Abstract
Introduction
Vascular devices such as stents, hemodialyzers, and membrane oxygenators can activate blood coagulation and often require the use of systemic anticoagulants to selectively prevent intravascular thrombotic/embolic events or extracorporeal device failure. Coagulation factor (F)XII of the contact activation system has been shown to play an important role in initiating vascular device surface-initiated thrombus formation. As FXII is dispensable for hemostasis, targeting the contact activation system holds promise as a significantly safer strategy than traditional antithrombotics for preventing vascular device-associated thrombosis.
Objective
Generate and characterize anti-FXII monoclonal antibodies that inhibit FXII activation or activity.
Methods
Monoclonal antibodies against FXII were generated in FXII-deficient mice and evaluated for their binding and anticoagulant properties in purified and plasma systems, in whole blood flow-based assays, and in an in vivo non-human primate model of vascular device-initiated thrombus formation.
Results
A FXII antibody screen identified over 400 candidates, which were evaluated in binding studies and clotting assays. One non-inhibitor and six inhibitor antibodies were selected for characterization in functional assays. The most potent inhibitory antibody, 1B2, was found to prolong clotting times, inhibit fibrin generation on collagen under shear, and inhibit platelet deposition and fibrin formation in an extracorporeal membrane oxygenator deployed in a non-human primate.
Conclusion
Selective contact activation inhibitors hold potential as useful tools for research applications as well as safe and effective inhibitors of vascular device-related thrombosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascular devices, including ventricular assist devices (VAD), stents, and extracorporeal membrane oxygenators (ECMO), are prone to surface-initiated thrombus formation.20,30 While these devices provide needed cardiovascular support to patients, their use inevitably exposes blood to non-biological surfaces and non-physiological shear stress, creating a highly pro-coagulant environment.16 The current standard of care to prevent thrombotic complications for some vascular devices, including venous and arterial thromboembolism, or occlusions within extracorporeal vascular device circuits (extracorporeal organ support, ECOS) sometimes mandates the administration of anticoagulation. Currently available anticoagulants (e.g., heparin, warfarin, direct coagulation factor (F) X inhibitors) target enzymes in the extrinsic, intrinsic, and common pathways of blood coagulation. While these anticoagulants prevent vascular device-associated thrombosis, the extrinsic and common pathways are critical for hemostasis, their inhibition universally increase the risk of bleeding, including life-threatening hemorrhage.30 Therefore, there is an urgent need to develop new strategies for safer anticoagulation capable of preventing vascular device-associated thrombosis. Our study was designed to develop and characterize inhibitors of the contact activation system (CAS), and in particular, FXII, which is activated by foreign surfaces, including vascular devices, yet is not required for normal hemostasis.16 Our efforts in developing exclusively intrinsic pathway inhibitors, including FXII, are consistent with the original concept that an anticoagulation strategy targeting FXII or activated FXII (FXIIa) could provide thromboprotection without hemostasis impairment.3,14,17
FXII (Hageman factor) is an 80 kDa, 596 residue long single-chain glycoprotein proenyzme that is produced and secreted by hepatocytes. It is encoded by a 12 kb gene comprised of 13 introns and 14 exons, located at chromosome 5q33-qter. Upon activation, it is cleaved into a 353 residue heavy chain joined to a 243 residue long light chain, which contains part of the catalytic domain joined by a disulfide bond (α-FXIIa) (Fig. 1a). There are seven structural domains: a fibronectin domain type II, two epidermal growth factor (EGF) domains, a fibronectin domain type I, a kringle domain, a proline-rich region, and the protease (catalytic) domain.29 The physiologic plasma concentration of FXII in humans is in the range of 30–40 µg/mL.24 Phylogenetic studies have demonstrated that FXII likely arose as a genetic duplication event; FXII is conserved across a number of species, including human, opossum, platypus, and frog, but is absent in birds and many sea creatures.18 Humans and mice with congenital deficiencies in FXII exhibit in vitro prolongation of the activated partial thromboplastin time (aPTT), yet lack an abnormal bleeding phenotype, in stark contrast to other coagulation factor deficiencies such as FVIII and FIX (hemophilia A and B, respectively).21 Pharmacologic inhibition or genetic knockout of FXII has been shown to reduce thrombus formation in both in vitro and in vivo experimental models, again whilst leaving hemostasis intact.15,22
