polis and Strong 1996; Jefferies 2000; Moore and de Ruiter 2012). Concerning the external organic inputs, a type of bottom-up pressure, it is known that most aquatic ecosystems are recipients of allochthonous materials that enhance in situ secondary productivity and affecting the life cycles of macroconsumers and their predators by consumption process of detritus substrates (Wallace et al. 1999; Kevrekidis 2004; Benke and Huryn 2006).
In temperate areas, allochthonous plant detritus from the native riparian vegetation should be considered as a seasonal trophic resource that influences the trophic structures of consumers (Polis et al. 2004; Bärlocher 2007). Generally, this happens because its availability is strongly dependent on the leaf abscission process (Cho et al. 2008; Mathuriau et al. 2008). Consequently, taking into consideration the plant species of the riparian vegetation structures (Friberg and Winterbourn 1997; Parkyn and Winterbourn 1997), the analysis of the effects of the plant detritus inputs into the aquatic ecosystems could be reasonably related to the leaf abscission calendar of each plant species (Ostrofsky 1997; Cummins 2002). This seasonal periodicity suggests to assume it as a driving force of the donor-controlled relationship between plant detritus availability and structuring and functioning processes of aquatic detritus-based communities (Werner and Gilliam 1984; Kominosky et al. 2009).
The current debate on the impact of exotic species on native biodiversity is still open (Schlaepfer 2018; Pauchard et al. 2018), less is known about the effects of plant debris of exotic origin on the ecological processes of aquatic ecosystems (Moore and Ruiter 2012).
The presence of exotic plant species in the native riparian vegetation structures is an established fact (Stohlgren et al. 1998, 1999) and the presence of non-native plant species seems to produce not only deep changes in community structures, but also in ecosystem functioning (Godoy et al. 2010; Furey et al. 2014; Bo et al. 2014; for examples in aquatic coastal ecosystems, see Reise et al. 2006). The effects of exotic plant detritus on the structure and functioning of donor-controlled food webs could provide suggestions on the possibility to increase the predictive power of the aquatic communities’ responses in disturbed conditions (Huxel and McCann 1998; Ponsard et al. 2000; Rosemond et al. 2001; Wollrab et al. 2012). In this regard, previous researchers have emphasized that the community of invertebrates had no difficulty in consuming exotic plant species and that the macroinvertebrate density and richness were higher on leaves of native than on those of exotic plant species (Alonso et al. 2010; Stiers et al. 2011).
In aquatic ecosystems, the macrozoobenthic assemblages appear to respond in a predictable way in terms of biomass and abundance to the quality and quantity of plant detritus input, as well as to stressors as, for example, the salinity fluctuations, reducing the biodiversity in terms of species richness (Telesh et al. 2013). The salinity fluctuation is an important and well-known abiotic factor, which can be another driving force in the biodiversity patterns particularly into the transitional aquatic ecosystems (Brock et al. 2005; Jørgensen et al. 2013; Cerfolli et al. 2013).
Although numerous investigations conducted on ecological community patterns show a significant reduction of biodiversity related to the salinity increase (Piscart et al. 2005; Amores et al. 2013), other studies highlight the response performances of invertebrate assemblages and changes in food webs in hyperhaline waters (i.e. physiological specialism increase; McEvoy and Goonan 2003; Angeletti et al. 2010; Bellisario et al. 2013).
Recent studies seem to emphasize that the increasing levels of salinity could affect the native vegetation growing along riverbanks, especially in transitional aquatic ecosystems, mediating competition between native and exotic plant species and thus changing the structure of plant communities (Tang et al. 2014; Canhoto and Graça 1996; Gonçalves and Canhoto 2009; Gonçalves et al. 2012). Contextually, the role that the exotic detritus plays as a food surrogate for macrozoobenthic assemblages in waters with different level of salinity should be explored. One hypothesis is that both the native and exotic plant detritus are used as trophic resources from macrozoobenthic assemblages under salt stress conditions (see also Wardle et al. 2004; Furey et al. 2014).
The aim of this paper is to show how an exotic plant detritus trophic resource affects the structural and functional responses of macrozoobenthic assemblages in several increasing salt stress conditions. Our endpoint variables were the taxonomic composition, abundance and biomass of macrozoobenthic assemblages. Here, the comparison between the breakdown dynamics of native and exotic detritus by macroinvertebrate assemblages has been adopted as a criterion to probe the relationship between structural and functional responses of donor-controlled macroinvertebrate communities in salt stress conditions. We also addressed the influence of the increase of salinity on the leaching as well as on the consumption by trophic macrozoobenthic assemblages, characterized by species belonging to different trophic groups.
