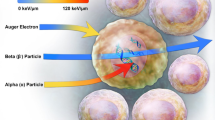
Abstract
Cancer is the second cause of death and morbidity in Europe. Unfortunately, currently available treatments cannot permanently cure most cancers, especially when metastatic. New therapy approaches are, therefore, urgently needed. Radionuclide therapy deposits cytotoxic radiation by means of energetic particles (alfa, beta, and auger) labeled to a carrier that specifically targets cancer cells. Targeted Alpha Therapy is very promising, because alpha particles deliver high energy (i.e., cytotoxic effect) in a small range, binding a target cell population without significant harm to healthy tissues. The high linear energy transfer typical of alpha particles determines irreversible double-strand DNA breaks with per-unit absorbed doses of acute biologic effects three-to-seven times greater than the damage produced by external beam with photons or beta radiation. As consequence, cells—not equipped to efficiently repair this type of damage—typically undergo death. Therefore, Targeted Alpha Therapy is such a new approach to treat tumors. This article aimed to provide an overview (five “W”s and “How”) on Targeted Alpha Therapy.
Graphical Abstract

Similar content being viewed by others

Avoid common mistakes on your manuscript.
1 Why? Background and rationale
More than 3.0 million people are newly diagnosed with cancer each year. Cancer is the second most important cause of death and morbidity in Europe. The most common causes of death for cancers are Lung (21%), Colorectal (12%), Breast (7%), Pancreas (7%), and Prostate (6%) [(Source: ECIS—European Cancer Information System. From https://ecis.jrc.ec.europa.eu, accessed on 6/12/2019 © European Union, 2019)].
The currently available treatments, even though often effective, cannot permanently cure most cancers mainly when disease have already formed distant metastases. New therapy approaches are, therefore, urgently needed.
Radionuclide therapy delivers cytotoxic radiation through energetic particles (alfa, beta, and auger) labeled to a carrier that specifically targets cancer cells.
Targeted Alpha Therapy (TAT) employing alpha particle emitters is such a new approach to cancer treatment.
Targeted Alpha Therapy is very promising, because alpha particles deposit high energy at a small distance. The short range allows delivering the cytotoxic effect to a specific cell population limiting the effect on non-targeted cells. The high linear energy transfer (LET) typical of alpha particles determines irreversible double-strand DNA breaks with per-unit absorbed doses of acute biologic effects three-to-seven times greater than the damage produced by an external beam with photons or beta radiation (Sgouros 2008; Allan 2013). As a consequence, cells—not equipped to efficiently repair this type of damage—typically undergo death (Kassis 2008).
The present paper aimed to provide an overview of TAT as a new approach to cancer treatment.
2 Who? Clinical need
One of the most appealing characteristics of radionuclide treatment is its adaptability. In principle, each radionuclide can combine with each targeting vehicle addressing specific requirements related to the way of administration, the stage of the disease, the accessibility of the target, and the site of action.
Accordingly, the development of a new efficient probe for radionuclide treatment depends upon the optimal match between the tumor-associated antigen and the targeting vehicle. Ideally, the “perfect” antigen is overexpressed by tumor cells and absent or scarce in healthy tissues (Dekempeneer et al. 2016).
Because of the high binding affinity to tumor-associated antigens, four crucial aspects—high specificity, high tumor-to-background ratio, high metabolic stability, and low immunogenicity—make antibodies suitable vehicles for the delivery of therapeutic radioisotopes.
Since the early 1950s, radioimmunotherapy (RIT) has been a significant research area for cancer treatment, despite the marketing authorization of an RIT agent in non-Hodgkin lymphoma, clinical trials in solid tumors are rarely successful and progress beyond the phase I/II. Accordingly, research in solid tumors aims to find alternative approaches to improve the clinical success of radionuclide therapy (Sollini et al. 2015). Different strategies have been proposed including the use of different antibody formats (e.g., diabodies), the identification of alternative targeting agents (e.g., folate and peptides), and the selection of radionuclides with more favorable characteristics in terms of physical properties (energy and half-life), labeling chemistry properties, cytotoxic effect, and toxicity profile (Sollini et al. 2015; Dekempeneer et al. 2016; Targeted Alpha Therapy Working Group et al. 2018).
3 What? Physics and radiochemistry
Alpha particles are helium nuclei with a positive charge. They are about 8000 times larger than beta particles and have some advantages compared to beta ones. Like alpha particles, with a +2 charge, deliver energy with a short range (50–100 μm versus several mm for beta particles) and high LET (from 25 to 230 keV/μm versus about 0.2 keV/μm for beta particles) (Gudkov et al. 2016). The energy per unit path length deposited by an α-particle is ≥ 500 times higher than an electron or β-particle (Baidoo et al. 2013).
The LET is ~ 80 to 100 keV/μm along most of their up-to-100-μm path before increasing to ~ 300 keV/μm toward the end of the track (Bragg peak) (Kassis 2008).
Overall, the cytocidal effect of α-emitters is not related to dose fractionation, dose rate, or hypoxia, and it overcomes the resistance to chemotherapeutics encountered in the conventional chemo- and radiation treatment (Baidoo et al. 2013).
The therapeutic effect of alpha emitters depends on:
the distance between the decaying atom and the nucleus of the targeted cell;
the heavy-ion recoil of the daughter atom, mainly if the alpha-particle emitter is covalently bound to nuclear DNA; and
the bystander effects and the magnitude of cross-dose (Sgouros 2008).
The alpha-emitting radionuclides of main interest for cancer treatment purposes are astatine-211 (211At), bismuth-212 (212Bi), bismuth-213 (213Bi), radium-223 (223Ra), actinium-225 (225Ac), lead-212 (212Pb), and thorium-227 (227Th). Table 1 summarizes the main characteristics (Sgouros 2008; Gudkov et al. 2016).
Emitters of interest are generally radiometals, in which the highest binding constants are obtained through chelation by polydentate ligands. The halogen 211At is the exception. Each radioisotope has exclusive aqueous coordination chemistry properties, and specific chemical demands, including ligand donor atom preferences (e.g., N, O, S, and hard/soft), coordination number, and coordination geometry. These characteristics must be considered for labeling strategy. The radiometal is bind to the antibody vectors (immunoconjugates) or peptides through a ligand system (i.e., chelator) which represent an essential component of a radiometal-based radiopharmaceutical. Robust chelate chemistry is crucial for the implementation of therapeutic agents based on alpha-emitting isotopes, because it can influence biodistribution and have an impact on the potentially fatal toxicity (Price and Orvig 2014). Indeed, non-bound radionuclides might non-specifically localize in non-target tissues. For example, bismuth localizes in kidney, terbium, and radium in bone, radium, actinium in the liver, and astatine in lung, spleen, thyroid, and stomach (Dadwal et al. 2011; Wilbur 2011; Price and Orvig 2014; Ramdahl et al. 2016; Pozzi and Zalutsky 2017).
3.1 Carriers
Vectors are suitable when specifically target the tumors to be treated. Many vectors are being used or are close to being introduced in clinical use. For instance, the recombinant humanized IgG1 monoclonal antibody HuM195reactive with CD33 targeting acute myelogenous leukemia; the murine 9.2.27 mAb highly specific for the melanoma-associated chondroitin sulfate proteoglycan NG2; the anti-CD20 for lymphoma; MX35 F(ab′)2 (targeting a cell surface glycoprotein), and trastuzumab (targeting Her2) for ovarian cancer; the murine 81C6 IgG2b mAb targeting an extracellular matrix glycoprotein expressed in many tumors but not in healthy tissues; the human-mouse chimeric anti-tenascin 81C6 for brain tumors, the PAI2 against urokinase-type plasminogen activator, the C595 a murine mAb against MUC-1, and the folate receptor alpha for many solid tumors. Prostate-specific membrane antigen (PSMA), investigated for various diagnostic applications, especially in prostate cancer (Vallabhajosula et al. 2004; Behe et al. 20111; Gao et al. 2014; Kiess et al. 2015; Evans et al. 2016; Hadaschik and Boegemann 2017), is one of the most promising theragnostic agents for TAT. Radium-223-dichloride, due to its natural affinity for bone, is applied in bone cancers and skeletal metastases (Allen 2012; Dekempeneer et al. 2016).
Generally, in RIT trials, whole immunoglobulins G (IgG) are used, since they are highly available, can be easily manufactured and handled. Data on preclinical studies demonstrated that whole IgG presented slow blood clearance that resulted in the highest tumor uptake as compared to F(ab′)2 or Fab′ fragments or engineered forms. However, whole IgG-based radiopharmaceuticals administration has been reported to be associate with increased risk of myelosuppression due to the exposure of red marrow to radiation even at low activities. Another disadvantage when using the whole IgG is related to low penetrance into the tumor mass due to high internal pressure within the tumor. Migration of targeted molecules is also significantly influenced by the “binding-site barrier”, which affected more higher affinity molecules. A way to partially overcome this limited localization is increasing the vehicle dose. However, an excess of unbounded product competing with the labeled one might result in a significant reduction of the amount of radioactive molecule in the tumor. Structural modification of antibodies has been attempted to obtain molecules with more favorable pharmacokinetic properties including F(ab′)2, Fab or its multivalent conjugate, minibody, diabody, and single-chain variable fragments (scFv). All these molecules have the same antigen-binding properties of the whole IgG, but are characterized by faster clearance. Notably, the scFv fragments having a mass significantly lower than a whole IgG extravasate easier and diffuse more in-depth into the tumor. As a consequence, the scFv fragments result in a faster tumor uptake, in higher tumor/non-tumor ratios and potentially in a more homogeneous distribution. Multivalent antibodies including IgG, small immunoprotein (SIP), or mini-antibodies, F(ab)2, (scFv)2 can bind at the same time to two antigens. Furthermore, if the antibody dissociates from the antigen, at one or both binding sites, a rebinding is more likely before the complete dissociation occurs. On the other hand, small molecules have the main disadvantage of low tumor uptake as compared to the whole IgG as a consequence of their fast clearance as well as high susceptibility to the binding-site barrier effect. A rapid tumor uptake typical of the whole IgG is more cytocidal, delivering the radiation at a higher dose rate. Nonetheless, the administered activity could be theoretically increased, since the exposure of bone marrow is reduced by their fast clearance. Practically, side effects limit this increase (i.e., high dose to the tumor). Molecules smaller than 50,000 Da cleared by kidneys may cause renal toxicity, mainly when radiometals are used (Sollini et al. 2015). So far, vehicles for TAT benefited from some improvements aimed to develop and to optimize target molecules. Nonetheless, there is still considerable room for amelioration, primarily related to the development of new coupling chemistries, and the improvement of pharmacokinetic properties (Dekempeneer et al. 2016).
