Abstract
Pollution with heavy metals is a major environmental problem, and plants that accumulate these metals might provide efficient and ecologically sound approaches for their removal. Therefore, the present study was conducted to investigate the phenological behavior and the potential to accumulate nutrients and heavy metals in the aboveground phytomass of two perennial grasses (Imperata cylindrica and Desmostachya bipinnata) along the watercourses in Nile Delta, Egypt. Twenty-five quadrats were selected seasonally, to represent the growth of the two grasses, along canals and drains of the Nile Delta. The phenological behavior of the studied species showed similar seasonal trends along the canals and drains. The average annual biomass of the living and dead parts of D. bipinnata (1901.3 g m−2) was higher than that of I. cylindrica (1626.4 g m−2). D. bipinnata accumulated higher concentrations of Na, and K (14.3, 26.2 mg g−1), while lower Ca, Mg, N, P and Fe (14.2, 11.4, 10.8, 0.3 and 1.4 mg g−1) than I. cylindrica (12.8, 24.8, 14.4, 14.7, 11.6, 0.4 and 2.0 mg g−1). The living parts of I. cylindrica accumulated the highest contents of carbohydrates and proteins during autumn and spring, respectively, while those of D. bipinnata had the highest ash content, but the lowest lipids during summer. D. bipinnata accumulated higher concentrations of Cu and Mn, but lower of Zn and Pb, than I. cylindrica in their living and dead parts. Heavy metals, except Zn, had BF more than unity, however, the uptake capability was in the order: Pb > Mn > Cu > Zn for I. cylindrica, while Pb > Cu > Mn > Zn for D. bipinnata. The analysis of the nutritive values for the two studied grasses evaluated them as poor forage. Finally, the high bioaccumulation factors of both species for Mn, Cu and Pb, in addition to their ability to accumulate the highest concentrations of macro- and micronutrient in the dead parts, render these species a powerful phytoremediator for the removal of these metals from contaminated ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Phenology is an important component of plant life history theory affecting both biotic (e.g., competition, herbivory, pollination) and abiotic (e.g., frost, drought) constraints of plant performance (Stanton et al. 2000). The study of plant phenology provides knowledge about the pattern of plant growth and development as well as the effects of environment and selective pressures on flowering and fruiting behavior (Zhang et al. 2006). Phenological changes, including changes in flowering dates, are some of the most obvious effects of climatic changes on biological communities (Chapin et al. 2008). Changes in the timing of important events such as flowering or fruiting could result in failure to produce offspring or they have adequately dispersed (Lesica and Kittelson 2010). Several trends have become apparent from these studies, at least some species were flowering earlier than in the past, and earlier flowering species demonstrated the greatest advance in flowering date (Lesica and Kittelson 2010).
Pollution of air, soil, and water with heavy metals is a major environmental problem (Srivastava and Purnima 1998). Metals cannot be easily degraded and the cleanup usually requires their removal (Lasat 2002). However, this energy intensive approach can be prohibitively expensive. Phytoremediation offers a cost-effective, nonintrusive, and safe alternative to conventional cleanup techniques. Utilizing the ability of certain grass species to remove, degrade, or immobilize harmful chemicals can reduce risk from contaminated soil, sludge, sediments, and ground water through contaminant removal, degradation or containment (Zavoda et al. 2001). With global heavy metal contamination on the rise, plants that can process heavy metals might provide efficient and ecologically sound approaches for their sequestration and removal (Lasat 2002).
Perennial halophytic grasses constitute a valuable fodder and cash crop resource on saline and alkaline wastelands besides ecological applications, such as landscaping or rehabilitation, of damaged ecosystems (Dagar et al. 2004). Warm season grasses are increasingly being researched for biomass production, in addition to their normal use as summer forage and for soil conservation (Madakadze et al. 1998). The produced biomass can be used for energy production or as industrial raw material for chemical production and/or pulp and paper (Sanderson et al. 1996). Utilization of biomass offers the main advantages of: (a) relatively inexpensive renewable resource, (b) reduction of carbon dioxide emissions (Graham et al. 1992), and (c) soil amelioration and reduced pollution of ground water because of reduced fertilization (Gebhart et al. 1994).
Nowadays, Egypt faces a serious problem of feed shortage, especially green summer fodder, which suppresses the improvement of animal production. Therefore, dependence on improving local food and food resources for both animals and humans is necessary for a sound policy. The vegetation covering the wetland areas plays an important role in sequestering large quantitative of nutrients (Cronk and Fennessy 2001) and metals (Baldantoni et al. 2004) from the environment by storing them in the roots and/or shoots. In addition, wetland plants have high remediation potential for macronutrients because of their general fast growth and high biomass production (Dhir et al. 2009).