When blood contacts negatively charged surfaces, including a number of biological molecules and artificial materials such as those that comprise vascular devices, the zymogen FXII is cleaved after Arg353, which generates the serine protease α-FXIIa.33 The fate of α-FXIIa is several-fold: (1) α-FXIIa cleaves factor XI (FXI) to form FXIa and ultimately produces thrombin (factor IIa [FIIa]) to drive platelet activation and fibrin formation; (2) α-FXIIa activates prekallikrein (PK) to form α-kallikrein, which both converts FXII to α-FXIIa in a reciprocal feedback mechanism, and activates major components of complement system C3 and C5, which subsequently activate both the classical and alternative complement activation pathways;16 moreover, kallikrein cleaves the cofactor high molecular weight kininogen (HK) to release bradykinin (BK), a systemic vasoregulatory and inflammatory mediator;20,30 (3) α-FXIIa is cleaved after Arg334 to generate β-FXIIa, which is comprised of the α-FXIIa catalytic domain and a 2 kDa heavy chain remnant (Fig. 1b); and (4) β-FXIIa, while unable to generate FXIa, is additionally able to activate PK to form α-kallikrein.
Herein, we describe the generation and characterization of antibodies against FXII used to study FXII activation pathways and the function of FXII in promoting thrombus formation under shear flow. We also validate that inhibiting FXII activity prevents platelet deposition and fibrin formation in blood perfused membrane oxygenators.
Materials and Methods
Generation of Anti-FXII Monoclonal Antibodies
Antibodies against FXII were generated as previously described.29 In brief, three FXII-deficient Balb-C mice were immunized with an intraperitoneal (IP) injection of a 30 µg mixture of recombinant murine and human FXII in Freund’s complete adjuvant on day 0, and 20 µg mixture in Freund’s incomplete adjuvant on day 18. A 10 µg booster dose in saline was administered on day 46. Spleens were harvested on day 49, and a standard polyethylene glycol-based protocol was used to fuse lymphocytes with P3X63Ag8.653 myeloma cells. Production runs of 50–400 mL hybridoma cultures were grown and antibodies were purified using a protein A matrix.
Expression of Recombinant FXII and Antibody Mapping
Complementary DNA (cDNA) from human FXII was inserted into vector pJVCMV.18 Using polymerase chain reaction (PCR), the sequence encoding individual domains from the FXII homolog hepatocyte growth factor activator (HGFA) was amplified from the human HGFA cDNA and used to replace the corresponding sequence in the FXII cDNA (Fig. 1c).21 HEK293 fibroblasts (ATCC-CRL1573) were transfected with pJVCMV/ FXII-HGFA constructs as described.18 Anti-FXII monoclonal antibodies were used as the primary antibody in Western blots to detect FXII in human and mouse plasma, as well as purified α- and β-FXII. Purified FXII antibodies were size-fractionated on 10% polyacrylamide–sodium dodecyl sulfate gels, and chemiluminescent Western blots were prepared using 1D7, 5C12, 7G11, 9G3, 15D10, or goat polyclonal-anti-human FXII immunoglobulin G (IgG) for detection.
Western Blot of Plasma FXII
Platelet-poor plasma (PPP) was obtained as previously described from the following wide range of species: giant anteater (Myrmecophaga tridactyla), cattle (Bos taurus), horse (Equus ferus caballus), pig (Sus scrofa domestica), rabbit (Oryctolagus cuniculus), raccoon (Procyon lotor), Amur tiger (Panthera tigris altaica), human (Homo sapiens), baboon (Papio anubis), domestic cat (Felis silvestris catus), chicken (Gallus gallus domesticus), domestic dog (Canis lupus familiaris), African elephant, (Loxodonta africana), llama (Lama glama), cynomolgus monkey (Macaca fascicularis), rhesus macaque (Macaca mulatta), rat (Rattus norvegicus domestica), African green monkey (Chlorocebus aethiops), and marmoset (Callithrix jacchus).1 One μL PPP was size fractionated on SDS-polyacrylamide gels under reducing or non-reducing conditions, followed by Western blotting using the different monoclonal antibodies.