3.2 Dosimetry and radiation protection
Radiation treatments, even if different, may be standardized and compared using dosimetry. Fundamentally, dosimetry provides data to understand and to quantify the effects of radiation on biological tissue(s). In the case of alpha emitters, dosimetric estimates require mathematical modeling to determine the activity distribution as a function of time at the cellular and subcellular levels. Determining the activity distribution, however, remains challenging. For high-LET irradiation, the effect of even a single event in the nucleus of the cell(s) is so substantial that the mean absorbed dose can result in an inaccurate index of biological effect. This fact may be related to different reasons, including the small number of alpha particles that generally crosses a cell nucleus making stochastic variations significant, and the path of the alpha particles through the cell nucleus. Accordingly, an alpha particle that solely grazes the surface of a cell nucleus will result in a small or even no energy deposit. Conversely, the right cross of the cell nucleus will determine a high deposit of energy. Microdosimetry, taking into account the stochastic nature of energy deposited in small targets, is another method for alpha particle dosimetry. Microdosimetry traditionally requires the calculation of specific energy (energy per unit mass) and lineal energy (energy per unit path length through the target) that could be computed using either analytical or Monte Carlo methods. Kellerer and Chmelevsky (Kellerer and Chmelevsky 1975) outlined the rationale for microdosimetry, suggesting that the stochastic variations of energy deposited within the target must be considered when the relative deviation of the local dose exceeds 20% (Sgouros et al. 2010). The therapeutic dose of an alpha-emitter clinically used may affect radiolabeling chemistry, since the activity (mCi or MBq) can differ within alpha-emitting radionuclides. TAT should be considered different from target beta-radiation therapy. In the latter case, the beta emissions benefit from cross-fire effect to kill cells. Therefore, the therapeutic effect of beta-emitters relies on the total amount of radiation administered to the patient, and localized in the target tissue, while the therapeutic effect of primarily comes from alpha-emissions on the targeted cells. Notably, alpha therapy is not affected by the dose rate or oxygen tension. While the biological half-life and nature of the targeting molecule play central roles, the number of radioactive atoms and not the activity is the primary factor when using alpha-emitters (Wilbur 2011). Radiation protection directives are implemented according to national and European regulations, in particular, the EU Directive 2103/59/EURATOM (European Commission 2014). Alpha emitting nuclei can also emit beta and gamma radiations. On one hand, alpha particles can be easily stopped and represent a significant risk only when placed in contact with biological tissues. On the other hand, beta and gamma radiations have a higher capacity to penetrate tissues and to pass physical barriers. This capacity should be considered when dealing with radiopharmaceuticals labeled with alpha-emitting isotopes.
4 Where? and When? Clinical applications of Targeted Alpha Therapy
Currently, oncological patients are treated using a multimodal approach that, guided by stage and prognostic factors, may include systemic neoadjuvant treatment alone or in association with surgery or external beam radiotherapy, and eventually, adjuvant chemotherapy with or followed by regional adjuvant radiotherapy. In hematological malignancies, TAT may be used to enhance the cytoreductive effect of the chemotherapy, to reduce the tumor burden, and to condition patients scheduled for transplantation. In the adjuvant setting of some epithelial cancers such as melanoma, ovarian cancer, mesothelioma, and neuroendocrine tumors (NETs), TAT may be proposed as boost or consolidating therapy after curative treatment (e.g., surgery and adjuvant chemotherapy). Intra-cavity administration of TAT has been evaluated in recurrent malignant gliomas and ovarian cancer. The main advantages of this route of administration are the minimizing risk of side effects (e.g., myelotoxicity) and unexpected toxicity due to unforeseen microscopic off-target localization of the radiolabeled complex in the body. The radioactive decay of emitters characterized by a short half-life, such as 213Bi and 211At, mainly occurs within the cavity, before the distribution of the molecule in the body. Palliative treatment can be administrated using the intravenous route as for symptom relief of bone metastases, or the intra-cavity route as in case of peritoneal carcinomatosis. As mentioned, the inherent biological features of solid tumors are less favorable for radio-conjugate binding access than hematological malignancies.
Moreover, in the case of manifest macroscopic disease (5–10 mm), TAT efficacy might be suboptimal. However, there are some clinical indications such as melanoma and refractory non-Hodgkin lymphoma. The use of pre-targeting strategies might increase the potential efficacy of TAT. Table 2 summarizes the state of development of targeted alpha-particle therapy radiopharmaceuticals classified per clinical setting.
4.1 Leukemia and multiple myeloma
As above-mentioned myeloid-lymphoproliferative malignancies are more prone to RIT than solid tumors. Broadly, the accumulation of radiolabeled antibodies in the bone marrow occurring when the targeted antigen is expressed on healthy hematopoietic cells or even when the bone-marrow minimally involved made RIT a suitable treatment for myeloid-lymphoproliferative malignancies. Accordingly, RIT may be used to enhance the cytoreductive effect of the chemotherapy, to reduce the cancer load alone or in combination with chemotherapeutics, and to condition patients scheduled for transplantation (Behr et al. 2002; Richman et al. 2005; Nademanee et al. 2005).
Particularly, myeloablative RIT may fill the gap between benefit and toxicity of conditioning therapies. RIT when used to condition patients scheduled for transplantation, induces hypoplasia/aplasia in bone marrow, determining a low rate of relapse and toxicity since organs which not express the target are spared. Moreover, it does not cause a significant increase in therapy-related mortality (Buchmann et al. 2009).
The first clinical trial with 213Bi-HuM195 in patients with relapsed/refractory AML or CML demonstrated the safety, feasibility, and anti-leukemic effects of TAT (Jurcic et al. 2002). Based on the promising results of this preliminary study, safety and efficacy of 213Bi-HuM195 after partially cytoreductive chemotherapy with cytarabine were tested in 31 patients with newly diagnosed or relapsed/refractory AML within a phase I/II study. Marrow blasts significantly decreased at all dose levels (Rosenblat et al. 2010). 213Bi-anti-CD33, binding the CD33 antigen expressed by AML cells, exerted its cytotoxic effect inducing apoptosis. Similarly, 211At-anti-CD33 exhibited a high cytotoxic effect in CD33-positive AML cell lines. This evidence suggested the reactivation of the apoptotic pathways, being TAT a promising opportunity for patients with resistant AML untreatable by the conventional therapies (Petrich et al. 2010; Friesen et al. 2013).
A phase I/II clinical trial on 211At-BC8-B10 treatment before donor stem cell transplant in patients with high-risk AML, ALL, or myelodysplastic syndrome is currently ongoing ([NCT03670966]).
The 225Ac-HuM195 proved to have anti-leukemic activity in advanced AML patients regardless of the activity ([NCT00672165]; Baidoo et al. 2013), which is now being investigated combined to low-dose cytarabine in elderly AML patients within a multicenter phase I/II trial ([NCT02575963]). A combined treatment to reduce tumor burden in AML consisting of chemotherapy followed by α-RIT (213Bi- or 225Ac-immunoconjugates) has been recently completed [(Home—ClinicalTrials.gov. https://clinicaltrials.gov/. Accessed 8 Jan 2020)].
Some phase I/II study combining 225Ac-lintuzumab with the other agents is planned in refractory or relapsed AML ([NCT03932318]; [NCT03867682]; [NCT03441048]). Of note, preliminary data of 225Ac-lintuzumab in 40 older AML patients unfit for standard induction chemotherapy showed moderate efficacy. The study was closed based on the hypothesis that other AML therapies, will likely have better outcomes when used in combination or in settings where myelosuppression is expected (Atallah et al. 2019).
A phase I trial to evaluate 225Ac-lintuzumab as monotherapy in multiple refractory myelomas is currently ongoing ([NCT02998047]) as well-known typically multiple myeloma has lytic bone involvement. Therefore, the application of bone-seeking agents would result inappropriate. However, the osteoclastic activity may be altered by bisphosphonates and other anti-responsive agents, which potentially turn the newly deposited bone matrix into a target for bone-seeking radiopharmaceuticals (Humm et al. 2015). A phase I/II trial evaluating 223Ra-dichloride in combination with bortezomib and dexamethasone in the early relapsed multiple myeloma patients is currently ongoing ([NCT02928029]).
