Abstract
Microbe-induced calcite precipitation is a sustainable improvement technique for sandy soil, which can alter the properties of sand via microbial activity. In this study, we investigated the loose-sand-consolidation effect by controlling the injection velocity, bacterial and cementing-solution concentrations, and hold times. The results demonstrate that, as the cyclic batch increases, the utilization rate of the bacterial fluid increases and both the optical density (OD600) of the bacteria and urease activity decrease. Moreover, it was determined that a 3-h hold time for a 0.5 mol/L cementing solution with a cementing fluid velocity of 2 mL/min has the greatest bonding effect. The final strength of the loose sand with an increase in calcium carbonate content was further discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the local and global acceleration of urbanization, the demand for civil infrastructure is growing at an unprecedented rate. During the construction of water conservancy projects, the soil mass that does not meet specific project requirements may be encountered. In such cases, the unsatisfactory soil must be strengthened.
Most foundation reinforcement methods use mechanical or artificial materials (e.g., cement, epoxy resin, sodium silicate, and polyurethane) to generate a bonding effect. Furthermore, the entire strengthening process requires the consumption of a large quantity of building materials [1]. For example, a large consumption of cement is considered as an important contribution to global warming [2, 3]. The disadvantages of cement usage, such as its high energy consumption, high pollution emission, and high cost, significantly limit its practical applications. As for chemical grouting, most chemical pulp is toxic except for sodium silicate [4]. The main components of soil curing agents, which are not environmentally friendly, will inevitably spread to the surrounding environment.
Microbial mineralization is a common phenomenon in nature. Several microbes can use their own metabolisms to generate a variety of mineral crystals. Calcium carbonate is one of the most widely distributed carbonates in nature, and it is durable; therefore, the microbial deposition of calcium carbonate has been a popular topic in the study of microbial mineralization.
The application of microbial mineralization technology in the field of building materials was successful [5,6,7,8]. This technology has also been widely used in various fields, such as waste water treatment [9], sand consolidation [10, 11], and decrease in permeability [12]. Studies have found that the greenhouse gases produced and the energy consumed during the process of microbial reactions can be ignored [13]. To summarize, microbe-induced calcium carbonate precipitation (MICP) is a promising bio-mediated technology and can be applied to many practices.
However, most studies of the microbe-induced calcium carbonate mineralization process mainly focused on the environment of microbial solution [14, 15]. These studies did not report the microbial mineralization process in loose sand granular environments [16]. Therefore, the purpose of this study is to achieve a better consolidation effect by controlling the injection velocity, concentrations of the bacterial and cementing solution, and hold times in loose sand. At the same time, we try to explain the effect of different CaCO3 concentrations on the mechanical properties of the sand.
MICP Technology
MICP technology is well-documented in Ref. [17]. In rock and soil applications, loose sand is the main environment for MICP. Given the presence of extracellular polymeric and double layer structures, microorganisms tend to approach the surface of particles (Fig. 1). The cell wall of microorganisms usually possesses a large number of negative ions, which facilitates the efficient adsorption of calcium ions. The reaction equation is described as follows [Eq. (1)]:
Moreover, the reaction of organic substrate urea-accelerated hydrolysis under the action of urease is expressed as follows:
Urea decomposition leads to an increase in the pH value of the microenvironment around the microbial cells [Eqs. (2), (3)]. The high pH conditions are conducive to the formation of \({\text{CO}}_{3}^{2 - }\) from \({\text{HCO}}_{3}^{ - }\) [18] [Eqs. (4), (5)]:
Finally, the following equation describes the nucleation and crystal growth of calcium carbonate around the cell [Eq. (6)]:
Materials and Methods
Bacterial Solution
An isolated bacterial culture of Sporosarcina pasteurii ATCC 11859 was used in this study. One mL of the strain was inoculated in 100 mL of the culture medium (Table 1). Then, the inoculated culture medium was placed on a shaker table at 220 rpm for 24 h during growth. Subsequently, the bacterial culture was harvested at the end of the growth phase and was stored at 4 °C for subsequent use.
Cementation Solution
The type and concentration of the calcium source were not the same as the amount of calcium carbonate that was formed by microbial mineralization [19]. Compared with other calcium sources, CaCl2 is the most common source of calcium. According to the bacterial consolidation mechanism, urea and calcium sources are the basic substances used in bacterial bonding. Here, a solution of CaCl2 and urea served as the fixation solution and cementation fluid.
Properties of Sand
The sand used in this experiment was standard sand. The sand was sieved by a 0.6 mm sieve prior to use and the smaller particles were chosen. A summary of the basic physical properties and parameters of the sand is shown in Tables 2 and 3. The grain size distribution of the sand is shown in Fig. 2.