2 Materials and methods
2.1 Study area
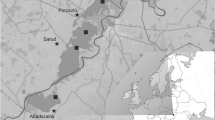
The study area was the coastal aquatic ecosystem of disused Tarquinia saltworks (central Italy, 42°12′ N, 11°43′ E), a patchy environment composed by a series of about 100 pools whose connection is ensured by a surrounding drainage system. The exchange of waters is provided by a single connection with the sea located north of the area (Fig. 1). Isolation and hydrological connectivity give rise to a wide salinity gradient spanning from hypohaline to hyperhaline waters (Bellisario et al. 2013).
2.2 Field and laboratory methods
Pools in the study area show a pattern in the variability of the salinity levels, pH and dissolved oxygen concentration. Four sampling sites (pools) were randomly selected, covering the maximal range of salinity variation from marine to hyperhaline waters (annual values, from 40 to 160 psu or ‰), excluding the freshwater conditions, to perform a macrozoobenthic consumption experiment of native and exotic detritus in different salt stress conditions (Table 1).
For the scope of this work, two trophic vegetable resources were offered as plant detritus to the macrozoobenthos of a transitional aquatic ecosystem. The first resource was the Common Reed, Phragmites australis (Cav.) Trin. ex Steud., a native plant species of Mediterranean aquatic vegetation communities; the second was the River Red Gam, Eucalyptus camaldulensis (Dehnh, 1832), an exotic plant species naturalized in Mediterranean eco-region (Celesti-Grapow et al. 2010).
Phragmites australis is an azonal species due to its wide range, that is, without belonging to a specific bioclimatic or altitude range (Lucchese 2017, 2018). In addition, P. australis is a species that tends to expand in areas affected by secondary succession processes and contributing with the input of allochthonous leaf debris to the maintenance of the macrozoobenthic communities of the area (Cerfolli et al. 2013). The presence of specimens of Eucalyptus camaldulensis in the area dates back to 1880 and is associated with their use in the recovery works of marshland, as natural water pumps and windbreaks to protect the salt-farm buildings (Abbruzzese 2014).
The choice of these two food resources was also determined by knowing the rates of breakdown by leaching of the respective detritus, a useful feature to analyze functional processes such as the trophic consumption and the colonization process by macroinvertebrates (Agoston-Szabò and Dinka 2008; Cerfolli et al. 2013).
Among the detritus resources, Phragmites australis was selected because it occurs in the riparian habitats of the area. An exotic plant species, Eucalyptus camaldulensis, was also selected because input records of leaf detritus were not observed in the water column of the pools (henceforth the two plant species will be mentioned by their genus).
To measure the short-term effects of the two plant detritus inputs on macrozoobenthic assemblage in terms of structuring and functioning of donor-controlled systems drove by salinity, we placed in each pool, 20 protected (mesh size: 1 mm2) and 20 unprotected (mesh size: 5 mm2) pre-weighted leaf packs for both detritus resources (total = 320 leaf packs). In protected packs the fungal decomposition can be an attractive food resource (e.g. Barlöcher and Kendrick 1973; Barlöcher and Graca 2002): however in this study we assumed that this phenomenon is negligible. Knowing the initial dry weight of each leaf pack (2.000 ± 0.004 g after storing at 60 °C for at least 72 h), we measured by an approximately monthly sampling (6 months, total 181 days), with r = 4 replicates:
- (i)
the remaining dry weight of leaf detritus in both protected and unprotected leaf packs, after again storing at 60 °C for at least 72 h (monthly means);
- (ii)
the number of colonizing taxa;
- (iii)
the number of individuals (abundance) for each taxon (monthly means);
- (iv)
the dry biomass of individuals for each taxon (monthly means), after storing at 60 °C for at least 72 h, and the determination of the ash free dry weight (AFDW) after ignition in a muffle furnace at 500 °C for 6 h.
AFDW was considered to provide a better comparison with other macroinvertebrates than dry weight (Fazi and Rossi 2000).
All benthic macroinvertebrates were identified to the appropriate taxonomic level (genus or species), then enumerated and recorded on electronic field data sheet (Table 2).