Blood Sample Analysis
Activated Partial Thromboplastin Time (aPTT) Nine parts venous blood was drawn by venipuncture from healthy male and female adult volunteers into one part 3.2% sodium citrate in accordance with the OHSU (Oregon Health & Science University) Institutional Review Board (No. 1673). Informed consent was received from all human blood donors. Platelet-poor plasma was prepared by centrifugation of citrated whole blood at 2150×g for 10 min. Further centrifugation of the plasma fractions at 2150×g for 10 min yielded platelet-poor plasma, which was pooled and stored at – 80 °C until use.
aPTT measurements were made in duplicate using SynthASil (Instrumentation Laboratory, Bedford, MA, USA) and a KC4 Analyzer (TCoag, Ltd, Wicklow, Ireland). Activated clotting time (ACT) of blood was determined in duplicates using LupoTek KCT (r2-Diagnostics, South Bend, IN, USA). Prothrombin time (PT) assay was conducted with Dade® Innovin® (Siemens Healthcare Diagnostics, Flanders, NJ, USA) according to the manufacturer’s protocol. PT of plasma samples was quantified using a KC4 Analyzer. Plasma thrombin-antithrombin complex (TAT) were measured with ELISA kits from Siemens (Flanders, NJ, USA) as previously described.
In separate studies, citrated platelet-poor plasma was obtained from the following species: human (Homo sapiens), baboon (Papio anubis), cynomolgus monkey (Macaca fascicularis), rhesus macaque (Macaca mulatta), mouse (Mus musculus), rat (Rattus norvegicus), and rabbit (Oryctolagus cuniculus) and serially diluted into FXII-depleted human plasma (Affinity Biologicals). These dilutions were incubated with 20 µg/mL anti-FXII monoclonal antibody and aPTT was measured using a KC-4 coagulometer (Tcoag, Ltd, Ireland; SynthasIL Reagent, Instrumentation Laboratory) as described above.
Non-activated thromboelastometry analysis (NATEM) NATEM was measured immediately after venous blood collection (within 5–10 min). Human blood was taken into syringes containing 3.2% (w/v) sodium citrate (one-tenth of blood volume) and FXII antibodies (40 µg/mL, final concentration) were added prior to recalcification (300 µL blood re-calcified with 20 µL 200 mM CaCl2) for use in the NATEM assay following standard protocols.
FXII Activation and FXIIa Inhibition
FXII activation and FXIIa inhibition were measured as previously described.19 In brief, to measure activation of FXII, human FXII (40 nM; Haematologic Technologies, Inc., Essex Junction, VT) was incubated with an anti-FXII monoclonal antibody (mAb) (0–80 nM) for 10 min at room temperature, followed by dextran sulfate (1 µg/mL) for 20 min at 37 °C. Spectrozyme FXIIa (0.5 mM; Sekisui Diagnostics GmbH, Germany) was then added to measure hydrolysis by activated FXII (FXIIa). To assess the effect on FXII activation by polyphosphates, FXII (100 nM) was co-incubated with an anti-FXII mAb (0–200 nM) for 10 min at room temperature. The mixture was diluted 50/50 with HK (12.5 nM, Enzyme Research Laboratories, South Bend, IN), PK (12.5 nM, Enzyme Research Laboratories, South Bend, IN), and polyphosphates [long (> 1000 phosphate units) or short (~ 70–100 phosphate units)].27 The antibody solution (10 µM, final concentration) was incubated for 60 min at 37 °C. Polybrene (120 µg/mL, Sigma-Aldrich, Saint Louis, MO) and soybean trypsin inhibitor (1 mg/mL, Sigma, Sigma-Aldrich, Saint Louis, MO) were added to the antibody solution and the amidolytic activity was quantified. To test FXIIa inhibition, Spectrozyme FXIIa was added to mixtures of FXIIa (20 nM) and anti-FXII mAb (0–40 nM). The samples were read at 405 nm.