4.2 Lymphoma
RIT with 90Y-ibritumomab tiuxetan (Zevalin®) is a commercially available agent for relapsed or refractory, low-grade or transformed B cell non-Hodgkin lymphoma. However, other molecules and approaches are under evaluation to treat lymphoma patients. As above-mentioned, RIT can be used with a dual purpose—targets tumor cells and suppresses immunocompetent recipient cells—in allogeneic hematopoietic cell transplantation. RIT with beta-emitters has been successfully used for intensification of myeloablative conditioning before allogeneic hematopoietic cell transplantation. The advantages offered by alpha-emitters to target hemopoietic cells have been wildly proved (Bethge et al. 2006). Heeger et al. (Heeger et al. 2003) conducted a phase I with 213Bi-CHX-A″-anti-CD20 radioconjugate (up to 1640 MBq) in non-Hodgkin lymphoma patients with promising results (response in 20% of cases). An open-label phase I with BAY1862864, an anti-CD22 antibody linked to thorium-227, in patients with refractory/relapsed CD22-positive non-Hodgkin lymphoma is currently ongoing ([NCT02581878]).
4.3 Primary brain tumors
The majority of brain tumors, glioblastomas (GBM) in particular, appear as a single lesion that will locally recur. Accordingly, loco-regional application of bifunctional molecules consisting of a target domain (e.g., the transferrin receptor, the interleukin-4 and interleukin-13 receptors, the neurokinin type-1 receptors, or a mAb against tenascin-C) and of an effector domain (e.g., bacterial toxins or radioisotopes) has been developed as potentially effective treatment (Kneifel et al. 2006). TAT for its intrinsic properties might accurately irradiate of tumors, protecting from side effects neighboring functional or critical areas of the brain. Based on the evidence of the over-expression of the peptide, carrier substance P (SP) targets the neurokinin type-1 receptors by GBM cells (Morgenstern et al. 2014), Cordier et al. (Cordier et al. 2010) conducted an encouraging pilot study in five newly diagnosed glioma patients treated with 213Bi-SP, providing the proof-of-concept for 213Bi-SP application in gliomas. These preliminary results were confirmed in 20 recurrent GBM patients treated with 213Bi-SPin. Intracavitary or intratumoral treatment resulted in safe, causing mild transient adverse reactions. 213Bi-SP improved patient’s median survival compared to reoperated patients (7.5 months versus < 6 months), resulting in a better outcome when repeated (up to 11.2 GBq) (Morgenstern et al. 2014; Królicki et al. 2019). Zalutsky et al. (Zalutsky et al. 2008) reported their experience with 211At-ch81C6 in 18 patients with recurrent malignant brain tumors. Astatine-211-ch81C6 was administered into a surgically created resection cavity, and after TAT patients received salvage chemotherapy. Treatment resulted feasible, safe, and with promising antitumor activity, providing the proof-of-concept for local TAT.
4.4 Melanoma
Successful treatment of melanoma remains a challenge in oncology. Therefore, several attempts have been made in drug development research to discover active molecules to improve patients’ outcome. Notably, in RIT, several efforts and strategies have been set up to find out novel targets, to develop novel ligands, bifunctional chelators, and forms of mAbs with suitable binding and pharmacokinetics properties, to test different administration strategies including pre-targeting and “cocktail” approaches (i.e., the mix of different radioisotopes with a variety of emission types and half-lives). Melanoma was thought to be relatively resistant to radiation, but the use of external beam radiotherapy as palliative treatment in metastatic cases has been proved to have particular effectiveness to control the disease locally. This evidence suggested that melanoma cells have peculiar radiobiology being more susceptible when higher radiation dose per fraction regimens has been applied, and supported the use of α-RIT (high LET in a short-range) in melanoma (Contessa and Roberson 2006).
The mAb anti-NG2 IgG (9.2.27 mAb) radiolabeled with 213Bi has been proved to be safe and effective in stage IV melanoma patients. Cell deaths occurred 2 weeks after intralesional treatment as confirmed by apoptosis, tumor debris, and both Ki67 and serum marker melanoma-inhibitory-activity (MIA) protein decrease (Allen et al. 2005). The maximum tolerance dose was not achieved, and no adverse events of any type or level were observed when TAT was intravenously administrated. Treatment determined stable disease or partial response in half of the patients (40% and 10%, respectively) at 8 weeks, resulting in an independent prognostic factor for survival (Raja et al. 2007; Allen et al. 2011).
4.5 Prostate cancer
Prostate-specific membrane antigen (PSMA) also named glutamate carboxypeptidase II (GCPII), N-acetyl-l-aspartyl-l-glutamate peptidase I (NAALADase I) or N-acetyl-aspartyl-glutamate (NAAG) peptidase, is an enzyme encoded by the folate hydrolase (FOLH1) gene (O’Keefe et al. 1998). The N-terminal cytoplasmic tail of PSMA interacts with membrane scaffold proteins that control the endocytosis of some molecules such as filamin A. The central portion of PSMA is represented by the extracellular component which included three domains—protease, apical, and C-terminal domain or dimerization domain—responsible for the substrate/ligand recognition (Bařinka et al. 2012; Evans et al. 2016). PSMA has different functions, and it is highly expressed by many tissues, including the prostatic one (Evans et al. 2016). Particularly, prostate cancer (PCa) overexpressed PSMA at higher levels than normal or hyperplastic prostates (Horoszkiewicz et al. 1987). In PCa, androgens act as PSMA repressor, while the other growth factors such as basic fibroblast growth factor, act positively (Israeli et al. 1993; Evans et al. 2016). The exact role of PSMA in tumors, including PCa, is not explored yet, but it has been reported to be associated with cancer progression and invasion (Yao et al. 2008). PSMA is rapidly internalized and recycled by positive cells. Its overexpression determines an increase in folate processing. Based on its characteristics, PSMA has been explored as a target agent to imaging and treat PCa (Behnam Azad et al. 2015; Evans et al. 2016). Preclinical data of radiolabeled PSMA showed, as mentioned above, that there was a high level of target-specific uptake in malignant PCa cells. These data were confirmed in vivo using both animal models and human tissues (Evans et al. 2016). Moreover, PSMA levels are related to Gleason score and disease progression (i.e., the more aggressive the disease and the more advanced stage, the higher the PSMA level). As for the unbound form of the molecule, also radiolabeled PSMA is internalized by positive cells (Hadaschik and Boegemann 2017). Therefore, the possibility to deliver cytotoxic radiation to a specific target makes PSMA a suitable and attractive molecule for RIT. RIT with PSMA radiolabeled with beta emitters (125/131I, 177Lu, and 90Y) determined a reduction of tumor volume or a delayed tumor growth in preclinical PCa animal models (Vallabhajosula et al. 2004; Behe et al. 2011; Gao et al. 2014; Kiess et al. 2015; Evans et al. 2016; Hadaschik and Boegemann 2017). In men, RIT with PSMA radiolabeled with beta emitters showed biochemical or radiological response associated with clinical benefit (e.g., pain relief) in the majority of cases (Bander et al. 2005; Vallabhajosula et al. 2005; Tagawa et al. 2013; Zechmann et al. 2014; Ahmadzadehfar et al. 2015, 2017; Kratochwil et al. 2015; Rahbar et al. 2017), even if about one-third of treated patients did not respond despite the in vivo demonstration of PSMA overexpression by 68Ga-PSMA PET/CT (Hadaschik and Boegemann 2017). In this regard, TAT might be used as a strategy to overcome the primary radio-resistance to ß-RIT and to reduce toxicity (Hadaschik and Boegemann 2017). It is worth mentioning that clinical trials with 225Ac-PSMA may have an impact on other clinical development activities featuring the use of TAT also in the other clinical settings. First-in-human retrospective experience with 225Ac-PSMA has been recently reported (Kratochwil et al. 2016, 2017). They treated 14 end-stage castration-resistant PCa patients with empirically dose-escalation activity of 225Ac-PSMA. The treatment schedule was defined on individual decisions resulting incomparable in terms of timing, number of cycles, and administered activities. Nonetheless, this experience suggested a potential benefit for castration-resistant PCa patients treated with 225Ac-PSMA. The same group reported a remarkably clinical antitumor activity (PSA response was used as surrogate endpoint) of 225Ac-PSMA (3 cycles 100 kBq/kg at 2-month interval) in metastatic castration-resistant PCa (Kratochwil et al. 2018). More recently, results of pilot experience in 20 metastatic castration-resistant PCa treated with 225Ac-PSMA as part of tandem therapy (combination with 177Lu-PSMA) have been published, suggesting that TAT powered the efficacy of single-agent PSMA-targeted radioligand therapy (Khreish et al. 2019). A phase I trial with 227Th-BAY 2315497 is currently ongoing in patients with metastatic castration-resistant PCa (Hammer et al. 2019). Overall than 90% of metastatic PCa had bone involvement, and typically, bone metastases from PCa are related to blastic new osseous formation, making them especially amenable to targeted therapies with bone-seeking agents such as 223Ra-dichloride (Lange and Vessella 1999; Bourgeois et al. 2011). Starting from the hypothesis that 223Ra-dichloride would localize to the bone and would spare the bone marrow, a phase I clinical trial was begun in breast and prostate cancers. Patients experienced pain relief without toxicity, and serum alkaline phosphatase levels decreased, providing the evidence of treatment effectiveness (Nilsson et al. 2005; Humm et al. 2015). These results were confirmed in symptomatic metastatic castration-resistant PCa by phase II and III trials (Nilsson et al. 2013; Parker et al. 2013a, b; Sartor et al. 2014). Notably, improved overall survival (14.9 months versus 11.3 months), low myelosuppression rates, and a few adverse events have been reported in patients treated with 223Ra-dichloride within the randomized, double-blind, placebo-controlled “ALSYMPCA” trial (Parker et al. 2013a). Moreover, phase IIIb study showed that patients with more advanced metastatic castration-resistant PCa were more unlikely to complete 223Ra-dichloride treatment (i.e., fewer cycles), and showed worst outcome compared to patients with less advance disease (Saad et al. 2019; Heidenreich et al. 2019). Treatment with 223Ra-dichloride induced variable pain control. The first or the second infusion(s) of the usual six planned administrations may result in a prompt and permanent pain relief in the majority of lesions even if, sometimes it may be incomplete or shorter. In the latter case, attention should be paid to possible other or concurrent causes of pain, including neuropathic pain, suboptimal analgesic control, or even no-related to the tumor. However, when the pattern of pain changes (pain-flare after a subside phase or different location), new metastatic localizations should be ruled out (Humm et al. 2015). In 2013, 223Ra-dichloride received marketing approval (Xofigo®) by both the U.S. Food and Drug Administration and the European Medicine Agency (EMA) for the treatment of adults with castration-resistant PCa, symptomatic bone metastases, and no known visceral metastases. However, in 2018, the EMA restricted its use after docetaxel, and at least one androgen receptor-targeted agent or in case of patients without the other therapeutic options (Medicines Agency 2018). This restrictive action was the effect of the significant safety risks (fractures and deaths) observed within the phase III trial, which combined 223Ra-dichloride to abiraterone plus steroids. Currently, the European Association of Urology Guidelines on prostate cancer recommends the 223Ra-dichloride as a life-prolonging drug for metastatic castration-resistant prostate cancer (Mottet et al. 2020). Some clinical trials evaluating the association of 223Ra-dichloride with other drugs (s) or interventions (e.g., olaparib, enzalutamide, sipuleucel-T, pembrolizumab, and radiotherapy) are currently ongoing ([Search of: radium-223|Recruiting, Active, not recruiting Studies|Interventional Studies|Prostate Cancer—List Results—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?term=radium-223&cond=Prostate+Cancer&recrs=a&recrs=d&age_v=&gndr=&type=Intr&rslt=&Search=Apply. Accessed 7 Jan 2020]).