The present study is the sixth of a series of studies dealing with the evaluation of biomass, nutrients and nutritive values of riparian plants along the watercourses of the Nile Delta (El-Kady 2002; Shaltout et al. 2010, 2012, 2013a, b). The present study aims at evaluating the phenological behavior, aboveground biomass, and nutrient status of two grasses (Imperata cylindrica and Desmostachya bipinnata) along the water courses of the Nile Delta, Egypt. It aims also at evaluating their ability to accumulate nutrients and heavy metals as well as their nutritive values to be used as forage plants.
2 Materials and methods
2.1 Study area
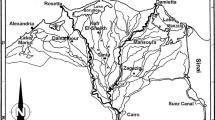
The study area lies in Nile Delta that is bounded by the two branches of the Nile: Rosetta to the west and Damietta to the east (Fig. 1). Nile Delta is about 22,000 km2, compared with 13,000 km2 for the area of the Valley; thus it comprises about 63 % of Egypt’s productive agricultural land. Most of the Egyptian cultivated land is irrigated by the river Nile through a network of canals and is drained by a similar network of drains. Most of these canals and drains were dug in the last 200 years. The total length of both networks (excluding private ditches and drains) exceeds 47,000 km: >31,000 km of canals and >16,000 km of drains (Shaltout et al. 2013a). Soil characters of the water courses in the middle Delta region indicated that most soils were consisted of silt (Table 1). The drains were characterized by higher salinity compared with canals. On the other hand, canals were characterized by higher values of N, K and Ca; but lower of organic matter, P, Na and Mg than drains.
The climatic conditions of the northern part of Nile Delta that lies in the arid zone (UNESCO 1977) are characterized by warm summer (20–30 °C) and mild winter (10–20 °C). Though occasional short rainstorms occur in winter, most of the days are sunny. The aridity index (P/PET) is between 0.03 and 0.20 at the North of the Delta (arid region) and less than 0.03 at the south (hyper-arid region); where P is the annual precipitation and PET is the potential evapotranspiration.
2.2 Study species
Imperata cylindrica (L.) Beauv. is a perennial grass native to the Old World and is found as well in the warm temperate zone (El-Komy 2002). In Egypt, it is very common in the Nile region, the oases of the Libyan Desert, the Mediterranean coastal strip from El-Sallum to Rafah, the Red Sea coastal region, Sinai proper and all the deserts of Egypt. It grows vigorously because it can thrive on many soil types and in a variety of habitats such as dry or moist sandy places, in waste lands, along channels and in old gardens (Boulos 2009). I. cylindrica is frequently used as a sand binder for coastal sand dunes and mobile sand hills in desert areas, and used to stabilize canals and railroad embankments (El-Komy 2002). It is palatable to livestock in the early growth stages, and so grasses often fired to stimulate growth of new sprouts. The value of this grass for forage may be dependent upon its stage of growth at cutting or grazing and upon a system of management, which keeps the crop in a palatable condition (Holm et al. 1977).
Desmostachya bipinnata (L.) Stapf, a sacrificial grass, is a drought and salt-tolerant grass with a deep, strong rhizome, making it an excellent sand binder. It is not normally regarded as a fodder but is used in some arid areas such as Afghanistan where it is chopped and in mixed with cereals. It is a common and widespread species growing along irrigation channels, in orchards, and associated with cultivation in many countries (Gulzar et al. 2007). D. bipinnata is cited by Holm et al. (1977) as a “serious” weed in Pakistan and a “common” weed in Egypt and Iraq. It is among the worst weeds of crops in Yemen (Al-Kouthayri and Hassan 1998). Once established, it can spread very aggressively by rhizome and dominate arid and semi-arid environments.
2.3 Plant sampling and analysis
Sampling process of the studied species was carried out four times a year (seasonally) using 25 quadrats (each of 1 × 1 m) distributed along 15 irrigation and 10 drainage canals within the study area (Fig. 1). In each quadrat, the seasonal variation in the phenological behavior of the two species was assessed visually, where the main described phases were sprouting, vegetative, flowering, fruiting and withering. For estimating the aboveground biomass, all shoots within the sampled quadrat were harvested and transferred to the laboratory in polyethylene bags. In the laboratory, the shoots were separated into living and dead parts, and then the biomass was estimated after oven drying of the plant materials at 60 °C to constant weight. For plant analysis, a composite sample of the living and dead parts was taken from each quadrat and ground into a powder using a metal-free plastic mill. Ash percentage was estimated by ignition at 550 °C for 2 h. Nutrients and heavy metals were extracted from 0.5 to 1 g samples using mixed-acid digestion method. Total nitrogen (N) was assessed by the Kjeldahl method; Na, Ca, and K using a flame photometer (CORNING M410); Mg, Fe, Cu, Mn, Zn and Pb using atomic absorption (Shimadzu AA-6200), and P by applying molybdenum blue method using a spectrophotometer (CECIL CE 1021). Ether extract (i.e., crude fat) and crude fiber were determined by the Soxhlet extraction method. All these procedures are according to Allen (1989).