Flow Chamber Analysis
Glass capillary tubes (0.2 × 2 × 50 mm; VitroCom, Mountain Lakes, NJ, USA) were coated with 100 µg/mL of fibrillar type I collagen (Chrono-Log Corp, Havertown, PA, USA) for 1 h at room temperature (RT), then washed with PBS and blocked with 5 mg/mL denatured bovine serum albuminutes for 1 h at RT. The capillary tubes were connected to a blood reservoir and a syringe pump. Human venous blood was collected by venipuncture into syringes containing 3.8% (w/v) sodium citrate (one-tenth of blood volume). Blood was incubated with 100 µg/mL 1B2, 1D7, or 5A12 for 10 min at RT. Re-calcification buffer (1:9, buffer:blood; 75 mmol/L CaCl2 and 37.5 mmol/L MgCl2) was added to allow for coagulation prior to perfusion at venous shear rate of 300 s−1 for 10 min at 37 °C. Capillaries were washed with PBS, fixed with 4% paraformaldehyde and sealed with Fluoromount-G Mounting Medium (ThermoFisher Scientific). Platelet aggregates and fibrin formation in the capillaries were imaged for analysis using a 63× Zeiss Axio Imager M2 microscope (Carl Zeiss MicroImaging GmbH, Germany) as previously described.36
Anticoagulation of Baboons
All animal experiments were approved by the Institutional Animal Care and Use Committee of Oregon Health & Science University. To establish the time course of coagulation parameters, one anti-FXII antibody that binds the FXII catalytic domain (1B2), and one that bind the FXII heavy chain (1D7) were selected. 1B2 (n = 2) or 1D7 (n = 1) were administered to baboons (Papio anubis) in subsequent i.v. doses of 1 mg/kg every 40 min over a 4-h period, totaling six doses. A baseline measurement was made prior to drug administration at time = 0. Blood samples were collected for up to three weeks post administration and aPTT, ACT, PT, TAT levels were measured. Complete blood count (CBC) was also measured to determine platelet count, hematocrit concentration, white blood cell count, and red blood cell count.
Baboon Model of Thrombogenesis in Extracorporeal Membrane Oxygenators
All animal experiments were approved by the Institutional Animal Care and Use Committee of Oregon Health & Science University. Two juvenile baboons (Papio anubis) were repeatedly employed for the perfusion experiments. Baboons were implanted with exteriorized femoral arteriovenous shunts as described previously.4,8,9 In brief, 111In-labeled autologous platelets and 125I-labeled homologous fibrinogen were used for measurement of thrombus formation in the saline-primed oxygenator cartridge that was interposed in the exteriorized shunt loop (Terumo-CAPIOX® RX05, coated hollow fiber design, Terumo Cardiovascular Group, Ann Arbor, MI). Animals were administered 1B2 intravenously at a 5 mg/kg initial dose with 2 mg/kg daily maintenance dose. The vehicle control experiments were performed in week one, prior to antibody treatment in week two. Platelet radioactivity within the oxygenator was recorded in real-time using gamma camera imaging (GE-Brivo NM 615 interfaced with Xeleris 3.1 software, GE Healthcare, Chicago, IL). Platelet deposition was calculated as previously described.8,9,11,31 For quantification of 125I-fibrin content at the 60 min endpoint, the cartridge was removed, rinsed, dried, and stored refrigerated until processing. In brief, the oxygenators were filled with digest buffer (10 mM Tris-H3PO4 pH 7.0, 35 mM SDS) for three days. The radioactivity of the digest solution was measured using a gamma counter (Wizard-3, PerkinElmer, Shelton, CT), and the amount of trapped fibrin/fibrinogen was calculated as previously described.9,11 Bleeding times were evaluated twice during each administration of 1B2 using the adult Surgicutt® device (International Technidyne, Piscataway, NJ), as described previously. Template bleeding time (BT) was recorded twice during each experiment and averaged.10,12 Briefly, a pressure cuff at 40 mm Hg was applied to the upper arm, and a 5 mm long × 1 mm deep incision was made on the volar surface of the lower arm. Blood drops were collected on a Whatman® filter paper every 30 s until the bleeding stopped.