2.3 Data analyses
We assumed that the difference between initial and remaining dry weights of leaf detritus in protected litterbags was a measure of leaching loss (sensu Bärlocher 2007). While the difference between initial and remaining dry weights of unprotected litterbags was due to the sum of two weight losses i.e. the leaching loss (where WD, protect litterbags ≈ WD, unprotected litterbags) and the feeding activity of macrozoobenthic assemblage.
For each leaf sample, we then measured three detritus categories: dissolved (WD), palatable (WP) and recalcitrant (WR) fractions where their sum is unitary (i.e. WR + WP + WD = 1). In protected litterbags, the dissolved matter fraction (WD) was a measure of matter lost by only leaching activity (WD = 1 − WR, where WR was the fraction of the dry weight of remnant matter and WP = 0), whereas, inversely, the recalcitrant fraction was a measure of remnant matter (WR = 1 − WD). In unprotected litterbags, the palatable matter fraction (WP) was defined as: WP = 1 − (WR + WD), where WR = 1 − (WD + WP) was the fraction of the dry weight of remaining matter and WD = 1 − (WR + WP), was a measure of organic matter lost by leaching activity.
The breakdown rates of native and exotic detritus resources were estimated using the exponential decay [k = (lnW0 − lnWt)/t], where k is the decay coefficient, W0 the initial dry weight and Wt the remaining dry weight at time t (Olson 1963).
A regression analysis between the percentage of remaining material (log-transformed) and collecting time was conducted for each of the two detritus (Phragmites and Eucalyptus) to estimate the weight loss coefficient k (equal to slope) and number of days to reach 50% of mass loss (T50) (Bärlocher 2007). The significance of linear regression was measured by means of ANOVA and, for both parameters (k and T50), the 95% confidence intervals were estimated. Differences in k or T50 values between two detritus plant species were considered significant when their 95% confidence limits did not overlap.
A principal component analysis (PCA) was used to identify the main component (salinity (psu); [O2] mg L−1; pH) involved in the annual change in chemical-physical parameters in the four pools of the study area.
Finally, a two-way permutational analysis of variance (PERMANOVA) was used to test for the effect of leaf detritus type (two levels fixed, native and exotic) and salinity (four levels fixed) on macrozoobenthic assemblages. Multivariate community structure was analyzed with PERMANOVA on 4th root transformed data (Bray–Curtis similarity coefficient; 4999 permutations; Clarke et al. 2006).
Statistical analyses were performed with R (R Development Core Team 2014).
3 Results
Fluctuations in the level of pH and dissolved oxygen concentration slightly affected the environmental conditions within the pools, as showed by the PCA ordination. Particularly, PCA showed the role of salinity as the main driving force in the ordination (PCA score = 0.971), while pH and dissolved oxygen concentration accounted for the remaining fraction (Table 1).
In six months, 1790 individuals (14.622 AFDW g) were collected, belonging to S = 12 macrozoobenthic taxa in P = four pools (Tables 2 and 4).
Highest values of abundance (481 and 245 individuals) were observed on native and exotic resources in 52.64 ± 6.01 psu due to Hydrobia complex acuta on the detritus leaves of Phragmites and Eucalyptus.
On the weight loss, the breakdown rates (Olson’s formula emphasize the role of native and exotic detritus resources, where knative > kexotic in low salt stress conditions, and knative = kexotic in high salt stress conditions (Table 3). More particularly, we observed an increase in the weight loss of detritus when, in 181 days under high salt stress conditions, only 5.46% and 4.87% of offered native and exotic resources are consumed by Chironomus salinarius (larvae) in terms of abundances.
In 43.41 ± 3.39 psu (or, equivalently, in marine water), native and exotic plant detritus resources were consumed, in 6 months, by higher richness of macroinvertebrates (9 and 10 taxonomic unities, respectively) than in 160.27 ± 43.42 psu (on both leaf detritus, 2 taxonomic unities). In 52.64 ± 6.01 psu, native plant detritus was consumed by higher abundance of macroinvertebrates than exotic resources (593 and 284 total individuals, respectively).
Regression analysis always showed a significant negative correlation between the percentage of remaining material and collecting time (r2 > − 0.90 and p < 0.05 for all cases) in all sampled pools. Both trophic resources (native and exotic) and environmental conditions (salinity levels) contribute in determining the observed pattern of macroinvertebrate assemblage (2-way PERMANOVA F > 5.432 and p < 0.05 in all cases). The results also showed a significant effect of the interaction terms (F = 3.762, p = 0.032), with both leaf litter types and environmental conditions affecting the abundance and biomass of sampled macroinvertebrates (Table 4).