The content of crude protein was calculated by multiplying the nitrogen concentration by the factor of 6.25 (Adesogon et al. 2000). Carbohydrate (NFE) was calculated according to the equation of Le Houérou (1980): NFE (in % dry matter) = 100 − (CP + CF + crude fat + ash), where CP = crude protein and CF = crude fiber. Digestible crude protein (DCP) was calculated according to the equation of Demarquilly and Weiss (1970): DCP (in % dry matter) = 0.929 CP (in % dry matter) − 3.52. Total digestible nutrients (TDN) were estimated according to the equation applied by Naga and El-Shazly (1971): TDN (in % dry matter) = 0.623 (100 + 1.25 EE) − P0.72, where EE = percentage of ether extract and P = percentage of crude protein. Digestible energy (DE) was estimated following this equation (NRC 1984): DE (Mcal kg−1) = 0.0504 CP (%) + 0.077 EE (%) + 0.02 CF (%) + 0.000377 (NFE)2 (%) + 0.011 (NFE) (%) − 0.152. Metabolized energy (ME) = 0.82 DE (Garrett 1980), Net energy (NE) = 0.50 ME (Le Houérou 1980), while Gross energy (GE) was calculated following this equation (NRC 1984): GE (Kcal 100 g−1) = 5.72 CP (%) + 9.5 EE (%) + 4.79 CF (%) + 4.03 NFE (%).
Soil analysis was taken from El-Sheikh (1989), while soil heavy metals (Mn, Cu, Zn and Pb) were gathered from Galal and Shehata (2013) on their studies on the water courses of the middle Nile Delta, respectively. One-way ANOVA was applied to assess the significance of variations in standing crop phytomass, elements, organic components and nutritive variables in relation to the type of water course and season (SAS 1985).
Heavy metal concentrations of soils and plants were calculated on the basis of dry weight. The bioaccumulation factor (BF), an index of the ability of the plant to accumulate a particular metal with respect to its concentration in the soil substrate (Ghosh and Singh 2005), was calculated as follows:
where C plant root and C soil represent the heavy metal concentrations in the plant root and soils, respectively.
3 Results
3.1 Phenological behavior
The phenological behavior of the two studied species in canals and drains had more or less the same seasonal behavior (Fig. 2). The maximum sprouting and vegetative activities of both grasses were attained during winter with higher activity of I. cylindrica in canals, but lower in drains than D. bipinnata. On the other hand, flowering and fruiting activities attained their maximum, in canals and drains, during spring and summer, respectively; however, the activity of D. bipinnata was greater than that of I. cylindrica, except fruiting in drains. Moreover, the maximum senescence was during autumn with higher activity of I. cylindrica in drains and lower in canals than the other species. I. cylindrical had no sprouting or vegetative activities during summer and autumn, while flowering and fruiting during winter in the drains. On the other hand, D. bipinnata did not form flowers and fruits during winter in both canals and drains.
3.2 Aboveground Biomass
The seasonal variation of the aboveground standing crop biomass (Fig. 3) indicated that the average annual biomass of the living and dead parts of D. bipinnata (1901.3 g m−2) was higher than that of I. cylindrica (1626.4 g m−2). Significant seasonal variations in the biomass of the living and dead parts of both species were recognized. The biomass of the living parts of I. cylindrica and D. bipinnata had its maximum (1681.4 and 2076.1 g m−2) in autumn and the minimum in winter (678.5 and 909.0 g m−2). In addition, the dead parts of the I. cylindrica attained its maximum biomass (662.7 g m−2) during summer, while that of D. bipinnata (847.7 g m−2) during autumn. However, the dead parts of I. cylindrica and D. bipinnata attained their minimum biomass during spring and summer, respectively.
3.3 Nutrient accumulation
The inorganic nutrient analysis of the aboveground living parts of study species showed significant seasonal variation in the concentration of Na, Mg and P for I. cylindrica, while Na, Ca, Mg and Fe for D. bipinnata. However, variations in the dead parts of both plants were non-significant (Table 2). I. cylindrica accumulated the highest concentrations of Ca and N (8.6 and 6.5 mg g−1) during spring, K (16.2 mg g−1) during summer, and Mg and P (8.5 and 0.4 mg g−1) during autumn, in their living parts, while Na and Fe (8.1 and 1.8 mg g−1) during summer and spring, respectively, in their dead parts. On the other hand, the living parts of D. bipinnata accumulated the highest concentrations of K and Ca (17.9 and 9.1 mg g−1) during autumn, N (7.1 mg g−1) during spring, and Fe (0.9 mg g−1) during summer, while the dead parts had the maximum of Na and Mg (10.7 and 8.8 mg g−1) during summer and spring, respectively. On the average, D. bipinnata accumulated concentrations of Na, K and Mg in its dead tissues, but K and N in the living ones higher than those of I. cylindrica. However, the later species had concentrations of Ca, N and Fe in the dead parts; and Ca, Mg and P in the living ones higher than those of the former one.