Results
Generation and Characterization of Monoclonal FXII Antibodies
FXII antibodies were generated by immunization of FXII knock-out mice with a mixture of human and mouse FXII zymogen. A total of 463 monoclonal antibodies were identified in an ELISA screen as binders of murine and/or human FXII. Using an aPTT clotting assay, we screened the cell culture supernatants using platelet-poor plasma serially diluted into FXII-deficient plasma (1:128 dilution), which gave a baseline aPTT of ~ 50–70 s. Of these, 78 clones were shown to prolong aPTT of human platelet-poor plasma (> twofold increase in clotting times over baseline); these antibodies were then tested for cross-reactivity with mouse FXII in plasma. We identified two antibodies that robustly prolonged aPTT of human plasma only (1B2 and 5C12) and four that prolonged the aPTT of both human and mouse plasma (1D7, 7G11, 15D10, and 9G3). Additionally, 5A12, despite binding FXII, did not prolong the aPTT of human platelet-poor plasma.
The binding epitopes for these antibodies on human and mouse FXII were determined by Western blot. We used plasma-derived α- and β-FXIIa to identify the binding region. Our data showed that only two human-specific antibodies, 1B2 and 5C12, recognized β-FXIIa, which only contains the catalytic domain of α-FXII; neither bound to murine FXII. The antibodies 1D7, 7G11, 9G3, 15D10, and 5A12 recognized both human and mouse FXII and did not bind β-FXIIa, which suggests that they bind to the heavy chain of FXII (Figs. 2a and 2b).
We next used FXII/HGFA chimeras to identify the FXII regions containing the binding sites for the anti-FXII antibodies. As the F12 gene arose from a duplication of the HGFA gene, FXII and HGFA have similar domain structures with the exception that FXII has a proline-rich region not found in HGFA. We prepared FXII with individual domains replaced by corresponding HGFA domains as previously described (Fig. 1c).17 The antibody 1D7 was the only antibody which recognized both the reduced and non-reduced forms of human FXII (Fig. 2a); 1D7 was found to recognize the second epidermal growth factor (EGF2) domain, as evidenced by the reduction in detection when this domain was swapped out for the H4 domain of HGFA. Similarly, 5A12 detected the second EGF2 domain of FXII. Deletion or exchange of the fibronectin type 2 (F2), as well as the first and second EGF domains (EGF1, EGF2) of FXII regions with the HGFA homologous region reduced the detection of these FXII chimeras by the antibodies 7G11, 9G3, and 15D10 by Western blot (Fig. 2c).
Cross-Reactivity of Monoclonal FXII Antibodies
As antibodies were raised in FXII-deficient mice, it is possible that some of them recognize epitopes on FXII that are conserved between species. We evaluated the species cross-reactivity of the anti-FXII antibodies with FXII derived from a range of vertebrate animals (Fig. 3a). The most universal antibodies in our set were 7G11 and 15D10, which recognized FXII from giant anteater, cattle, horse, pig, raccoon, tiger, baboon, dog, elephant, and llama. A slight band was detected by 7G11 and 15D10 for rabbit FXII. Despite 9G3 binding to the same three domains as 7G11 and 15D10, 9G3 was only found to detect FXII in plasma from raccoon, tiger, dog, elephant, and llama. 9G3 detected FXII from human but not baboon plasma. The antibody 1D7 detected FXII in the plasma from cattle, horse, rabbit, raccoon, tiger, baboon, dog, elephant, llama, and uniquely, cat FXII. The antibody 5A12 detected FXII in plasma from cattle, pig, rabbit, tiger, baboon, elephant, and llama. The antibodies 1B2 and 5C12 were shown to detect FXII in plasma from giant anteater and baboon. All of the antibodies were shown to detect both the positive control (human FXII), with FXII-depleted plasma serving as a negative control (Fig. 3a). These studies confirm that immunizing mice with a protein in which they are deficient can generate antibodies that cross-react with presumed conserved epitopes across species.
We extended our study to characterize the binding of a subset of anti-FXII antibodies to plasmas from animals commonly used in preclinical research including NHPs. Our data showed that 1B2 and 5C12 detected FXII in plasma from African green monkeys in addition to baboons and humans (Fig. 3b). Likewise, the antibodies 1D7 and 5A12 detected FXII in plasma from baboons and humans as well as cynomolgus monkey, rat, and marmoset; a slight band was shown for both antibodies for detection of rhesus macaque FXII. 5A12 detected FXII from African green monkey, while 1D7 detected FXII in beagle plasma (Fig. 3b).