4.6 Renal cell carcinoma
Metastatic renal cell carcinoma has limited therapeutic options. It frequently presents bone involvement and bone metastases are associated with worse survival. Data on 223Ra-dichloride effectiveness in PCa supported an exploratory phase I which combined 223Ra-dichloride (55 kBq/kg every 4 weeks × 6 cycles) to vascular endothelial growth factor targeted therapy (sorafenib or pazopanib) in renal cell carcinoma patients with bone metastases ([NCT02406521]). Preliminary results in 12 patients suggested that treatment with 223Ra-dichloride plus sorafenib was safe and effective on bone turnover markers (McKay et al. 2017).
4.7 Breast cancer
Data on 223Ra-dichloride in PCa support its use also in the other cancers with bone metastases with blastic characteristics (Coleman 2016). An open-label, phase IIa nonrandomized study investigated the effects of 223Ra-dichloride (50 kBq/kg IV every 4 weeks for four cycles) in 23 patients with advanced breast cancer and progressive bone-dominant disease no longer amenable to endocrine treatment. 223Ra-dichloride determined a significant reduction of disease biomarkers associated with a metabolic response in more than one-third of lesions (32% at week 9 after two injections, and 41% at week 17 upon completion of treatment). Treatment was safe and well tolerated (Coleman et al. 2014). 223Ra-dichloride is under evaluation within three international phase II trials focused on bone predominant metastatic breast cancer either hormone-positive (223Ra-dichloride + hormonal treatment + denosumab ([NCT02366130])) or hormone-positive HER2 negative (223Ra-dichloride or placebo + hormonal treatment ([NCT02258464]), or exemestane + everolimus ([NCT02258451])).
4.8 Ovarian cancer
The majority of ovarian cancer cases are diagnosed when carcinomatosis is already present. Surgery followed by the conventional adjuvant chemotherapy results commonly in complete response. Nonetheless, the disease will recur in approximately half of cases. Intraperitoneal chemotherapy and radionuclides have shown to be effective, improving survival or decreasing abdominal failure (Armstrong et al. 2006; Verheijen et al. 2006). Previous experience with intraperitoneal radionuclide treatment suggested that some benefits (higher activity without excess in toxicity) could be obtained from an isotope with a shorter half-life and fewer γ emissions than β-emitter commonly used for RIT (Macey and Meredith 1999). Experiments in mouse ovarian cancer model showed the therapeutic potential of trastuzumab, MOv18 (mAb targeting cell membrane glycoprotein), and MX35 labeled with α-emitter (Andersson et al. 2000; Elgqvist et al. 2006; Palm et al. 2007). Data obtained in the first 3 HER-2 positive ovarian cancer patients treated with intraperitoneal 212Pb-TCMC-trastuzumab within a phase I trial, were consistent with biodistribution and safety results obtained in preclinical experiments ([NCT02258451]; Meredith et al. 2014b). Overall, 16 patients (15 recurrent ovarian cancer and one colorectal cancer) were treated with minimal toxicity drug-related, which was expected, according to dosimetry (Meredith et al. 2014a). Intraperitoneal 211At-MX35 F(ab′)2 as adjuvant treatment for the microscopic disease was safety administered in nine patients in complete clinical remission after second-line chemotherapy for recurrent ovarian carcinoma (Andersson et al. 2009; Cederkrantz et al. 2015).
4.9 Osteosarcoma
Osteosarcoma is a primary bone tumor in which spindle cells produce osteoid. Accordingly, osteosarcoma, as osteoblastic metastases from PCa and breast tumors, is suitable for bone-seeking agents treatment. The positive clinical therapeutic experience based on β-emitting radiopharmaceuticals opens the possibility for the use of 223Ra-dichloride in selected osteosarcoma patients (Humm et al. 2015). A phase I/II dose trial is active in osteosarcoma ([NCT01833520]).
4.10 Neuroendocrine tumors
Peptide radioreceptor therapy (PRRT) with somatostatin analogues labeled with beta-emitters is an effective treatment for NETs (Zaknun et al. 2013; Hicks et al. 2017). However, some patients are resistant to PRRT. As abovementioned, α-emitters may overcome resistance to β-emitters (Friesen et al. 2007), and that both 225Ac-DOTATOC and 213Bi-DOTATOC have shown promising antitumor effects in preclinical studies (Norenberg 2006; Miederer et al. 2008). Kratochwil et al. (Kratochwil et al. 2014) reported the first-in-human experience with 213Bi-DOTATOC in eight patients refractory to 90Y/177Lu-DOATOC with progressive advanced neuroendocrine liver metastases. Treatment was administered by intra-arterial (n = 7) or systemic (n = 1) infusion (up to 5 cycles with a cumulative activity of 3.3–20.8 GBq). Six patients were evaluable for treatment response which resulted in a complete response (> 28 months, ongoing), two partial responses (up to 17 months), and three stable diseases (up to > 31 months, ongoing). Acute hematological toxicity was low. Three patients developed chronic anemia. Twenty-four months after TAT (5 years after the first administration of 90Y-DOTATOC), one patient developed a myelodysplastic syndrome that progressed 6 months after into acute myeloid leukemia, and the patient died. Recently, short-term results of a prospective study recruiting patients with metastatic gastroenteropancreatic neuroendocrine tumors stable or progressive on 177Lu-DOTATATE therapy showed partial response or stable disease (24/32 and 9/32, respectively) in all cases after 225Ac-DOTATATE treatment (100 kBq/kg body weight, 1–5 cycles at 8 weeks) (Ballal et al. 2019).