The seasonal variations in the organic contents of the living parts of I. cylindrica were significant for carbohydrates (NTF), crude fibers (CF) and ash content, while the dead parts had significant ether extract (EE) variations (Table 3). However, the living and dead parts of D. bipinnata had non-significant seasonal variations. The living parts of I. cylindrica accumulated the highest contents of NTF (57.6 %) during autumn, TP and Ash (4.1 and 10.3 %) during spring, CF (33.4 %) during summer, and EE (2.4 %) during winter, while those of D. bipinnata had the highest TP (4.4 %) during spring, EE (2.4 %) during winter, and ash content (11.9 %) during summer. Moreover, the dead parts of D. bipinnata attained the highest contents of NTF and CF (57.4 and 37.3 %) during summer and autumn, respectively. On the average, I. cylindrica accumulated concentrations of NTF and EE, in the living tissues, while TP, EE, CF and ash contents in the dead ones higher than those of D. bipinnata. On the contrary, the later species accumulated concentrations of TP, CF and ash contents in their living, and NTF in dead tissues higher that the former one.
3.4 Heavy metals accumulation
The heavy metals analysis data indicated that the dead parts of the study species accumulated higher annual concentrations of Cu and Mn, in addition to Zn in I. cylindrica and Pb in D. bipinnata, than the living parts (Fig. 4). In addition, D. bipinnata accumulated higher concentrations of Cu and Mn, but lower of Zn and Pb, than I. cylindrica in their living and dead parts. The aboveground dead parts of D. bipinnata accumulated the highest concentrations of Cu, Mn (33.9 and 86.4 µg g−1), while those of I. cylindrica had the highest of Zn (89.3 µg g−1) and the lowest of Pb (23.6 µg g−1) during spring and summer, respectively. Moreover, the highest Pb concentration (106.9 µg g−1) was recorded in the living tissues of I. cylindrica during spring. It is worth to note that the living parts of both species had more or less the same trend in accumulating heavy metals, while the dead parts showed variations.
The bioaccumulation factors (BF) of the studied species indicated that all the investigated heavy metals, except Zn, had BFs more than unity (Fig. 5). I. cylindrica had higher BF of Pb and Mn (90.1 and 1.6) and lower of Cu (1.5) than those of D. bipinnata (62.4, 1.2 and 1.7, respectively). The uptake capability from soil to plant was in the order of Pb > Mn > Cu > Zn for I. cylindrica, and in the order of Pb > Cu > Mn > Zn for D. bipinnata.
3.5 Nutritive value
The nutritive values of the aboveground parts of the studied species indicated that the dead parts of D. bipinnata had the highest Ca/P ratio (71.1), while the living parts had the highest Ca/Mg (3.0) during summer (Table 4). The dead parts of I. cylindrica had the highest values of digestible crude protein and total digestible nutrients (1.3 and 63.9 %) during winter, while the lowest of total digestible nutrients (1.3 %), digestible energy and net energy (1.7 and 0.7 Mcal kg−1, respectively) during spring. Moreover, the dead parts of D. bipinnata had the highest values of digestible and metabolized energy (2.7 and 2.2 Mcal kg−1), while those of I. cylindrica had the highest of gross energy (418.1 kcal 100 g−1) during summer. On the average, the living parts of I. cylindrica had higher digestible crude protein, total digestible nutrients, digestible and metabolized energy, but lower Ca/P, Ca/Mg and gross energy than those of D. bipinnata.
4 Discussion
Plant phenology is the seasonal timing of environment-mediated events such as growth and reproduction (Rathcke and Lacey 1985). It is expected to be one of the most sensitive and easily observable natural indicators of climate change (Badeck et al. 2004). The phenological behavior of the plant species in the present study indicated that maximum sprouting and vegetative activities of both grasses were attained during winter, while those of flowering and fruiting were recorded during spring and summer. This behavior coincided with that of Cynodon dactylon in the water courses of Nile Delta (Shaltout et al. 2013a). It was reported that water availability in winter played an essential role in seed germination and recruitment of new individuals (Farahat et al. 2015). However, flowering has most often been correlated with warmer temperatures in the months prior to anthesis (Lesica and Kittelson 2010). Moreover, the two species exhibited high withering during autumn and high vegetative growth during winter. Xi and Zhang (2005) demonstrated that drought and low temperature in winter reflected withered and yellow leaves on Zoysia japonica, while maintained normal vegetative growth by extended duration of light at similar water and temperature conditions.
The seasonal variation in biomass indicated that the two studied species had the same pattern with its maximum in autumn, while the minimum in winter for D. bipinnata and spring for I. cylindrica. A similar finding was postulated by El-Kady (2002) on P. australis, and Shaltout et al. (2010) on E. stagnina and Shaltout et al. (2013a) on C. dactylon and Panicum repens. The biomass of I. cylindrica and D. bipinnata (1626.4 and 1901.3 g m−2) was greater than 1499.2 g m−2 of D. bipinnata on the canal banks (Galal and Shehata 2013), 921.7 g m−2 of P. australis (El-Kady 2002) and 803.6 g m−2 of Echinochloa stagnina (Shaltout et al. 2010). This may be due to the strong rhizomatus nature of these plants which is believed to be more resistant to trampling along the canal terraces by farmers and their grazing animals (e.g., buffalos, cows, sheep, goats and donkeys) than the other life forms (Shaltout et al. 2010). In addition, the rapid development of these plants caused by the rich reserve material at the beginning of the growing season enables the photosynthetic apparatus to be active for a longer period under favorable conditions (Engloner 2009).