Effect of Monoclonal FXII Antibodies on Clotting Times of Plasma and Whole BLOOD
We measured the effect of each antibody on the aPTT using pooled human platelet-poor plasmas (PPP) that were serially diluted with human FXII-depleted PPP. Our reference data, serially diluted normal pooled human plasma with FXII-depleted plasma alone, showed that aPTT remained near 30 s until the level of FXII was reduced by > 95%, at which point there was a sharp increase in aPTT that exceeded 250–300 s as the amount of FXII remaining fell near or below 1% of normal (Fig. 4a). This pattern was conserved across the seven species tested (human, baboon, mouse, rat, cynomolgus, rhesus macaque, and rabbit (Figs. 4b–4h).
Serially diluted normal pooled human plasma with FXII-depleted plasma alone was used as reference data (a). Platelet-poor plasma from (b) human, (c) baboon, (d) cynomolgus, (e) rhesus, (f) mouse, (g) rat, or (h) rabbit was serially diluted into FXII-deficient human plasma. These dilutions were incubated with 20 µg/mL of FXII antibody candidates. Clotting times were measured following addition of an aPTT reagent. Changes in aPTT were plotted as a function of FXII levels on a logarithmic scale.
The diluted aPTT assay was employed to detect the potential inhibitory effects of the FXII antibodies on clotting. In human plasma, the aPTT began increasing upon reduction of FXII to 10% or less, and plateaued at approximately 260 s once FXII was reduced to 0.01%. Of note, the antibodies were used at a concentration of 20 µg/mL (125 nM), thus roughly four times lower than the concentration of FXII in plasma (~ 40 µg/mL, 500 nM). The anti-FXII mAbs 1B2 and 5C12 caused a leftwards shift in the curve for human and baboon plasma clotting times indicative of an inhibitory effect (Figs. 4b–4c). Antibodies 1D7, 7G11 and 15D10 inhibited the aPTT in human, baboon, cynomolgus, rhesus, mouse, rat, and rabbit plasmas (Figs. 4b–4h), although it should be noted that the degree of inhibition as reflected by the aPTT prolongation in human and baboon plasmas was less than observed for 1B2 and 5C12. An inhibitory effect was also observed for 9G3 in human, baboon, cynomolgus, rhesus, and mouse plasmas (Figs. 4b–4f). The FXII antibody 5A12 did not affect clotting times in any of the plasmas tested (Figs. 4b–4h).
We next employed non-activated thromboelastometry analysis (NATEM) to measure the effect of the panel of FXII antibodies on clotting time (CT), clot formation time (CFT), the time to reach a clot strength of 20 mm force, time to reach maximum clot firmness (MCF-t), maximum clot firmness (MCF), and the time from the start of clotting to maximum velocity (α-angle). We first performed an aPTT assay using whole human blood, wherein we confirmed that the FXII antibodies 1B2, 5C12, 1D7, 7G11, 15D10, and 9G3 prolonged clotting times, with the greatest level of inhibition observed for 1B2 and 5C12 (Fig. 5a). For NATEM assay (for schematic, see Fig. 5b), we used an antibody concentration of 40 µg/mL, thus roughly half the equimolar concentration to antigen FXII levels in whole blood.
Thrombus formation and growth was measured using non-activated thromboelastometry (NATEM). Human plasma from healthy volunteers was inhibited with 40 µg/mL antibody. (a) FXII inhibition confirmed by aPTT; (b) thromboelastometry curve explaining the parameters reported below; (c) clotting time; (d) clot formation time, (e) time to 20 mm clot firmness; (f) time to maximum clot firmness; (g) maximum clot firmness; (h) time to maximum velocity. Data represents mean ± SEM and were analyzed using one-way ANOVA (GraphPad Software, San Diego, CA) with Dunnett’s posthoc analysis versus control. *p < 0.05, **p < 0.01, ***p < 0.001.
The clotting time (CT), and the CT added to the clot formation time (CT+CFT) measured via NATEM, were prolonged for 1B2, 5C12, 1D7, 7G11, and 15D10, whereas 9G3 and 5A12 did not affect the CT (Figs. 5c and 5e). No increases for CFT alone, MCF-t, MCF or α-angle were observed for 1B2, 5C12, 1D7, 7G11, and 15D10 (Figs. 5d, 5f–5h). 5A12 did not affect any of the parameters measured (Figs. 5c–5h).