4 Discussion
The main result was that the donor-controlled community responded better, in terms of loss of palatable fraction (excluding leaching and recalcitrant roles), on native resources than on exotic resources.
In particular, comparing the responses of macrozoobenthic assemblages to the different salt stress conditions and resources, we obtained two complex patterns: one denoting changes in structuring process (abundance and biomass macrozoobenthic assemblages) and another denoting change in donor functionality (reduction of palatable fraction, increase of recalcitrant and leaching fractions; Fig. 2).
When considering the characteristics at community level (total richness, abundance and biomass), the macrozoobenthic assemblages responded showing highest values at low salt stress conditions, then simplifying their structure in stressed conditions of high salinity.
When increasing the salinity, the native detritus was colonized by a higher abundance of macroinvertebrates than the exotic detritus, according to Alonso et al. (2010) and Stiers et al. (2011). In a previous experimental work (Cerfolli et al. 2013), we observed two different thresholds in macrozoobenthic assemblages: between about 44 and 50 psu and between 50 and 87 psu, respectively. The first threshold was due to a qualitative turnover in taxon composition between assemblages, the second threshold was due to a quantitative change in taxon richness (lower in pools with higher salinity: i.e. > 50 psu).
Even in this experimental work, concerning the palatable fraction, a threshold (52.64 ± 6.01 psu), immediately close to the values just higher than the marine water, was for feeding activity of macroconsumers. This barrier seems to overcome by hyper-specialists to withstand conditions of high salinity or able to tolerate such stressed conditions for reproductive reasons or by slowly consumption of the plant detritus, regardless of its origin (native or exotic substrates).
When considering the trophic consumption, the macrozoobenthic assemblages showed an immediate reduction in their activity transferring from marine water conditions to pools with progressively higher salinity. This pattern was similar on both the resources (native and exotic leaf detritus).
At very high level of salinity, detritus resources are not easily available due to their physical degradation (salt encrustation) and, therefore, the trophic consumption by macrozoobenthic assemblage is strongly affected. In these extreme conditions, the exotic detritus represents a food surrogate available for these structurally simplified assemblages.
A change in salinity represents a natural or human-induced disturbance (sensu Sousa 1984; White and Pickett 1985; Petraitis et al. 1989; Dornelas et al. 2011; for a review on disturbance ecology: Battisti et al. 2016). In particular, at macrozoobenthic assemblage level, when salinity increases a shortening of the food chains tend to be evident, with a disruption of production, competition and other benthic processes (Por 1980).
At our scale of study, the patterns observed apparently did not generally match with the intermediate disturbance hypothesis (Wilson 1994; Collins and Glenn 1997; Mackay and Currie 2001; Roxburgh et al. 2004); i.e. we did not observe a peak of structure complexity or higher consumption rates at intermediate level of salinity. Only the pattern of structure of assemblages on native resource apparently showed a peak in intermediate conditions (52.64 ± 6.01 psu) likely due to the use of the leaf detritus as site for egg deposition (Hydrobia complex acuta) rather than for feeding activities.
These results suggest that the exotic leaf detritus, characterized by a low rate of decomposition and reduced palatability, can be an excellent analytical probe to increase the predictive power of ecological responses of macrozoobenthic assemblages subjected to salt stress conditions.
Humans may actively change the wetland salinity, e.g. altering the hydroperiod (e.g., through the creation of dry wilderness areas or inappropriate water management policy), so having synergistic effect on diversity of invertebrate communities, including some keystone species (Waterkeyn et al. 2010). In this work, we highlighted how these effects are evident also at structural and functional level. Moreover, we observed a counter-intuitive response of macrozoobenthic assemblages to an exotic trophic resource that was more consumed in hyperaline conditions compared to a native resource. This may have implications for conservation of altered wetlands. In coastal transitional aquatic ecosystems characterized by a disturbance due to a natural or human-induced hyper-salinity, these stressed conditions may affect the trophic quality of native resource, thereby resulting unavailable for the structurally simplified macrozoobenthic assemblages (Bonsdorff and Pearson 1999; Lee 2008; Dolbeth et al. 2011). Since this complex assemblage represents a strategic trophic basic level in wet ecosystems, this structural simplification and the consequent low native resource consumption could have cascade effects at higher trophic levels (primary and secondary consumers and predators, mainly wetland-related birds; Brawn et al. 2001; Herbst 2006). In this context, an exotic resource as the leaf-detritus of Eucalyptus, may represent a trophic surrogate that allows maintenance of remnant and highly dominated macrozoobenthic assemblages. Although these disrupted assemblages are often collapsed in terms of their structure (few species, high concentration of dominance, low diversity; e.g. Helmus et al. 2010), they represent a basic trophic level for higher consumers (migratory birds, Aphanius fasciatus, etc.; e.g. Vizzini and Mazzola 2008; Wollheim and Lovvorn 1995).