Plants with potentially high annual primary production can extract large amounts of nutrients from their environment and store them in biomass and litter (Eid et al. 2010). It has long been recognized that metal accumulation capacity varies greatly between different species and varieties, and is affected by various edaphic conditions (Shu et al. 2002). The present study indicated that D. bipinnata accumulated higher values of Na, K, Ca and Mg; while I. cylindrica higher values of N, P and Fe. Both species had lower concentrations of Na, Ca and Mg than C. dactylon and P. repens (Shaltout et al. 2013a) and P. australis (El-Kady 2002), but lower than E. stagnina (Shaltout et al. 2010). In addition, Na, K, Ca and Mg concentrations of the study species were higher, while those of N and P were lower than those recorded by Galal and Shehata (2013) in D. bipinnata along the canal banks of Nile Delta.
The dead parts of the study species accumulated higher annual concentrations of Cu and Mn, in addition to Zn in I. cylindrica and Pb in D. bipinnata, than the living parts. This disagrees with Mortimer (1985) who reported that the rates of metal absorption are lower in senescent than in active growing plants. According to Welsh (1977), some exceptions in which rates of heavy metals absorption were higher in old and dead than in the young leaves are recognized. The aboveground parts of I. cylindrica accumulated higher concentrations of Mn, Zn and Pb and lower of Cu than those of D. bipinnata. Elevated Pb, Zn and Cu might impose phytotoxic effects to higher plants (Shaltout et al. 2013a). Moreover, the study species had higher Mn, Cu and Pb, while lower Zn concentrations than that recorded by Galal and Shehata (2013) on D. bipinnata. In the present study, I cylindrica accumulated concentrations of heavy metals in the order of Pb > Mn > Zn > Cu and D. bipinnata in the order of Mn > Pb > Zn > Cu, which is more or less in agreement with the previous studies (Yan et al. 2012; Zhang et al. 2012; Shaltout et al. 2013a; Galal and Shehata 2013). It can be concluded that even though the types of grasses are different, the order for heavy metal absorbing capability from soil to grass is more or less similar (Yan et al. 2012).
Plant species and populations differ widely in their ability to accumulate heavy metals (Denga et al. 2004). In the present study, Cu and Zn concentration did not exceed the toxic levels for normal plants (66 and 20 μg g−1) reported by Borkert et al. (1998). In addition, the concentrations of Mn, Cu and Pb in the aboveground tissues were higher than that of the soils in which the plant grows, which agrees with the study of Galal and Shehata (2013). Moreover, the relationship between the heavy metal content of soils and corresponding grasses was investigated using the bioaccumulation factor (BF), which has been applied to assess plants’ capability to absorb heavy metals from the soil (Ratko et al. 2011; Xiao et al. 2011). The BF generally showed the movement of heavy metals from sediment to root and shoot, indicating the efficiency to uptake of the bio-available metals from the environment and gives an idea whether the plant is an accumulator, excluder or indicator (Bose et al. 2008). The order of uptake capability is Pb > Mn > Cu > Zn for I. cylindrica, while Pb > Cu > Mn > Zn for D. bipinnata, which disagree with Zn > Cu > Mn (Galal and Shehata 2013). Zu et al. (2005) reported that BF >1 in metal accumulating plants, whereas it was typically <1 in metal excluding plants. In the present study, the heavy metals had BF >1 for Mn, Cu and Pb, which indicate that both species are powerful accumulator for these metals.
The organic nutrients contents of the study grasses indicated that carbohydrates, ether extract, total protein and ash content were lower than that recorded for D. bipinnata (Galal and Shehata 2013), P. australis (El-Kady 2002), C. dactylon and P. repens (Shaltout et al. 2013a) E. stagnina (Shaltout et al. 2010), while crude fibers were higher. The total protein and crude fibers lay far the maintenance range of sheep, goat, dairy cattle and beef cattle (NRC 1975, 1981, 1978, 1984). Ministry of Agriculture, Fisheries and Food in England (Anonymous 1975) reported that minimum protein in the animal diet ranges between 6 and 12 % depending on the animal species. NRC (1975) indicated that sheep are known to require 8.9 % protein for maintenance. Our investigations of both grasses agreed with the scale of the protein content of some rough fodder materials (2.7–13.4 %: Shoukry 1992). The same is true regarding ash content (1.3–23.1 %). On the other hand, lipids lay within the scale of some rough fodder materials (0.5–3.1 %: Shoukry 1992), but lower than that of T. alexandrinum (2.9 %: Chauhan et al. 1980). Moreover, total carbohydrates exceed the range of some rough fodder material (27.8–51.9 %: Shoukry 1992) and higher than that of T. alexandrinum (43.4 %: Chauhan et al. 1980). This result coincided with Galal and Shehata (2013) on D. bipinnata.