Effect of Monoclonal FXII Antibodies on FXII Activation and FXIIa Activity
We assessed the ability of the anti-FXII antibodies to inhibit FXII activation or activity. Dextran sulfate or long- or short-chain polyphosphates (polyP) were added to purified FXII to induce FXII activation and amidolytic activity toward a FXIIa-specific chromogenic substrate. We found that FXII activation or activity was inhibited by 1B2, 5C12, 1D7, 7G11, and 15D10 as measured by the amidolytic activity of FXIIa (Figs. 6a–6c) regardless of whether FXII was activated by long- or short-chain polyP or dextran sulfate. Differing degrees of efficacy were observed for the antibodies with the greatest potency being reported for 1B2 and 5C12. 1D7 was observed to inhibit FXII activation by short-chain polyP as compared to long-chain polyP or dextran sulfate, whereas 9G3 was ineffective in blocking FXII activation by dextran sulfate while exhibiting inhibitory effects against long- or short-chain polyP-mediated activation of FXII (Figs. 6a–6c). FXII activation was unaffected 5A12. Lastly, we characterized the effect of the anti-FXII antibodies on inhibiting FXIIa activity directly in a purified system. Only the antibodies that bound to the catalytic domain of FXII, 1B2 and 5C12, were capable of inhibiting the amidolytic activity of purified FXIIa (Fig. 6d).
Anti-FXII antibodies inhibit FXIIa activity. Increasing concentrations of anti-FXII antibodies were added to a solution of purified FXII. FXII was activated by either long PolyP (a), short PolyP (b), or dextran sulfate (c). Amidolytic activity was quantified as hydrolysis of the chromogenic substrate, Spectrozyme FXIIa. Activated FXII (FXIIa; 20 nM) was incubated with increasing concentrations of anti-FXII antibodies for 5 min before addition of Spectrozyme FXIIa. The velocity of hydrolysis was measured for 20 minutes and Vmax was calculated for each reaction (d). Each data point was measured in duplicate. Data represent mean ± SEM from n = 2.
Effect of Monoclonal FXII Antibodies on an In Vitro Model of Thrombus Formation Under Flow
Experiments were designed to examine the effect of anti-FXII antibodies on fibrin generation and platelet deposition in an in vitro model of thrombus formation under flow. Sodium citrate-anticoagulated whole human blood was recalcified prior to perfusion over immobilized collagen for 10 min at a shear rate of 300 s−1 to produce fibrin- and platelet-rich clots (Fig. 7). Consistent with a necessary role for FXII activation and activity in this assay,34,36 the anti-FXII antibodies 1B2, 5C12, and 1D7 abrogated fibrin formation on collagen under flow; only platelet aggregates were observed in the presence of these antibodies. In contrast, the anti-FXII antibody, 5A12, did not affect the extent of fibrin deposition or thrombus formation.
Effect of Monoclonal FXII Antibodies on an In Vivo Model of Vascular DEVICE-Initiated Thrombus Formation
We next selected two anti-FXII antibodies for advancement for testing in a baboon NHP model. We have already demonstrated the efficacy of antibody 5C12 in this model.33 Based on favorable binding and inhibitory profiles in the in vitro assays, we chose one antibody that binds the FXII catalytic domain (1B2), and one that binds the FXII heavy chain (1D7). In a preliminary dose range finding study, both 1B2 and 1D7 were administered to baboons in a stepwise manner consisting of six doses of 1 mg/kg given 40 min apart. Our results show that 1D7 caused a 1.5 fold increase in the plasma aPTT above baseline after the sixth dose, while 1B2 caused a greater than threefold increase in the plasma aPTT from baseline after the second dose (Fig. 8a). Corresponding increases in activated clotting time (ACT) were noted after 4 h (Fig. 8b). Neither of the antibodies altered the PT, circulating TAT levels, platelet count, hematocrit, or WBC or RBC levels (Figs. 8d–8h). Based on the favorable pharmacodynamic profile of 1B2 over 1D7, 1B2 was chosen for use in the baboon model of vascular device-initiated thrombus formation.