Instrumentation
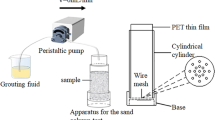
A PVC mold with a height and a diameter of 9 and 3 cm, respectively, was used to fabricate the specimens (Figs. 3, 4). According to the test conditions in the first phase (Table 4), a 65 g metage of standard sand was placed into the mold (6 cm in height and 3 cm in diameter). To provide a better flow channel for the next phase of injection, the sand samples were passed into distilled water at a flow rate of 2 mL/min for each column. Subsequently, 16.5 mL of the bacterial solution was introduced for each test condition, and the bottom of the mold was closed for a 1-h hold time. This step was immediately followed by the injection of 16.5 mL of a cementing solution. The hold times were 3, 3, and 6 h after the injection of the cementing solution to each of the sand columns. All samples were injected with 16.5 mL of the cementing solution three times every 3, 6, and 3 h. One cycle consisted of three additions of cementing solution and bacterial liquid, and a total of five cycles were performed for each sample.
Results and Discussion
Results for Bacteria
To analyze the adsorption effect of bacteria in the sand column and the urea hydrolysis ability, the oxygen demand (OD) value and urease activity were calculated for each batch of bacterial liquid outflow. The test results in Fig. 5 are discontinuous and exhibit a certain dispersion. However, the overall trend shows that the adsorption rate of the first batch of the bacterial liquid is low. With an increasing number of cyclic batches, the utilization rate of the bacterial fluid increased. Furthermore, the OD value of the bacteria and urease activity showed a decreasing trend.
For the 2 mL/min injection velocity and 0.5 mol/L concentration conditions of cementing fluid, the OD value changed due to the bacteria at a 6-h hold time. The change was slower with an increasing number of cyclic batches compared with the static 3-h condition. This result occurred because of an increase in the hold time. Specifically, the nutrient was consumed so that the rate and quantity of the extracellular polymer that was caused by microbial cell death and microbial metabolism decreased [19]. In contrast, for a shorter settling time, the bacteria showed increased activity. With the solidification and cementation of the sand column, the internal space of the loose sand column was gradually reduced. If a “bridge” was established among the particles, then the number of bacteria that was adsorbed on the surface of the particles increased and both the bacterial OD value and urease activity decreased.
A test condition that included a 5 mL/min injection velocity and a 1 mol/L cementing-solution concentration was compared with a test condition of 2 mL/min injection velocity and a 0.5 mol/L cementing-solution concentration for the same static time of 3 h. Early cementation possessed its own adequate calcium source, which was beneficial for the solidification and cementation of the sand column and eased the enrichment of cementing formation. Nevertheless, bacteria and sand remained as the internal cementation material in the column because the injection velocity was large and the injected cement liquid was washed away easily. Finally, this test condition showed a high OD value and a high urease activity of bacteria.
Calcium Ion Utilization Ratio
The abovementioned results show that an increase in the cyclic batches increased the utilization rate of the bacterial fluid. Therefore, the calcium ions in the solution can be adsorbed through the cell wall surface of the microorganisms, which are on the surface of the sand particles that have a large number of bacteria. Quantitative analyses of calcium carbonate in the sand columns under the three test conditions were conducted to better analyze the calcium ion utilization ratio.
Precipitated CaCO3 serves as a binding material, which binds soil particles and improves the mechanical properties of the soil. Therefore, a reasonable method to quantify the amount of calcite is important. This study used three techniques to evaluate the calcite content: thermal decomposition method, acid leaching method (ALM), and CO2 volume evaluation (CVE) method. According to Neupane et al. [20], the calcite amount obtained using the ALM and thermal decomposition methods was obviously higher than the calcite amount that was evaluated by the CVE method. In the ALM, the flushing of soluble impurities led to mass loss during washing, which resulted in higher calcite percent values. In the thermal decomposition method, the presence of ammonium as a by-product of the calcite precipitation reaction and the boiling of the ammonium noticeably reduced the decomposition of CaCO3; thus, the mass loss is attributed to CaCO3 decomposition and includes ammonium evaporation. Detailed test procedures were introduced by Neupane et al. [20] to improve experimental accuracy and to evaluate the effect of calcium carbonate precipitation under the selected conditions. This study utilized CVE as the calcite quantification method. The concentration of hydrochloric acid was 1 mol/L. A schematic of the experiment is shown in Fig. 6.
The sand calcium was divided into three parts: top, middle, and bottom. Every part of the sand column is fully reflected in Fig. 7d. The test results are shown in Table 5.
The chemical equation shown below was obtained after determining whether 3.285 g of CaCO3 was thoroughly dissolved in 90 mL of hydrochloric acid. Table 5 shows that, after the actual reaction, the calcium carbonate quantity was noticeably less than 3.285 g. Therefore, the CaCO3 was fully dissolved in 90 mL of hydrochloric acid.