Digestible crude protein attained its maximum values (1.3 %) in I. cylindrica, which is lower than the value required for the diet of sheep (6.1 %; NRC 1975), while TDN had a value (63.9 %) for I. cylindrica, which is lower than 68.8 and 66.4 % reported by Heneidy and Bidak (1996) on P. australis and Shaltout et al. (2010) on E. stagnina, respectively. In the present study, D. bipinnata attained the maximum value of digestible energy (2. 7 Mcal/kg) in their living tissues; however, sheep are known to require the same value for diet (NRC 1975).
Abd El-Salam (1985) recognized the importance of the adequate Ca:P ratio of 2:1 as a major factor affecting the utilization of the whole diet. In the present study, this ratio is far higher than the optimum, while El-Kady (1987) in his study on range ecosystems in the western Mediterranean region of Egypt reported a ratio of 25.8 and Shaltout et al. (2012) on Azolla filiculoides reported a ratio of 33.3. Such a high ratio would lead to lower utilization of both Ca and P by animals. It is also asserted that if too little P is available, the N absorption, and hence biomass production are reduced (Shaltout et al. 2012). On the other hand, the Ca:Mg ratio was 0.9 for I. cylindrica and 1.5 for D. bipinnata, while it was 1.2–2.8 in the browse plants in North Africa (Le Houérou 1980). It seems that the nutritive values of the living and dead parts of both species are too low from the range of nutritive value of sheep (NRC 1975), goat (NRC 1981), dairy cattle (NRC 1978) and beef cattle (NRC 1984). Thus, using the scale suggested by Boudet and Riviere (1968), the living and dead parts of the two grasses under investigations were evaluated as poor forage.
5 Conclusion
Nutrients and heavy metals are removed in constructed wetland systems by many different processes including plant uptake and accumulation. Harvesting the aboveground biomass is therefore an option to remove accumulated elements. In order to maximize removal, harvest should be done during the period of maximum content of heavy metals in the plant tissues. Autumn is the suitable season to harvest both I. cylindrica and D. bipinnata for removal of nutrients and heavy metals. The high bioaccumulation factors of both species for Mn, Cu and Pb, in addition to their ability to accumulate the highest concentrations of macro- and micronutrient in the dead parts, render this species a powerful phytoremediator for the removal of these metals from contaminated ecosystems. The analysis of the nutritive values for the two study grasses evaluated them as poor forage.
References
Abd El-Salam H (1985) Grazing capacity per feddan at Omayed grazing area, the northern coastal zone, western to Alexandria. Ph.D. Thesis, Alexandria Univ. p 166
Adesogon AT, Givens DI, Owen E (2000) Measuring chemical composition and nutritive value in forages. In: Mannetje L. t’, Jones RM (Eds.), Field and laboratory methods for grassland and animal production research. CABI Publishing, Wallingford, Oxon, p 263-278
Al-Kouthayri GR, Hassan AA (1998) Survey of major weeds in Hadramout Valley. Yemen Arab J Plant Protect 16(1):19–26
Allen SE (1989) Chemical analysis of ecological materials. Blackwell Scientific Publications, London
Anonymous (1975) Energy allowances and feeding system for ruminants. Ministry of Agriculture, Fisheries and Food, Her Majesty’s Stationary Office, Technical Bulletin, London, p 33
Badeck FW, Bondeau A, Bottcher K, Doktor D, Lucht W, Schaber J, Sitch S (2004) Responses of spring phenology to climate change. New Phytol 162(2):295–309
Baldantoni D, Alfani A, Di Tommasi P, Bartoli G, Virzo De Santo A (2004) Assessment of macro- and micro-element accumulation capability of two aquatic plants. Environ Poll 130:149–156
Borkert CM, Cox FR, Tucker MR (1998) Zinc and copper toxicity in peanut, soybean, rice and corn in soil mixtures. Comm Soil Sci Plant Anal 29:2991–3005
Bose S, Vedamati J, Rai V, Ramanathan AL (2008) Metal uptake and transport by Typha angustata L. grown on metal contaminated waste amended soil: an implication of phytoremediation. Geoderma 145(1–2):136–142
Boudet G, Riviere R (1968) Emploi practique des analyses fourageres pour l’appreciation pasturages tropicaux. Revue d’Elevage et de Medecine Veterinaire des Pays Tropicaux 2(21):227–266
Boulos L (2009) Flora of Egypt: checklist. Al Hadara Publishing, Cairo, Revised Annotated Edition 410 pp