Before the scenarios of rising sea levels and the resulting salinization of transitional aquatic ecosystems, the ecological role of exotic plant species tolerant to salt and the effects of the input of alien plant detritus on donor-controlled trophic structures, are interesting research topics. Use of the detritus of exotic plants to collect data on structural and functional changes of macrozoobenthic assemblages might be useful to manage the biodiversity in many aquatic ecosystems (e.g. de Souza et al. 2010). Moreover, the different average concentrations—on an annual scale—of the salinity values observed during the experiment, at the four sampling sites, represent, in a predictable perspective, what could happen to the macrozoobenthic communities, in the different succession scenarios that would be plausibly verify due to the effects of climate change (i.e. increase in temperature). In previous works in the area, the driving force that structures the macrozoobenthic communities seems to be precisely salinity (Cerfolli et al. 2013). One of the effects of the increase in salinity is a simplification of the macrozoobenthic communities both in terms of species and biomass (Cerfolli et al. 2013).
The collected data provide some interesting indications on the role played by Eucalyptus trees, extremely widespread in Mediterranean reclamation landscapes. Eucalyptus trees are often considered a foreign element and have recently undergone cutting and removal, even in the context of projects carried out in Europe (LIFE13 NAT/IT/000471; LIFE13 BIO/ES/001407: https://ec.europa.eu/environment/life/project/Projects/index.cfm). In this regard, in salt coastal areas deprived of the original vegetation, even the presence of exotic species can play a strategic ecological role. Therefore, the present results add important information to the critical debate about the paradoxical role of non-native species in complex ecosystems (Schlaepfer et al. 2012; Battisti et al. 2018; Schlaepfer 2018; Pauchard et al. 2018).
References
Abbruzzese G (2014) Considerazioni sulla vegetazione arbustiva e arborea della Riserva Naturale di Popolamento Animale Saline di Tarquinia. In: Colletti L (ed) 2014 La Riserva Naturale Statale “Saline di Tarquinia” Corpo forestale dello Stato. Ufficio territoriale per la Biodiversità di Roma, pp 101–109
Agoston-Szabò E, Dinka M (2008) Decomposition of Thypa angustifolia and Phragmites australis in the littoral zone of a shallow lake. Biologia 63:1104–1110
Alonso A, González-Munoz N, Castro-Díez P (2010) Comparison of leaf decomposition and macroinvertebrate colonization between exotic and native trees in a freshwater ecosystem. Ecol Res 25:647–653
Amores MJ, Verones F, Raptis C, Juraske R, Pfister S, Stoessel F, Antón A, Castells F, Hellweg S (2013) Biodiversity impacts from salinity increase in a coastal wetland. Environ Sci Technol 47:6384–6392
Angeletti D, Cimmaruta R, Nascetti G (2010) Genetic diversity of the killifish Aphanis fasciatus paralleling the environmental changes of Tarquinia salterns habitat. Genetica 138:1011–1021
Bärlocher F (2007) Leaching. In: Graça MAS, Bärlocher F, Gessner MO (eds) Methods to study litter decomposition. Springer, Berlin, pp 33–36
Barlöcher F, Graca MAS (2002) Exotic riparian vegetation lowers fungal diversity but not leaf decomposition in Portuguese streams. Freshw Biol 47:1123–1135
Barlöcher F, Kendrick B (1973) Fungi and food preferences of Gammarus pseudolimnaeus. Arch Hydrobiol 72:501–516
Battisti C, Poeta G, Fanelli G (2016) An introduction to disturbance ecology. A road map for wildlife management and conservation. Springer, Berlin
Battisti C, Fanelli G, Bertolino S, Luiselli L, Amori G, Gippoliti S (2018) Non-native invasive species as paradoxical ecosystem services in urban conservation education. Web Ecol 18:37–40
Bellisario B, Carere C, Cerfolli F, Angeletti D, Nascetti G, Cimmaruta R (2013) Infaunal macrobenthic community dynamics in a manipulated hyperhaline ecosystem: a long term study. Aquat Biosyst 9:20