5 How? New technologies and targets
Targeted therapies delivery their cytocidal effect after the binding between the vector and its target. Improvements made in genetic engineering result in the development of several vectors suitable for RIT. Nonetheless, the suboptimal pharmacokinetic properties of these molecules often hamper treatments. As above-mentioned, an accurate trade-off between tumor uptake and clearance is crucial. In this regard, fast clearance limits the organs irradiation. However, too rapid clearance may affect the targeting time. In turn, a targeting time too short could deliver an insufficient adsorbed dose to target cells. Pre-targeted strategies may be used to solve the pharmacokinetic challenge. This approach physically and temporally separates the distribution phase from the delivery one. This approach requires first the administration of a pre-targeting molecule which should be followed by sufficient time for the antigen-site binding. Normal organs are not affected by this binding phase, since the pre-targeting molecule is free from any cytotoxic substance. Before the injection of the radiolabeled vector, an agent is used to remove from the circulation of the unbound molecule (effector molecule). The effector molecule is specifically designed to bind to the antigen-associated pre-targeting molecules and diffuse more in-depth into the tumor. The rapid removal of circulating unbound effector molecule is in favor of a higher tumor-to-normal tissue ratio. Pre-targeting does not require a compromise between efficient binding/diffusion/tumor residence time and protection of dose-limiting normal tissues (Elgqvist et al. 2014). Examples of pre-targeted strategies are based on streptavidin–biotin (Lesch et al. 2010) or bispecific antibodies (Chang et al. 2002). 211At and 213Bi having some favorable properties (e.g., availability, daughter nuclides) has emerged as most promising α-emitters candidates. Indeed, even if their half-life could be too short of ensuring a sufficient targeting time, a pre-targeting strategy may be successfully used to increase the therapeutic index, overcoming the issue related to these short-lived α-particle emitters (Elgqvist et al. 2014). The positive effects of pre-targeted strategies on TAT have been reported in preclinical studies, mainly focused on hematological malignancies (Zhang et al. 2003; Park et al. 2010; Pagel et al. 2011). The advances in engineering lead to a growing interest in the use of nanobodies—the smallest, antigen-binding fragments from unique heavy-chain-only antibodies naturally occurring in Camelidae (Hamers-Casterman et al. 1993)—as vectors for RIT. Nanobodies have some favorable properties, including high antigen affinity and specificity, facile production, and high stability even in harsh conditions (e.g., high temperatures and extreme pHs), which offer the possibility to use a broader range of radiochemistry methods. Nanobodies being extremely specific for the antigen regardless of the isotope used for labeling are suitable vehicles for both nuclear imaging and RIT. Moreover, nanobodies have certain advantages over mAbs such as a lower molecular weight (15 kDa versus 150 kDa), lower immunogenicity, and a more efficient penetration in the tumor, which results in a rapid site binding (Dekempeneer et al. 2016). The limited unspecific binding to non-target tissues together with the fast clearance of nanobodies determines an early high tumor-to-background ratio (as early as 1 h after injection). Accordingly, nanobodies might be the solution to the off-target toxicity related to the long-lasting blood circulation of mAb (Dekempeneer et al. 2016). A variety of labeled nanobodies directed against membrane-bound biomarkers have been developed to image by SPECT or PET different conditions in animal models (Vaneycken et al. 2010, 2011; D’Huyvetter et al. 2014; Dekempeneer et al. 2016). Moreover, a promising experience of anti-HER2 nanobodies labeling with Astatine-211 has recently been reported in an animal model (Dekempeneer et al. 2019). Recoiling daughter nuclides may be an issue if the α-particles are not retained at the target site, since they may harm to healthy tissues. Some strategies may be applied to limit this phenomenon, including the encapsulation of α-particles in nanocarriers, or the development of multivalent forms rapidly internalized inside the target cells. Polymersomes proved to be safe carriers to encapsulate 225Ac, but they resulted inadequate to retain recoiling daughters isotopes which were significantly re-distributed throughout the body (Kruijff et al. 2019). Multivalent nanobody forms—differently from the monovalent counterparts which are characterized by a low level of internalization—may be used to augment the number of α-particles trapped inside target cells (Oliveira et al. 2010; Dekempeneer et al. 2016). Innovative approaches for micro- and small-scale dosimetry are required to calculate reliable estimates of α-emitters used in TAT. The recent development of techniques to image α-particle deposits at a cellular level is emerging among novel dosimetric methods. Moreover, the recent identification of mutations in some damage-repair associated genes in patients resistant to TAT paves the way to test combined approach with DNA-damage-repair targeting agents such as Poly(ADP-ribose)–Polymerase inhibitors (Kratochwil et al. 2019). A new strategy for α-treatment is the use of implantable seeds embedded with a low activity of radium-224. This strategy, called DaRT (Diffusing Alpha-emitters Radiation Therapy), does not target a binding site, but represents a novel alpha-emitting brachytherapy. It has been investigated in some solid tumors, including skin and head and neck cancers with promising results in terms of efficacy and safety (Popovtzer et al. 2019).
6 Conclusions
In conclusion, α-emitting isotopes are characterized by physical properties that render them extremely potent cell-killing agents. Progress in gene engineering allowed the creation of novel targeting vehicles, and characterized by high specificity and favorable pharmacokinetics. The possibility to label highly specific carriers with such radionuclides offers multiple possibilities of combinations to tailor the treatment to a specific disease. Optimal biodistribution, toxicity profile, dosimetry, and vectors design are challenges, that researchers will face in the next years. Nonetheless, there is growing evidence on the effectiveness of TAT in many oncological diseases that constitute a significant cause of morbidity and mortality worldwide.
References
https://clinicaltrials.gov/ct2/results?term=alpharadin&cond=“Prostatic+Neoplasms”. Accessed 6 Jan 2020
Study of Radium-223 dichloride versus placebo and hormonal treatment as background therapy in subjects with bone predominant her2 (human epidermal growth factor receptor 2) negative hormone receptor positive metastatic breast cancer. https://clinicaltrials.gov/ct2/show/NCT02258464. Accessed 30 Dec 2019
Lintuzumab-Ac225 in Older Acute Myeloid Leukemia (AML) Patients. https://clinicaltrials.gov/ct2/show/NCT02575963. Accessed 16 Dec 2019
Study Testing Radium-223 Dichloride in Relapsed Multiple Myeloma. https://clinicaltrials.gov/ct2/show/NCT02928029. Accessed 16 Dec 2019
Phase I Dose Escalation of Monthly Intravenous Ra-223 Dichloride in Osteosarcoma. https://clinicaltrials.gov/ct2/show/record/NCT01833520?term=NCT01833520. Accessed 9 Jan 2020
A Phase I Study of Lintuzumab-Ac225 in Patients With Refractory Multiple Myeloma. https://clinicaltrials.gov/ct2/show/record/NCT02998047. Accessed 9 Dec 2019
Targeted Atomic Nano-Generators (Actinium-225-Labeled Humanized Anti-CD33 Monoclonal Antibody HuM195) in Patients With Advanced Myeloid Malignancies. https://clinicaltrials.gov/show/NCT00672165. Accessed 9 Dec 2019
211At-BC8-B10 Before donor stem cell transplant in treating patients with high-risk acute myeloid leukemia, acute lymphoblastic leukemia, myelodysplastic syndrome, or mixed-phenotype acute leukemia. https://clinicaltrials.gov/ct2/show/NCT03128034. Accessed 23 Dec 2019
211At-BC8-B10 and donor stem cell transplant in treating relapsed or refractory AML, ALL, or myelodysplastic syndrome. https://clinicaltrials.gov/ct2/show/NCT03670966?term=astatine&cond=Leukemia&draw=2&rank=2. Accessed 14 Jan 2020
A first in human study of BAY2701439 to look at safety, how the body absorbs, distributes and excretes the drug, and how well the drug works in participants with advanced cancer expressing the HER2 protein. https://clinicaltrials.gov/ct2/show/NCT04147819?term=alpha-particle&draw=2&rank=11. Accessed 18 Dec 2019
A Phase 1 study of [225Ac]-FPI-1434 injection. https://clinicaltrials.gov/ct2/show/NCT03746431?term=actinium&draw=2&rank=8. Accessed 14 Jan 2020
A study of radium-223 in combination with tasquinimod in bone-only metastatic castration-resistant prostate cancer. https://clinicaltrials.gov/ct2/show/NCT02396368?term=alpha-particle&draw=2&rank=18. Accessed 18 Dec 2019
Ahmadzadehfar H, Rahbar K, Kürpig S et al (2015) Early side effects and first results of radioligand therapy with 177Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res 5:36. https://doi.org/10.1186/s13550-015-0114-2