A comparison of calcite in the test samples at different positions is shown in Fig. 8. The CaCO3 content was the largest at the top location of the three test cases, and it was the lowest at the bottom. The CaCO3 content at the same concentration was compared at different hold times. The results indicate that for 0.5 mol/L cementing solutions, the top CaCO3 content was lower at a 3-h hold time compared with that of a 6-h hold time. Furthermore, the CaCO3 content decreased by 13.7%. In contrast, the CaCO3 content in the middle location at a 3-h hold time was higher than that of a 6-h hold time. This tendency was also observed at the bottom of the sand column. The entire concentration achieved better uniformity at the 3-h hold time. This result is consistent with the view that bacteria exhibit a high degree of activity over a short settling time. As the number of bacteria that were adsorbed on the surface of particles increased, their OD value and urease activity decreased. With the solidification and cementation of the sand column, the internal space of the loose sand column was gradually reduced. This phenomenon is beneficial for the solidification and cementation of the sand column.
At a 5 mL/min injection velocity, the CaCO3 content was lower at the top, middle, and bottom of the sand column. A lower content was also observed for the 2 mL/min condition. This result occurred because the flow rate was too fast and not conducive to the use of calcium ions; the utilization rate was, therefore, reduced so that less CaCO3 was generated.
Strength Analysis
The consolidated sand column specimens are shown in Fig. 9. Subsequently, the sand columns were utilized for uniaxial testing at a loading rate of 0.4 mm/min (or 2% strain). The uniaxial compression test results are shown in Table 6.
The test results from Table 6 show that when the cementing-solution concentration was 0.5 mol/L, the strength value for the static 3-h condition was obviously higher than that for the 6-h condition. Specifically, the specimen strength was 2 times that of the 6-h condition (Fig. 10). This phenomenon occurred because of the static 3-h condition and low density of the calcium carbonate crystals, which were formed in the urea hydrolysis by weak bacteria. Weak bacteria can infiltrate the cementing solution and improve the overall spatial distribution. For the static 6-h condition, there was a weak bond effect because the cementing solution and bacteria showed a longer reaction time and the top density was improved. These settings are not conducive to the infiltration of the cementing solution. As a result, the material required for the formation of calcium carbonate cannot be well provided, and the content of calcium carbonate was lower in the sand column. However, an increase in the calcium carbonate content helped improve the strength index [21]. The strength increased with an increase in CaCO3 content [20]. For the 3-h hold time condition, the number of microorganisms that were adsorbed on the surface of loose particles increased and, hence, the CaCO3 quantity increased. The CaCO3 content was 39%, which was higher than that for the 6-h condition with a CaCO3 content of 38%. Therefore, the 3-h hold time yields better strength. Thus, the strength value for static 3-h condition was obviously higher than that for the 6-h condition.
For the 1 mol/L–3 h test condition, the calcium ion utilization rate was low and led to the lowest strength among the three conditions because of the high flow rate of the cementing fluid. At higher cementing fluid concentrations (greater than 1 mol/L), most of the urea and calcium ions were not fully utilized [21]. Harkes et al. [22] concluded that low concentrations of cementing fluid are beneficial for solidified cementation. Our result shows that high concentrations and flow rates do not improve the strength.
Additionally, an examination of Table 6 indicates that the failure position was located at the bottom of the sand column. The loading position on the sand column under the 1 mol/L–3 h test condition was switched to verify the failure phenomenon. Once again, the failure position occurred on the bottom. Therefore, the compactness of the top of the sand column is greater, whereas the overall spatial distribution of the sand column is uneven. This occurred because the top of the sand column preferentially reacted in the bonding period, which leads to improved compactness. With circular injections of the bacterial solution and cementing solution, the sand voids near the top sand position were easily blocked by calcium carbonate crystals. The internally porous sand column was gradually solidified and compacted, and the calcium carbonate in the sand samples was not uniformly distributed [10, 23]. The permeability gradually decreased so that the bond strength at the bottom of the sand column deteriorated. Thus, during the uniaxial test, the bottom end was preferentially damaged.
The results demonstrate that during the entire uniaxial compression test, all the curing processes followed the same type of calcification with certain differences. Because these processes belong to the same type of calcification process, the influence of boundary effects was not considered in our analysis of the compressive strength.
Based on the abovementioned results, both the cementing fluid velocity and concentration have a large effect on the influence of calcium carbonate cementing regardless of whether the speed or flow rate are beneficial for the cement.
Conclusions
This study demonstrates that both the cementing fluid concentration and velocity significantly influence the uniformity, strength index, and CaCO3 content of sand columns. With an increase in cyclic batches, the utilization rate of the bacterial fluid increased, and the OD value of the bacteria and urease activity showed a decreasing trend. Higher concentrations and flow rates did not improve the specimen strength.