Benke AC, Huryn AD (2006) Secondary production of macroinvertebrates. In: Hauer FR, Lamberti GA (eds) Methods in stream ecology, 2nd edn. Academic Press, Burlington, pp 691–710
Bo T, Cammarata M, López-Rodríguez MJ, Tierno de Figueroa JM, Baltieri M, Varese P, Fenoglio S (2014) The influence of water quality and macroinvertebrate colonization on the breakdown process of native and exotic leaf types in sub-alpine stream. J Freshw Ecol 29:159–169
Bonsdorff E, Pearson TH (1999) Variation in the sublittoral macrozoobenthos of the Baltic Sea along environmental gradients: a functional-group approach. Austral J Ecol 24:312–326
Brawn JD, Robinson SK, Thompson FR III (2001) The role of disturbance in the ecology and conservation of birds. Ann Rev Ecol System 32:251–276
Brock MA, Nielsen DL, Crossle K (2005) Changes in biotic communities developing from freshwater wetland sediments under experimental salinity and water regimes. Freshw Biol 50:1376–1390
Canhoto C, Graça MAS (1996) Decomposition of Eucalyptus globulus leaves and three native leaf species (Alnus glutinosa, Castanea sativa and Quercus faginea) in a Portuguese low order stream. Hydrobiologia 333:79–85
Celesti-Grapow L, Pretto F, Carli E, Blasi C (eds) (2010) Flora vascolare alloctona e invasiva delle regioni d’Italia. Casa Editrice Università La Sapienza, Rome
Cerfolli F, Bellisario B, Battisti C (2013) Detritus-based assemblage responses under salinity stress conditions in a disused aquatic artificial ecosystem. Aquat Biosyst 9:1–9
Cho SK, Larue CT, Chevalier D, Wang H, Jinn TL, Zhang S, Walker JC (2008) Regulation of floral organ abscission in Arabidopsis thaliana. Proc Natl Acad Sci USA 105:15629–15634
Clarke KR, Somerfield PJ, Chapman MG (2006) On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray–Curtis coefficient for denuded assemblages. J Exp Mar Biol Ecol 330:55–80
Collins SL, Glenn SM (1997) Intermediate disturbance and its relationship to within- and between-patch dynamics. N Z J Ecol 21:103–110
Cummins KW (2002) Riparian-stream linkage paradigm. Verh Internat Verein Limnol 28:49–58
de Souza RR, Goncalves JF, Mello Petrucio M (2010) Leaf breakdown and invertebrate colonization of Eucalyptus grandis (Myrtaceae) and Hirtella glandulosa (Chrysobalanaceae) in two Neotropical lakes. Acta Limnol Brasil 22:23–34
Dolbeth M, Cardoso PG, Grilo TF, Bordalo MD, Raffaelli D, Pardal MA (2011) Long-term changes in the production by estuarine macrobenthos affected by multiple stressors. Eustar Coast Sh Res 92:10–18
Dornelas M, Soykan CU, Ugland KI (2011) Biodiversity and disturbance. In: Magurran A, McGill BJ (eds) Biological diversity. Frontiers in measurements and assessments. Oxford University Press, Oxford, pp 237–251
Fazi S, Rossi L (2000) Effects of macro-detritivores density on leaf detritus processing rate: a macrocosm experiment. Hydrobiologia 435:127–134
Friberg N, Winterbourn M (1997) Effects of native and exotic forest on benthic stream biota in New Zealand: a colonization study. N Z J Mar Freshw Res 31:267–275
Furey C, Tecco PA, Perez-Harguindeguy N, Giorgis MA, Grossi M (2014) The importance of native and exotic plant identity and dominance on decomposition patterns in mountain woodlands of central Argentina. Acta Oecol 54:13–20
Godoy O, Castro-Dıez P, Van Logtestijn RSP, Cornelissen JHC, Valladares F (2010) Leaf litter traits of invasive species slow down decomposition compared to Spanish natives: a broad phylogenetic comparison. Oecologia 162:781–790
Gonçalves AL, Canhoto C (2009) Decomposition of eucalypt and alder mixtures: responses to variation in evenness. Fund Appl Limn 173:293–303
Gonçalves JF, de Souza RR, França J, Callisto M (2012) Invertebrate colonisation during leaf processing of native, exotic and artificial detritus in a tropical stream. Mar Freshw Res 63:428–439
Helmus MR, Keller WB, Paterson MJ, Yan ND, Cannon CH, Rusak JA (2010) Communities contain closely related species during ecosystem disturbance. Ecol Lett 13:162–174
Herbst DB (2006) Salinity controls on trophic interactions among invertebrates and algae of solar evaporation ponds in the Mojave Desert and relation to shorebird foraging and selenium risk. Wetlands 26:475–485