Ahmadzadehfar H, Zimbelmann S, Yordanova A et al (2017) Radioligand therapy of metastatic prostate cancer using 177Lu-PSMA-617 after radiation exposure to 223Ra-dichloride. Oncotarget 8:6–7. https://doi.org/10.18632/oncotarget.15698
Allan BJ (2013) Systemic targeted alpha radiotherapy for cancer. J Biomed Phys Eng 3:67–80
Allen BJ (2012) Systemic targeted alpha radiotherapy for cancer. J Proc R Soc New South Wales 145:19–33. https://doi.org/10.1358/dnp.2008.21.6.1246832.disrupting
Allen BJ, Raja C, Rizvi S et al (2005) Intralesional targeted alpha therapy for metastatic melanoma. Cancer Biol Ther 4:1318–1324
Allen BJ, Singla AA, Rizvi SMA et al (2011) Analysis of patient survival in a Phase I trial of systemic targeted α-therapy for metastatic melanoma. Immunotherapy 3:1041–1050. https://doi.org/10.2217/imt.11.97
Andersson H, Lindegren S, Bäck T et al (2000) The curative and palliative potential of the monoclonal antibody MOv18 labelled with 211At in nude mice with intraperitoneally growing ovarian cancer xenografts—a long-term study. Acta Oncol 39:741–745. https://doi.org/10.1080/028418600750063820
Andersson H, Cederkrantz E, Back T et al (2009) Intraperitoneal-particle radioimmunotherapy of ovarian cancer patients: pharmacokinetics and dosimetry of 211At-MX35 F(ab’)2—a phase I study. J Nucl Med 50:1153–1160. https://doi.org/10.2967/jnumed.109.062604
Armstrong DK, Bundy B, Wenzel L et al (2006) Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 354:34–43. https://doi.org/10.1056/nejmoa052985
Atallah E, Berger M, Jurcic J et al (2019) A phase 2 study of actinium-225 (225Ac)-lintuzumab in older patients with untreated acute myeloid leukemia (AML). J Med Imaging Radiat Sci 50:S37. https://doi.org/10.1016/j.jmir.2019.03.113
Baidoo KE, Yong K, Brechbiel MW (2013) Molecular pathways: targeted α-particle radiation therapy. Clin Cancer Res 19:530–537. https://doi.org/10.1158/1078-0432.ccr-12-0298
Ballal S, Yadav MP, Bal C et al (2019) Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: first clinical experience on the efficacy and safety. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-019-04567-2
Bander N, Milosky M, Nanus D et al (2005) Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol 23:4591–4601. https://doi.org/10.1200/jco
Bařinka C, Rojas C, Slusher B, Pomper M (2012) Glutamate carboxypeptidase II in diagnosis and treatment of neurologic disorders and prostate cancer. Curr Med Chem 19:856–870. https://doi.org/10.2174/092986712799034888
Behe M, Alt K, Deininger F et al (2011) In vivo testing of 177Lu-labelled anti-PSMA antibody as a new radioimmunotherapeutic agent against prostate cancer. In Vivo (Brooklyn) 25:55–59
Behnam Azad B, Banerjee SR, Pullambhatla M et al (2015) Evaluation of a PSMA-targeted BNF nanoparticle construct. Nanoscale 7:4432–4442. https://doi.org/10.1039/c4nr06069e
Behr TM, Griesinger F, Riggert J et al (2002) High-dose myeloablative radioimmunotherapy of mantle cell non-Hodgkin lymphoma with the iodine-131-labeled chimeric anti-CD20 antibody C2B8 and autologous stem cell support. Results of a pilot study. Cancer 94:1363–1372. https://doi.org/10.1002/cncr.10307
Bethge WA, Wilbur DS, Sandmaier BM (2006) Radioimmunotherapy as non-myeloablative conditioning for allogeneic marrow transplantation. Leuk Lymphoma 47:1205–1214. https://doi.org/10.1080/00423110500485822
Bourgeois DJ, Kraus S, Maaloof BN, Sartor O (2011) Radiation for bone metastases. Curr Opin Support Palliat Care 5:227–232. https://doi.org/10.1097/spc.0b013e3283499caa
Buchmann I, Meyer RG, Mier W, Haberkorn U (2009) Myeloablative radioimmunotherapy in conditioning prior to haematological stem cell transplantation: closing the gap between benefit and toxicity? Eur J Nucl Med Mol Imaging 36:484–498. https://doi.org/10.1007/s00259-008-0996-6
Cederkrantz E, Andersson H, Bernhardt P et al (2015) Absorbed doses and risk estimates of 211At-MX35 F(ab′) < inf > 2</inf > in intraperitoneal therapy of ovarian cancer patients. Int J Radiat Oncol Biol Phys 93:569–576. https://doi.org/10.1016/j.ijrobp.2015.07.005
Chang C, Sharkey RM, Rossi EA et al (2002) Molecular advances in pretargeting radioimunotherapy with bispecific antibodies. Mol Cancer Ther 1:553–563
Coleman R (2016) Treatment of metastatic bone disease and the emerging role of radium-223. Semin Nucl Med 46:99–104. https://doi.org/10.1053/j.semnuclmed.2015.10.012
Coleman R, Aksnes AK, Naume B et al (2014) A phase IIa, nonrandomized study of radium-223 dichloride in advanced breast cancer patients with bone-dominant disease. Breast Cancer Res Treat 145:411–418. https://doi.org/10.1007/s10549-014-2939-1
Contessa JN, Roberson PL (2006) Targeted radiation for melanoma: alpha therapy prepares for a beta test. Cancer Biol Ther 5:118–119. https://doi.org/10.4161/cbt.5.1.2401
Cordier D, Forrer F, Bruchertseifer F et al (2010) Targeted alpha-radionuclide therapy of functionally critically located gliomas with 213Bi-DOTA-[Thi8, Met(O2)11]- substance P: a pilot trial. Eur J Nucl Med Mol Imaging 37:1335–1344. https://doi.org/10.1007/s00259-010-1385-5
Dadwal M, Kang CS, Song HA et al (2011) Synthesis and evaluation of a bifunctional chelate for development of Bi(III)-labeled radioimmunoconjugates. Bioorg Med Chem Lett 21:7513–7515. https://doi.org/10.1016/j.bmcl.2011.06.107.synthesis
Data explorer|ECIS. https://ecis.jrc.ec.europa.eu/explorer.php?$0-0$1-AE28$4-1,2$3-All$6-0,14$5-2008,2008$7-8$CEstByCancer$X0_8-3$CEstRelativeCanc$X1_8-3$X1_9-AE28. Accessed 6 Dec 2019
de Kruijff RM, Raavé R, Kip A et al (2019) The in vivo fate of 225Ac daughter nuclides using polymersomes as a model carrier. Sci Rep 9:11671. https://doi.org/10.1038/s41598-019-48298-8
Dekempeneer Y, Keyaerts M, Krasniqi A et al (2016) Targeted alpha therapy using short-lived alpha-particles and the promise of nanobodies as targeting vehicle. Expert Opin Biol Ther 16:1035–1047. https://doi.org/10.1080/14712598.2016.1185412
Dekempeneer Y, Bäck T, Aneheim E et al (2019) Labeling of anti-HER2 nanobodies with astatine-211: optimization and the effect of different coupling reagents on their in vivo behavior. Mol Pharm 16:3524–3533. https://doi.org/10.1021/acs.molpharmaceut.9b00354
D’Huyvetter M, Xavier C, Caveliers V et al (2014) Radiolabeled nanobodies as theranostic tools in targeted radionuclide therapy of cancer. Expert Opin Drug Deliv 131:1–16. https://doi.org/10.1517/17425247.2014.941803
Elgqvist J, Andersson H, Bäck T et al (2006) Fractionated radioimmunotherapy of intraperitoneally growing ovarian cancer in nude mice with 211At-MX35 F(ab′)2: therapeutic efficacy and myelotoxicity. Nucl Med Biol 33:1065–1072. https://doi.org/10.1016/j.nucmedbio.2006.07.009
Elgqvist J, Frost S, Pouget J-P, Albertsson P (2014) The potential and hurdles of targeted alpha therapy—clinical trials and beyond. Front Oncol 3:324. https://doi.org/10.3389/fonc.2013.00324
Enzalutamide with or without radium Ra 223 dichloride in patients with metastatic, castration-resistant prostate cancer. https://clinicaltrials.gov/ct2/show/NCT03344211?term=alpha-particle&draw=2&rank=13. Accessed 18 Dec 2019
European Commission (2014) Council Directive 2013/59/Euratom of 5 December 2013. Off J Eur Union. https://doi.org/10.3000/19770677.l_2013.124.eng
Evans JC, Malhotra M, Cryan JF, O’Driscoll CM (2016) The therapeutic and diagnostic potential of the prostate specific membrane antigen/glutamate carboxypeptidase II (PSMA/GCPII) in cancer and neurological disease. Br J Pharmacol. https://doi.org/10.1111/bph.13576
Exploratory study of radium-223 and VEGF-targeted therapy in patients with metastatic renal cell carcinoma and bone mets. https://clinicaltrials.gov/show/NCT02406521
First-in-human study of BAY2287411 injection, a thorium-227 labeled antibody-chelator conjugate, in patients with tumors known to express mesothelin. https://clinicaltrials.gov/ct2/show/NCT03507452. Accessed 9 Jan 2020
Friesen C, Glatting G, Koop B et al (2007) Breaking chemoresistance and radioresistance with [213Bi]anti- CD45 antibodies in leukemia cells. Cancer Res 67:1950–1958. https://doi.org/10.1158/0008-5472.can-06-3569
Friesen C, Roscher M, Hormann I et al (2013) Anti-CD33-antibodies labelled with the alpha-emitter Bismuth-213 kill CD33-positive acute myeloid leukaemia cells specifically by activation of caspases and break radio- and chemoresistance by inhibition of the anti-apoptotic proteins X-linked inhibitor o. Eur J Cancer 49:2542–2554. https://doi.org/10.1016/j.ejca.2013.04.008
Gao X-F, Zhou T, Chen G-H et al (2014) Radioiodine therapy for castration-resistant prostate cancer following prostate-specific membrane antigen promoter-mediated transfer of the human sodium iodide symporter. Asian J Androl 16:120–123. https://doi.org/10.4103/1008-682x.122354
Gudkov SV, Shilyagina NY, Vodeneev VA, Zvyagin AV (2016) Targeted radionuclide therapy of human tumors. Int J Mol Sci 17:33. https://doi.org/10.3390/ijms17010033
Hadaschik B, Boegemann M (2017) Why targeting PSMA is a valuable addition in the management of castration-resistant prostate cancer: the Urologists’ point of view. J Nucl Med. https://doi.org/10.2967/jnumed.117.194753
Hamers-Casterman C, Atarhouch T, Muyldermans S et al (1993) Naturally occurring antibodies devoid of light chains. Nature 363:446–448. https://doi.org/10.1038/363446a0