An increase in the hold times decreased the calcite precipitation. Furthermore, the uniaxial compressive strength decreased. Overall, the calcium carbonate content at the top of the sand column exceeded the content at the bottom of the sand column, and the 3-h hold time for the 0.5 mol/L cementing solutions with a cementing fluid velocity of 2 mL/min provided a homogeneous calcium carbonate distribution.
Furthermore, the results of the uniaxial compression tests showed that the precipitated calcium carbonate can improve the mechanical properties of the soil. The maximum strength value reached 3.29 MPa. Decreasing the hold times and maintaining a reasonable velocity will facilitate an improved cementing process for field applications.
References
Schneider M, Romer M, Tschudin M et al (2011) Sustainable cement production—present and future. Cem Concr Res 41:642–650
Cayan DR, Bromirski PD, Hayhoe K et al (2008) Climate change projections of sea level extremes along the California coast. Clim Change 87:57–73
Karol RH, Mortensen BM, Martinez BC et al (2010) Bio-mediated soil improvement. Ecol Eng 36:197–210
Karol HR (2003) Chemical grouting and soil stabilization, Revised and Expanded, 3rd edn. Rutgers University, Marcel Dekker, Inc., New Jersey
Ramachandran SK, Ramakrishnan V, Bang SS (2001) Remediation of concrete using micro-organisms. ACI Mater J 98:3–9
De Muynck W, De Belie N, Verstraete W (2010) Microbial carbonate precipitation in construction materials: a review. Ecol Eng 36:118–136
Salifu E, MacLachlan E, Iyer KR et al (2016) Application of microbially induced calcite precipitation in erosion mitigation and stabilisation of sandy soil foreshore slopes: a preliminary investigation. Eng Geol 201:96–105
Khaliq W, Ehsan MB (2016) Crack healing in concrete using various bio influenced self-healing techniques. Constr Build Mater 102:349–357
Hammes F, Boon N, de Villiers J et al (2003) Strain-specific ureolytic microbial calcium carbonate precipitation. Appl Environ Microbiol 69:4901–4909
Whiffin VS, van Paassen LA, Harkes MP (2007) Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol J 24:417–423
Ivanov V, Chu J (2008) Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev Environ Sci Bio Technol 7:139–153
Nemati M, Voordouw G (2003) Modification of porous media permeability, using calcium carbonate produced enzymatically in situ. Enzyme Microb Technol 33:635–642
Achal V, Mukherjee A (2015) A review of microbial precipitation for sustainable construction. Constr Build Mater 93:1224–1235
Okwadha GD, Li J (2010) Optimum conditions for microbial carbonate precipitation. Chemosphere 81:1143–1148
Huang Y, Luo XG, He J et al (2009) Studies on the experiment of microbiologically-induced calcite precipitation in silica. J Southwest Univ Sci Technol 24:65–69 (in Chinese)
Rong H, Qian CX, Zhang L et al (2015) Cementation process of microbe cement. J Chin Ceram Soc 43:1067–1075 (in Chinese)
Mitchell AC, Dideriksen K, Spangler LH et al (2010) Microbially enhanced carbon capture and storage by mineral-trapping and solubility-trapping. Environ Sci Technol 44:5270–5276
Knoll AH (2003) Biomineralization and evolutionary history. Rev Mineral Geochem 54(1):329–356
Rong H (2014) Preparation and binding mechanism of microbe cement. Dissertation, Southeast University, Nanjing, China (in Chinese)
Neupane D, Yasuhara H, Kinoshita N et al (2015) Distribution of mineralized carbonate and its quantification method in enzyme mediated calcite precipitation technique. Soils Found 55:447–457
Zhao Q (2014) Experimental study on soil improvement using microbial induced calcite precipitation (MICP). Dissertation, China University of Geosciences, Beijing, China (in Chinese)
Harkes MP, van Paassen LA, Booster JL et al (2010) Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol Eng 36:112–117
Stocks-Fischer S, Galinat JK, Bang SS (1999) Microbiological precipitation of CaCO3. Soil Biol Biochem 31:1563–1571
Acknowledgements
This study was supported by the National Key Research & Development Program of China (no. 2016YFC0401904), the Science Fund for Creative Research Groups of the National Natural Science Foundation of China (no. 51621092), the National Natural Science Foundation of China (nos. 51309177 and 51409187), and the Program of Introducing Talents of Discipline to Universities (No. B14012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, Y., Lian, J., Xu, G. et al. Effect of Cementation on Calcium Carbonate Precipitation of Loose Sand Resulting from Microbial Treatment. Trans. Tianjin Univ. 23, 547–554 (2017). https://doi.org/10.1007/s12209-017-0084-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-017-0084-8