Chapin FS, Randerson JT, Mc Guire AD, Foley JA, Field CB (2008) Changing feedbacks in the climate-biosphere system. Front Ecol Environ 6:313–320
Chauhan TR, Gill RS, Ichhponani JS (1980) Nutritive value of berseem and clusterbean forages. Ind J Animal Sci 50(12):1052–1055
Cronk JK, Fennessy MS (2001) Wetland plants: biology and ecology. Lewis Publisher, Boca Raton
Dagar JC, Tomar OS, Kumar Y, Yadav RK (2004) Growing three aromatic grasses in different alkali soils in semi-arid regions of northern India. Land Degrad Dev 15:143–151
Demarquilly C, Weiss P (1970) Tableau de la valeur alimentaire des fourrages. Et. 42: Versailles INRA-SEI
Denga H, Ye ZH, Wonga MH (2004) Accumulation of lead, zinc, copper and cadmium by 12 wetland plant species thriving in metal-contaminated sites in China. Environ Poll 132:29–40
Dhir B, Sharmila P, Pardah Saradhi P (2009) Potential of aquatic macrophytes for removing contaminants from the environment. Crit Rev Environ Sci Technol 39:754–781
Eid EM, Shaltout KH, Al-Sodany YM, Soetaert K, Jensen K (2010) Modeling growth, carbon allocation and nutrient budget of Phragmites australis in Lake Burullus. Egypt Wetlands 30:240–251
El-Kady HF. (1987). A study of range ecosystems of the western Mediterranean coastal desert of Egypt. Ph.D. Thesis. Technical University, Berlin, p 138
El-Kady H (2002) Seasonal variation in phytomass and nutrient status of Phragmites australis along the water courses in the Middle Delta region. Taeckholmia 20(2):123–138
El-Komy TM. (2002). Evaluation of the range plants along the water courses in the Nile Delta. Ph.D. Thesis. Tanta Univ., Tanta, Egypt, p 200
El-Sheikh MA. (1989). A study of the vegetation environmental relationships of the canal banks of the middle Delta region. M.Sc. Thesis, Tanta Univ., Tanta, Egypt, p 139
Engloner AI (2009) Structure, growth dynamics and biomass of reed (Phragmites australis)—a review. Flora 204:331–346
Farahat E, Galal TG, El-Midany M, Hassan LM. (2015). Effect of urban habitat heterogeneity on functional traits plasticity of the invasive species Calotropis procera (Aiton) W.T. Aiton. Rendiconti 26(2): 193-201
Galal TM, Shehata HS (2013) Morphological variations, biomass and ion accumulation of the aboveground shoots of Desmostachya bipinnata (L.) Stapf. Flora 208:556–561
Garrett WN. (1980). Energy utilization of growing cattle as determined in seventy-two comparative slaughter experiments. In Mount LE (eds.), energy metabolism, EAAP Publ. No. 26, London
Gebhart DL, Johnson HB, Mayeux HS, Polley HW (1994) The CRP increases soil organic carbon. J. Soil Water Cons 49:488–492
Ghosh M, Singh SP (2005) A comparative study of cadmium phytoextraction by accumulator and weed species. Environ Poll 133:365–371
Graham RL, Wright LL, Turhollow AF (1992) The potential for short-rotation woody crops to reduce US CO2 emissions. Clim Change 22:222–238
Gulzar S, Khan MA, Liu Z (2007) Seed germination strategies of Desmostachya bipinnata: a fodder crop for saline soils. Rangeland Ecol Manage 60:401–407
Heneidy SZ, Bidak LM (1996) Halophytes as forage source in the western Mediterranean coastal region of Egypt. Desert Inst Bull 46(2):261–283
Holm LG, Plucknett DL, Pancho JV, Herberger JP (1977) The world’s worst weeds: distribution and biology. University Press of Hawaii, Honolulu
Lasat MM (2002) Phytoextraction of toxic metals: a review of biological mechanisms. J Environ Qual 31(1):109–120
Le Houérou HN (1980) Chemical composition and nutritive value of browse in Tropical West Africa. In: Houérou Le (ed) Browse in Africa. ILCA, Addis Ababa, pp 261–289
Lesica P, Kittelson PM (2010) Precipitation and temperature are associated with advanced flowering phenology in a semi-arid grassland. J Arid Environ 74:1013–1017
Madakadze IC, Stewart K, Petrson PR, Coulmans BE, Samson R, Smith DL (1998) Light interception, use-efficiency and energy yield of switchgrass (Panicum virgatum L.) grown in a short season area. Biom Bioener 15(6):475–482
Mortimer DC (1985) Freshwater aquatic macrophytes as heavy metal monitors. The Ottawa River experience. Environ Monit Assess 5:311–323
Naga MA, El-Shazly K (1971) The prediction of the nutritive value of animal feeds from chemical analysis. J Agri Sci 77:25
NRC (1975). Nutrient requirements of domestic animals: nutrient requirement of cheep. (5th Ed). National Research Council No. 5, Washington DC, Nat. Acad. Sci. p 72