Hammer S, Hagemann UB, Zitzmann-Kolbe S et al (2019) Preclinical efficacy of a PSMA-targeted thorium-227 conjugate (PSMA-TTC), a targeted alpha therapy for prostate cancer. Clin Cancer Res Clin 2268:2019. https://doi.org/10.1158/1078-0432.ccr-19-2268
Heeger S, Moldenhauer G, Egerer G et al (2003) Alpha-radioimmunotherapy of B-lineage non-Hodgkin’s lymphoma using 213Bi-labelled anti-CD19-and anti-CD20-CHX-A″-DTPA conjugates. Abstr Pap Am Chem Soc 225:U261
Heidenreich A, Gillessen S, Heinrich D et al (2019) Radium-223 in asymptomatic patients with castration-resistant prostate cancer and bone metastases treated in an international early access program. BMC Cancer 19:12. https://doi.org/10.1186/s12885-018-5203-y
Hicks RJ, Kwekkeboom DJ, Krenning E et al (2017) ENETS consensus guidelines for the standards of care in neuroendocrine neoplasia: peptide receptor radionuclide therapy with radiolabeled somatostatin analogues. Neuroendocrinology 105:295–309. https://doi.org/10.1159/000475526
Home-ClinicalTrials.gov. https://clinicaltrials.gov/. Accessed 8 Jan 2020
Horoszkiewicz J, Kawinski E, Murphy G (1987) Monoclonal antibodies to a new antigenic market in epithelial cells and serum of prostatic cancer patients. Anticancer Res 7:927–936
Humm JL, Sartor O, Parker C et al (2015) Radium-223 in the treatment of osteoblastic metastases: a critical clinical review. Int J Radiat Oncol Biol Phys 91:898–906. https://doi.org/10.1016/j.ijrobp.2014.12.061
Israeli RS, Powell CT, Fair WR, Heston WD (1993) Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res 53:227–230
Jurcic JG, Larson SM, Sgouros G et al (2002) Targeted alpha particle immunotherapy for myeloid leukemia. Blood 100:1233–1239
Kassis A (2008) Therapeutic radionuclides: biophysical and radiobiologic principles. Semin Nucl Med 38:358–366. https://doi.org/10.1053/j.semnuclmed.2008.05.002.therapeutic
Kellerer AM, Chmelevsky D (1975) Concepts of microdosimetry. I. Quantities. Radiat Environ Biophys 12:61–69. https://doi.org/10.1007/bf02339810
Khreish F, Ebert N, Ries M et al (2019) 225Ac-PSMA-617/177Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: pilot experience. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-019-04612-0
Kiess AP, Minn I, Chen Y et al (2015) Auger radiopharmaceutical therapy targeting prostate-specific membrane antigen. J Nucl Med 56:1401–1407. https://doi.org/10.2967/jnumed.115.155929
Kneifel S, Cordier D, Good S et al (2006) Local targeting of malignant gliomas by the diffusible peptidic. Clin Cancer Res 12:3843–3850. https://doi.org/10.1158/1078-0432.CCR-05-2820
Kratochwil C, Giesel FL, Bruchertseifer F et al (2014) 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. Eur J Nucl Med Mol Imaging 41:2106–2119. https://doi.org/10.1007/s00259-014-2857-9
Kratochwil C, Giesel FL, Eder M et al (2015) [177Lu]Lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. Eur J Nucl Med Mol Imaging 42:987–988. https://doi.org/10.1007/s00259-014-2978-1
Kratochwil C, Bruchertseifer F, Giesel FL et al (2016) 225Ac-PSMA-617 for PSMA-targeted -radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med 57:1941–1944. https://doi.org/10.2967/jnumed.116.178673
Kratochwil C, Bruchertseifer F, Rathke H et al (2017) Targeted Alpha Therapy of mCRPC with 225 actinium-PSMA-617: dosimetry estimate and empirical dose finding. J Nucl Med. https://doi.org/10.2967/jnumed.117.191395
Kratochwil C, Bruchertseifer F, Rathke H et al (2018) Targeted α-therapy of metastatic castration-resistant prostate cancer with 225 Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. J Nucl Med 59:795–802. https://doi.org/10.2967/jnumed.117.203539
Kratochwil C, Giesel FL, Heussel CP et al (2019) Patients resistant against PSMA-targeting alpha-radiation therapy often harbor mutations in DNA-repair associated genes. J Nucl Med. https://doi.org/10.2967/jnumed.119.234559
Królicki L, Bruchertseifer F, Kunikowska J et al (2019) Safety and efficacy of targeted alpha therapy with 213Bi-DOTA-substance P in recurrent glioblastoma. Eur J Nucl Med Mol Imaging 46:614–622. https://doi.org/10.1007/s00259-018-4225-7
Lange PH, Vessella RL (1999) Mechanisms, hypotheses and questions regarding prostate cancer micrometastases to bone. Cancer Metastasis Rev 17:331–336. https://doi.org/10.1023/a:1006106209527
Lesch HP, Kaikkonen MU, Pikkarainen JT, Ylä-Herttuala S (2010) Avidin–biotin technology in targeted therapy. Expert Opin Drug Deliv 7:551–564. https://doi.org/10.1517/17425241003677749
Lintuzumab-Ac225 in combination with CLAG-M chemotherapy in patients with relapsed/refractory acute myeloid leukemia. https://clinicaltrials.gov/ct2/show/NCT03441048?term=actinium&draw=2&rank=9. Accessed 14 Jan 2020
Macey DJ, Meredith RF (1999) A strategy to reduce red marrow dose for intraperitoneal radioimmunotherapy. Clin Cancer Res 5:1–2
McKay RR, Gray KP, Polacek L et al (2017) An exploratory study of radium-223 and vascular endothelial growth factor targeted therapy (VEGF TT). J Clin Oncol 35:suppl:Abstract 466
Medicines Agency E (2018) EMA restricts use of prostate cancer medicine Xofigo
Meredith R, Torgue J, Shen S et al (2014a) Dose escalation and dosimetry of first-in-human radioimmunotherapy with 212Pb-TCMC-trastuzumab. J Nucl Med 55:1636–1642. https://doi.org/10.2967/jnumed.114.143842
Meredith RF, Torgue J, Azure MT et al (2014b) Pharmacokinetics and imaging of 212 Pb-TCMC-trastuzumab after intraperitoneal administration in ovarian cancer patients. Cancer Biother Radiopharm 29:12–17. https://doi.org/10.1089/cbr.2013.1531
Miederer M, Henriksen G, Alke A et al (2008) Preclinical evaluation of the alpha-particle generator nuclide 225Ac for somatostatin receptor radiotherapy of neuroendocrine tumors. Clin Cancer Res 14:3555–3561. https://doi.org/10.1158/1078-0432.ccr-07-4647
Morgenstern A, Krolicki L, Kunikowska J et al (2014) Targeted alpha therapy of glioblastoma multiforme: first clinical experience with 213Bi-substance P. J Nucl Med 55:390
Mottet N, Bellmunt J, Briers E et al (2020) EAU – ESTRO – ESUR – SIOG Guidelines on Prostate Cancer, Edn. In: Presented at the EAU annual congress amsterdam 2020. EAU Guidelines Office, Arnhem, The Netherlands. https://uroweb.org/guideline/prostate-cancer/
Nademanee A, Forman S, Molina A et al (2005) A phase 1/2 trial of high-dose yttrium-90-ibritumomab tiuxetan in combination with high-dose etoposide and cyclophosphamide followed by autologous stem cell transplantation in patients with poor-risk or relapsed non-Hodgkin lymphoma. Blood 106:2896–2902. https://doi.org/10.1182/blood-2005-03-1310
Nilsson S, Larsen RH, Fossa SD et al (2005) First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res 11:4451–4459. https://doi.org/10.1158/1078-0432.ccr-04-2244
Nilsson S, Franzen L, Parker C et al (2013) Two-year survival follow-up of the randomized, double-blind, placebo-controlled phase II study of radium-223 chloride in patients with castration-resistant prostate cancer and bone metastases. Clin Genitourin Cancer 11:20–26. https://doi.org/10.1016/j.clgc.2012.07.002
Norenberg JP (2006) 213Bi-[DOTA0, Tyr3]octreotide peptide receptor radionuclide therapy of pancreatic tumors in a preclinical animal model. Clin Cancer Res 12:897–903. https://doi.org/10.1158/1078-0432.ccr-05-1264
O’Keefe DS, Su SL, Bacich DJ et al (1998) Mapping, genomic organization and promoter analysis of the human prostate-specific membrane antigen gene. Biochim Biophys Acta 1443:113–127
Oliveira S, Schiffelers RM, van der Veeken J et al (2010) Downregulation of EGFR by a novel multivalent nanobody-liposome platform. J Control Release 145:165–175. https://doi.org/10.1016/j.jconrel.2010.03.020
Open label phase two trial of radium Ra 223 dichloride with concurrent administration of abiraterone acetate plus prednisone in symptomatic castration-resistant (hormone-refractory) prostate cancer subjects with bone metastasis (eRADicAte). https://clinicaltrials.gov/ct2/show/record/NCT02097303?term=alpha-particle&draw=2&rank=16. Accessed 18 Dec 2019
Pagel JM, Kenoyer AL, Bäck T et al (2011) Anti-CD45 pretargeted radioimmunotherapy using bismuth-213: high rates of complete remission and long-term survival in a mouse myeloid leukemia xenograft model. Blood 118:703–711. https://doi.org/10.1182/blood-2011-04-347039
Palm S, Bäck T, Claesson I et al (2007) Therapeutic efficacy of astatine-211-labeled trastuzumab on radioresistant SKOV-3 tumors in nude mice. Int J Radiat Oncol Biol Phys 69:572–579. https://doi.org/10.1016/j.ijrobp.2007.06.023
Park SI, Shenoi J, Pagel JM et al (2010) Conventional and pretargeted radioimmunotherapy with bismuth-213 to target and treat CD20-expressing non-Hodgkin lymphoma: a preclinical model for consolidation therapy to eradicate minimal residual disease. Blood 116:4231–4239. https://doi.org/10.1182/blood-2010-05-282327.the
Parker C, Nilsson S, Heinrich D et al (2013a) Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 369:213–223. https://doi.org/10.1056/nejmoa1213755
Parker CC, Pascoe S, Chodacki A et al (2013b) A randomized, double-blind, dose-finding, multicenter, phase 2 study of radium chloride (Ra 223) in patients with bone metastases and castration-resistant prostate cancer. Eur Urol 63:189–197. https://doi.org/10.1016/j.eururo.2012.09.008
Petrich T, Korkmaz Z, Krull D et al (2010) In vitro experimental (211)At-anti-CD33 antibody therapy of leukaemia cells overcomes cellular resistance seen in vivo against gemtuzumab ozogamicin. Eur J Nucl Med Mol Imaging 37:851–861. https://doi.org/10.1007/s00259-009-1356-x