Huxel GR, McCann K (1998) Food web stability: the influence of trophic flows across habitats. Am Nat 152:460–469
Jefferies RL (2000) Allochthonous inputs: integrating population changes and food-web dynamics. Trends Ecol Evol 15:19–22
Jørgensen S, Tundisi JG, Matsumura-Tundisi T (2013) Handbook of inland aquatic ecosystem management. Taylor and Francis Group, LLC, New York
Kevrekidis T (2004) Seasonal variation of the macrozoobenthic community structure at low salinities in a Mediterranean lagoon (Monolimni Lagoon, Northern Aegean). Int Rev Hydrobiol 89:407–425
Kominosky JS, Hoellein TJ, Leroy CJ, Pringle CM, Swan CM (2009) Beyond species richness: expanding biodiversity ecosystem functioning theory in detritus-based streams. River Res Appl 26:67–75
Lee SY (2008) Mangrove macrobenthos: assemblages, services, and linkages. J Sea Res 59:16–29
Lucchese F (2017) Atlante della Flora Alloctona del Lazio: Cartografia, Ecologia e Biogeografia. vol 1: Parte generale e Flora Alloctona. Regione Lazio, Direzione Ambiente e Sistemi Naturali, Roma, p 352
Lucchese F (2018) Atlante della Flora Vascolare del Lazio, cartografia, ecologia e biogeografia, vol 2. La flora di maggiore interesse conservazionistico. Regione Lazio, Direzione Capitale Naturale, Parchi e Aree Protette, Roma, pp 400
Mackay RK, Currie DJ (2001) The diversity-disturbance relationship: is it generally strong and peaked? Ecology 82:3479–3492
Mathuriau C, Thomas AGB, Chauvet E (2008) Seasonal dynamics of benthic detritus and associated macroinvertebrate communities in a neotropical stream. Fund Appl Limn Arch Hydrobiol 171:323–333
McEvoy P, Goonan P (2003) Salinity is not necessarily bad for biodiversity: case studies of invertebrates from South Australian streams and River Murray wetlands. Rec South Aust Museum 7:131–134
Moore JC, de Ruiter PC (2012) Energetic food webs, an analysis of real and model ecosystems. Oxford Series in Ecology and Evolution, Oxford
Moore JC, Berlow EL, Coleman DC, de Ruiter PC, Dong Q, Hastings A, Johnson NC, McCann KS, Melville K, Morin PJ, Nadelhoffer K, Rosemond AD, Post DM, Sabo JL, Scow KM, Vanni MJ, Wall DM (2004) Detritus, trophic dynamics and biodiversity. Ecol Lett 7:584–600
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
Ostrofsky ML (1997) Relationship between chemical characteristics of autumn-shed leaves and aquatic processing rates. J N Am Benthol Soc 16:750–759
Parkyn SM, Winterbourn JM (1997) Leaf breakdown and colonization by invertebrates in a headwater stream: comparisons of native and introduced tree species. N Z J Mar Freshw Res 31:301–312
Pastor J (2008) Mathematical ecology of populations and ecosystems. Wiley-Blackwell, New York
Pauchard A, Meyerson LA, Bacher S, Blackburn TM, Brundu G, Cadotte MW et al (2018) Biodiversity assessments: origin matters. PLoS Biol 16(11):e2006686. https://doi.org/10.1371/journal.pbio.2006686
Petraitis PS, Latham RE, Niesenbaum RA (1989) The maintenance of species diversity by disturbance. Quart Rev Biol 64:393–418
Piscart C, Lecerf A, Usseglio-Polatera P, Moreteau JC, Beisel JN (2005) Biodiversity patterns along a salinity gradient: the case of net-spinning caddisflies. Biodivers Conserv 14:2235–2249
Polis GA, Strong DR (1996) Food web complexity and community dynamics. Am Nat 147:813–846
Polis GA, Power MA, Huxel GR (2004) Food webs at the landscape level. University Chicago Press, Chicago
Ponsard S, Arditi R, Jost C (2000) Assessing top-down and bottom-up control in a litter-based soil macroinvertebrate food chain. Oikos 89:524–540
Por FD (1980) A classification of hypersaline waters, based on trophic criteria. Mar Ecol 1:121–131
R Development Core Team (2014) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reise K, Olenin S, Thieltges DW (2006) Are aliens threatening European aquatic ecosystems? Helgol Mar Res 60:77–83
Rosemond AD, Pringle CM, Ramírez A, Paul MJ (2001) A test of top-down and bottom-up control in a detritus-based food web. Ecology 82:2279–2293