NRC (1978). Nutrient requirements of domestic animals: nutrient requirement of dairy cattle. (5th Edn.). National Research Council No. 3, Washington DC, Nat Acad Sci p 76
NRC (1981). Nutrient requirements of domestic animals: nutrient requirement of goats. National Research Council No. 15, Washington, DC, Nat Acad Sci p 80
NRC (1984). Nutrient requirements of domestic animals: nutrient requirement of beef cattle (6th Edn.). National Research Council No. 5, Washington DC, Nat Acad Sci p 90
Rathcke B, Lacey EP (1985) Phenological patterns of terrestrial plants. Annu Rev Ecol Syst 16:179–214
Ratko K, Snežana B, Dragica OP, Ivana B, Nada D (2011) Assessment of heavy metal content in soil and grasslands in national park of the lake plateau of the NP “Durmitor” Montenegro. Afr J Biotechnol 10:5157–5165
Sanderson MA, Reed RL, McLaughlin SB, Wullschleger SD, Conger BV, Parrish DJ, Wolf DD, Taliaferro C, Hopkins AA, Ocumpaugh WR, Husley MA, Read JC, Tischler CR (1996) Switchgrass as a sustainable bioenergy crop. Bioresour Technol 56:83–93
SAS (1985) SAS/STAT User’s Guide. SAS Instruction Incorporation, Cary, NC
Shaltout KH, Galal TM, El-Komy TM (2010) Evaluation of the nutrient status of some hydrophytes in the water courses of Nile Delta. Egypt Ecol Medit 36(1):77–87
Shaltout KH, El-Komi TM, Eid ME (2012) Seasonal variation in the phytomass, chemical composition and nutritional value of Azolla filiculoides Lam along the water courses in Nile Delta, Egypt. Fed Repert 123(1):37–49
Shaltout KH, Galal TM, El-Komy TM. (2013a). Nutrients and heavy metals accumulation in the aboveground biomass of two perennial grasses along the water courses of Nile Delta, Egypt. Egyptian J Bot 201–218
Shaltout KH, Galal TM, El-Komy TM (2013b) Biomass, nutrients and nutritive value of Persicaria salicifolia Willd. in the water courses of Nile Delta. Egypt Rendiconti 25:167–179
Shoukry MM (1992) An actual vision about the availability of the utilization of water hyacinth in feeding ruminants. National Symposium on Water Hyacinth, Assiut Uni, pp 75–92
Shu WS, Ye ZH, Lan CY, Zhang ZQ, Wong MH (2002) Lead, zinc and copper accumulation and tolerance in populations of Paspalum distichum and Cynodon dactylon. Environ Poll 120:445–453
Srivastava AK, Purnima X (1998) Phytoremediation for heavy metals: a land plant based sustainable strategy for environmental decontamination. Proc Nat Acad Sci India Sect B Biol Sci 68(35): 199–215
Stanton ML, Roy BA, Thiede DA (2000) Evolution in stressful environments. I. Phenotypic variability, phenotypic selection, and response to selection in five distinct environmental stresses. Evolution 54:93–111
UNESCO (1977). Map of the world distribution of arid regions. MAB Technical Notes, 7
Welsh RP. (1977). Studies on the uptake, translocation and accumulation of trace metals and phosphorus in aquatic plants. Ph.D. Thesis, Wastfield College, University of London. p 331
Xi JB, Zhang HX (2005) Winter-green technology on Zoysia japonica L. No. 3 sport field turf in south subtropic region. J Zhongshan Univ 44:93–95
Xiao R, Bai J, Zhang H, Gao H, Liua X, Wilkes A (2011) Changes of P, Ca, Al and Fe contents in fringe marshes along a pedogenic chronosequence in the Pearl River estuary. South China Cont Shelf Res 31:739–747
Yan X, Zhang F, Zeng C, Zhang M, Devkota LP, Yao T (2012) Relationship between heavy metal concentrations in soils and grasses of roadside farmland in Nepal. Int J Environ Res Pub Heal 9:3209–3226
Zavoda J, Cutright T, Szpak J, Fallon E (2001) Uptake, selectivity, and inhibition of hydroponics treatment of contaminants. J Environ Eng 127(6):502–508
Zhang G, Song Q, Yang D (2006) Phenology of Ficus racemosa in Xishuangbanna, Southwest China. Biotropica 38:334–341
Zhang F, Yan X, Zeng C, Zhang M, Shrestha S, Devkota LP, Yao T (2012) Influence of traffic activity on heavy metal concentrations of roadside farmland soil in mountainous areas. Int J Environ Res Pub Heal 9:1715–1731
Zu YQ, Li Y, Chen JJ, Chen HY, Qin L, Christian S (2005) Hyperaccumulation of Pb, Zn and Cd in herbaceous plants grown on lead–zinc mining area in Yunnan. China Environ Int 31:755–762
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaltout, K.H., Galal, T.M. & El-Komi, T.M. Phenology, biomass and nutrients of Imperata cylindrica and Desmostachya bipinnata along the water courses in Nile Delta, Egypt. Rend. Fis. Acc. Lincei 27, 215–228 (2016). https://doi.org/10.1007/s12210-015-0459-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-015-0459-5