Phase 1 study of AlphaMedixTM in adult subjects with SSTR (+) NET. https://clinicaltrials.gov/ct2/show/NCT03466216?term=alpha-particle&draw=2&rank=12. Accessed 18 Dec 2019
Popovtzer A, Rosenfeld E, Mizrachi A et al (2019) Initial safety and tumor control results from a “first-in-human” multicenter prospective trial evaluating a novel alpha-emitting radionuclide for the treatment of locally advanced recurrent squamous cell carcinomas of the skin and head and neck. Int J Radiat Oncol. https://doi.org/10.1016/j.ijrobp.2019.10.048
Pozzi OR, Zalutsky MR (2017) Radiopharmaceutical chemistry of targeted radiotherapeutics, part 4: strategies for 211 At labeling at high activities and radiation doses of At α -particles. Nucl Med Biol 46:43–49. https://doi.org/10.1016/j.nucmedbio.2016.11.009
Price EW, Orvig C (2014) Matching chelators to radiometals for radiopharmaceuticals. Chem Soc Rev 43:260–290. https://doi.org/10.1039/c3cs60304k
Rahbar K, Ahmadzadehfar H, Kratochwil C et al (2017) German multicenter study investigating 177 Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med 58:85–90. https://doi.org/10.2967/jnumed.116.183194
Raja C, Graham P, Abbas Rizvi SM et al (2007) Interim analysis of toxicity and response in phase 1 trial of systemic targeted alpha therapy for metastatic melanoma. Cancer Biol Ther 6:846–852. https://doi.org/10.4161/cbt.6.6.4089
Ramdahl T, Bonge-hansen HT, Ryan OB et al (2016) An efficient chelator for complexation of thorium-227. Bioorgan Med Chem Lett 26:4318–4321. https://doi.org/10.1016/j.bmcl.2016.07.034
Richman CM, Denardo SJ, O’Donnell RT et al (2005) High-dose radioimmunotherapy combined with fixed, low-dose paclitaxel in metastatic prostate and breast cancer by using a MUC-1 monoclonal antibody, m170, linked to indium-111/yttrium-90 via a cathepsin cleavable linker with cyclosporine to prevent human. Clin Cancer Res 11:5920–5927. https://doi.org/10.1158/1078-0432.ccr-05-0211
Rosenblat TL, McDevitt MR, Mulford DA et al (2010) Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia. Clin Cancer Res 16:5303–5311. https://doi.org/10.1158/1078-0432.ccr-10-0382
Saad F, Gillessen S, Heinrich D et al (2019) Disease characteristics and completion of treatment in patients with metastatic castration-resistant prostate cancer treated with radium-223 in an international early access program. Clin Genitourin Cancer 17:348.e5–355.e5. https://doi.org/10.1016/j.clgc.2019.05.012
Safety study of 212Pb-TCMC-trastuzumab radio immunotherapy. https://clinicaltrials.gov/ct2/show/NCT01384253. Accessed 23 Dec 2019
Safety and tolerability of BAY1862864 injection in subjects with relapsed or refractory CD22-positive non-Hodgkin’s lymphoma. https://clinicaltrials.gov/show/NCT02581878. Accessed 23 Dec 2019
Sartor O, Coleman R, Nilsson S et al (2014) Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol 15:738–746. https://doi.org/10.1016/s1470-2045(14)70183-4
Search of: radium-223|recruiting, active, not recruiting studies|interventional studies|prostate cancer—list results—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?term=radium-223&cond=Prostate+Cancer&recrs=a&recrs=d&age_v=&gndr=&type=Intr&rslt=&Search=Apply. Accessed 7 Jan 2020
Sgouros G (2008) Alpha-particles for targeted therapy. Adv Drug Deliv Rev 60:1402–1406. https://doi.org/10.1016/j.addr.2008.04.007
Sgouros G, Roeske JC, McDevitt MR et al (2010) MIRD Pamphlet no. 22 (abridged): radiobiology and dosimetry of -particle emitters for targeted radionuclide therapy. J Nucl Med 51:311–328. https://doi.org/10.2967/jnumed.108.058651
Sollini M, Boni R, Traino ACC et al (2015) New approaches for imaging and therapy of solid cancer. Q J Nucl Med Mol Imaging 59:168–183
Study evaluating the addition of pembrolizumab to radium-223 in mCRPC. https://clinicaltrials.gov/ct2/show/NCT03093428?term=alpha-particle&draw=2&rank=17. Accessed 18 Dec 2019
Study of radium-223 dichloride versus placebo and treatment with exemestane/everolimus in subjects with bone predominant HER2 (human epidermal growth factor receptor 2) negative hormone receptor positive metastatic breast cancer. https://clinicaltrials.gov/ct2/show/NCT02258451. Accessed 23 Dec 2019
Study to test the safety and how radium-223 dichloride an alpha particle-emitting radioactive agent works in combination with pembrolizumab an immune checkpoint inhibitor in patients with stage IV non-small cell lung cancer with bone metastases. https://clinicaltrials.gov/ct2/show/NCT03996473?term=alpha-particle&draw=2&rank=9. Accessed 18 Dec 2019
Tagawa ST, Milowsky MI, Morris M et al (2013) Phase II study of lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin Cancer Res 19:5182–5191. https://doi.org/10.1158/1078-0432.ccr-13-0231
Targeted Alpha Therapy Working Group, Parker C, Lewington V et al (2018) Targeted alpha therapy, an emerging class of cancer agents: a review. JAMA Oncol 4:1765–1772. https://doi.org/10.1001/jamaoncol.2018.4044
Total body irradiation and astatine-211-labeled BC8-B10 monoclonal antibody for the treatment of nonmalignant diseases. https://clinicaltrials.gov/ct2/show/NCT04083183?term=astatine&draw=2&rank=1. Accessed 14 Jan 2020
Trial of Ra-223 dichloride in combination with hormonal therapy and denosumab in the treatment of patients with hormone-positive bone-dominant metastatic breast cancer. https://clinicaltrials.gov/show/NCT02366130. Accessed 23 Dec 2019
Vallabhajosula S, Smith-Jones PM, Navarro V et al (2004) Radioimmunotherapy of prostate cancer in human xenografts using monoclonal antibodies specific to prostate specific membrane antigen (PSMA): studies in nude mice. Prostate 58:145–155. https://doi.org/10.1002/pros.10281
Vallabhajosula S, Goldsmith SJ, Kostakoglu L et al (2005) Radioimmunotherapy of prostate cancer using 90Y- and 177Lu-labeled J591 monoclonal antibodies: effect of multiple treatments on myelotoxicity. Clin Cancer Res 11:7195s–7200s. https://doi.org/10.1158/1078-0432.ccr-1004-0023
Vaneycken I, Govaert J, Vincke C et al (2010) In vitro analysis and in vivo tumor targeting of a humanized, grafted nanobody in mice using pinhole SPECT/micro-CT. J Nucl Med 51:1099–1106. https://doi.org/10.2967/jnumed.109.069823
Vaneycken I, Devoogdt N, Van Gassen N et al (2011) Preclinical screening of anti-HER2 nanobodies for molecular imaging of breast cancer. FASEB J 25:2433–2446. https://doi.org/10.1096/fj.10-180331
Venetoclax, azacitidine, and lintuzumab-Ac225 in AML patients. https://clinicaltrials.gov/ct2/show/NCT03932318?term=actinium&draw=2&rank=5. Accessed 14 Jan 2020
Venetoclax and lintuzumab-Ac225 in AML patients. https://clinicaltrials.gov/ct2/show/NCT03867682?term=actinium&draw=2&rank=6. Accessed 14 Jan 2020
Verheijen RH, Massuger LF, Benigno BB et al (2006) Phase III trial of intraperitoneal therapy with yttrium-90-labeled HMFG1 murine monoclonal antibody in patients with epithelial ovarian cancer after a surgically defined complete remission. J Clin Oncol 24:571–578. https://doi.org/10.1200/jco.2005.02.5973
Wilbur DS (2011) Chemical and radiochemical considerations in radiolabeling with α-emitting radionuclides. Curr Radiopharm 4:214–247. https://doi.org/10.2174/1874471011104030214
Yao V, Parwani A, Maier C et al (2008) Moderate expression of prostate-specific membrane antigen, a tissue differentiation antigen and folate hydrolase, facilitates prostate carcinogenesis. Cancer Res 68:9070–9077. https://doi.org/10.1158/0008-5472.can-08-2328
Zaknun JJ, Bodei L, Mueller-Brand J et al (2013) The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging 40:800–816. https://doi.org/10.1007/s00259-012-2330-6
Zalutsky MR, Reardon DA, Akabani G et al (2008) Clinical experience with -particle emitting 211At: treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J Nucl Med 49:30–38. https://doi.org/10.2967/jnumed.107.046938
Zechmann CM, Afshar-Oromieh A, Armor T et al (2014) Radiation dosimetry and first therapy results with a 124I/131I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging 41:1280–1292. https://doi.org/10.1007/s00259-014-2713-y
Zhang M, Zhang Z, Garmestani K et al (2003) Pretarget radiotherapy with an anti-CD25 antibody-streptavidin fusion protein was effective in therapy of leukemia/lymphoma xenografts. Proc Natl Acad Sci USA 100:1891–1895. https://doi.org/10.1073/pnas.0437788100
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MS, KM, and MK declare no conflict of interest. AC received speaker honoraria from General Electric and Blue Earth Diagnostics, acted as a scientific advisor for Blue Earth Diagnostics and Advanced Accelerator Applications, and benefited from an unconditional Grant from Sanofi to Institution. All honoraria and Grants are outside the scope of the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The author M. Sollini received the international prize “Francesco de Luca” for Cancer Research in 2019, attributed by the Accademia Nazionale dei Lincei, Rome.
Rights and permissions
About this article
Cite this article
Sollini, M., Marzo, K., Chiti, A. et al. The five “W”s and “How” of Targeted Alpha Therapy: Why? Who? What? Where? When? and How?. Rend. Fis. Acc. Lincei 31, 231–247 (2020). https://doi.org/10.1007/s12210-020-00900-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-020-00900-2