Roxburgh SH, Shea K, Wilson JB (2004) The intermediate disturbance hypothesis: patch dynamics and mechanisms of species coexistence. Ecology 85:359–371
Schlaepfer MA (2018) On the importance of monitoring and valuing all forms of biodiversity. PLoS Biol 16(11):e3000039
Schlaepfer MA, Sax DF, Olden JD (2012) Toward a more balanced view of non-native species. Conserv Biol 26:1156–1158
Sousa WP (1984) The role of disturbance in natural communities. Ann Rev Ecol Syst 15:353–391
Stiers I, Crohain N, Josens G, Triest L (2011) Impact of three aquatic invasive species on native plants and macroinvertebrates in temperate ponds. Biol Invasion 13:2715–2726
Stohlgren TJ, Bull KA, Otsuki Y, Villa CA, Lee M (1998) Riparian zones as havens for exotic plant species in the central grasslands. Plant Ecol 138:113–120
Stohlgren TJ, Binkley D, Chong GW, Kalkhan MA, Schell LD, Bull KA, Otsuki Y, Newman G, Bashkin M, Son Y (1999) Exotic plant species invade hot spots of native plant diversity. Ecol Monogr 69(1):25–46
Tang L, Gao Y, Li B, Wang Q, Wang CH, Zhao B (2014) Spartina alterniflora with high tolerance to salt stress changes vegetation pattern by outcompeting native species. Ecosphere 5(9):116
Telesh I, Schubert H, Skarlato S (2013) Life in the salinity gradient: discovering mechanisms behind a new biodiversity pattern. Estuar Coast Shelf Sci 135:317–327
Vizzini S, Mazzola A (2008) The fate of organic matter sources in coastal environments: a comparison of three Mediterranean lagoons. Hydrobiologia 611:67–79
Wallace JB, Eggert SL, Meyer JL, Webster JR (1999) Effects of resource limitation on a detrital-based ecosystem. Ecol Monogr 69:409–442
Wardle DA, Bardgett RD, Klironomos JN, Setala H, Van der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304(1629):1633
Waterkeyn A, Vanschoenwinkel B, Grillas P, Brendoncka L (2010) Effect of salinity on seasonal community patterns of Mediterranean temporary wetland crustaceans: a mesocosm study. Limnol Oceanogr 55:1712–1722
Werner EE, Gilliam JF (1984) The ontogenetic niche and species interactions in size-structured populations. Ann Rev Ecol Syst 15:393–425
White PS, Pickett STA (1985) Natural disturbance and patch dynamics: an introduction. In: Pickett STA, White PS (eds) The ecology of natural disturbance and patch dynamics. Academic Press, Orlando, pp 3–13
Wilson JB (1994) The intermediate disturbance hypothesis of species coexistence is based on patch dynamics. N Z J Ecol 18:176–181
Wollheim WM, Lovvorn JR (1995) Salinity effects on macroinvertebrate assemblages and waterbird food webs in shallow lakes of the Wyoming High Plains. Hydrobiologia 310:207–233
Wollrab S, Diehl S, De Roos AM (2012) Simple rules describe bottom-up and top-down control in food webs with alternative energy pathways. Ecol Lett 15:935–946
Acknowledgements
FC and CB contributed to the conceptual development of the work. FC wrote the manuscript and carried out the statistical analyses with CB which developed the section on disturbance ecology. FC contributed to the experimental design and the organization of sampling activity. We wish to thank Monica Alfano and Damiano Pastorelli for sampling activity to this study and Claudio Carere and Bruno Bellisario for their useful comments to the first draft of the manuscript. An anonymous reviewer and the Assistent Editor provided useful comments and suggestions which improved the first draft of the manuscript. Dr PhD A. Zocchi reviewed the English style and language.
Funding
This research did not provide funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
No human participants and/or animals have been involved in this research.
Informed consent
No human participants have been involved in this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cerfolli, F., Battisti, C. Impact of exotic plant detritus on macrozoobenthic assemblages: evidence from a transitional aquatic ecosystem. Rend. Fis. Acc. Lincei 31, 419–429 (2020). https://doi.org/10.1007/s12210-020-00908-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-020-00908-8