Time course of coagulation parameters after 1B2 or 1D7 administration in a non-human primate. Baboons were administered i.v. doses of 1 mg/kg 1B2 or 1D7 every 40 min over a 4-h period. Blood samples were collected for up to three weeks post administration. (a) aPTT, (b) ACT, (c) PT, and (d) TAT levels were measured. Complete blood count (CBC) was measured: (e) platelet counts, (f) hematocrit, (g) white blood cell count, and (h) red blood cell count. The upper and lower limits of reference values are indicated by dashed lines. aPTT, activated partial thromboplastin time; ACT activated clotting time, PT prothrombin time, TAT thrombin-antithrombin complex.
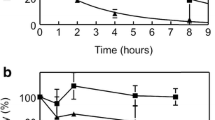
A pediatric extracorporeal membrane oxygenator was inserted into the loop of an exteriorized arteriovenous shunt implanted in a baboon. We quantified autologous radiolabeled platelet deposition in the oxygenator in the absence of an anticoagulant, finding that after 30 min over 1.5 billion platelets per minute were being deposited in the oxygenator, resulting in the retention of upwards of 80 billion platelets in the oxygenator by 60 min (Figs. 9a and 9b). Robust fibrin formation was observed in the oxygenator at the 60-min time point under non-anticoagulant conditions (Fig. 9c). Our data show that pretreatment of the baboon with 1B2 resulted in a significant reduction in both the rate (0.86 ± 0.03 billion platelets per minute) and the extent of platelet deposition and fibrin formation (Figs. 9a–9c). We did not detect any change in template bleeding time following treatment with 1B2 as compared to vehicle control (Fig. 9e).
Effect of 1B2 on platelet deposition and fibrin formation in ECMO. (a) Real-time platelet deposition was monitored in an ECMO device inserted in the extended loop of a chronic AV shunt. Study arms included vehicle control (n = 2) or 1B2 (5 mg/kg initial day + 2 mg/kg on the following days 30 min before perfusion, n = 4). (b) Average platelet deposition rate was calculated from 30 to 60 min. (c) Measurement of terminal fibrin content in the oxygenator and (d) correlation of fibrin and platelet content. (e) Two Surgicutt® bleeding time measurements were performed during each experiment and each data point represents the average of the two measurements. ECMO extracorporeal membrane oxygenator, AV arteriovenous, FXII factor XII.
Discussion
In this study, a library of monoclonal antibodies against FXII were generated in FXII-deficient mice. One non-inhibitory and six inhibitory antibodies were selected for further characterization. The relative potencies of the inhibitors on FXII activation and activity were determined using functional assays. These antibodies exhibited cross-reactivity with FXII from a variety of vertebrate animals as assessed by Western blots of mammalian plasmas. We used aPTT and NATEM assays to characterize the inhibitory effects of the antibodies on initiation, strengthening, and growth of clots. A series of activity assays, including an in vitro flow chamber model, were employed to define the effect of the antibodies on FXII activation and activity. Based on the results, two antibodies that bind to the catalytic domains of FXII, α-FXIIa and β-FXIIa, 1B2 and 5C12, were chosen to assess the effects of FXII inhibition in a model of vascular device-initiated thrombus formation using a pediatric membrane oxygenator inserted into a high flow arteriovenous shunt in a baboon. Results for 5C12 were reported previously.33 Here we discuss results with antibody 1B2.
Vascular interventions that involve placement of artificial devices such as intravenous access catheters, stents, vascular grafts, valves, and ventricular assist devices (VADs) may be complicated by thrombosis. Prothrombotic effects of some vascular devices, which can cause both device failure and thromboembolic complications, include: (1) non-physiologic, shear-stress induced platelet activation as shown by an increase in soluble P-selectin and enhanced platelet aggregation; (2) release of thrombotic platelet microparticles; (3) excessive red blood cell hemolysis; (4) complement activation; (5) inflammation; and (6) exaggerated coagulation via contact-driven FXII activation and thrombin generation.6,28 Several of these effects also paradoxically contribute to the non-trivial bleeding diathesis occasionally encountered with some types of vascular devices, most notably: consumptive coagulopathy; platelet shedding of hemostatic surface molecules including GPVI, GPIb, and CD63; and non-physiologic shear-mediated loss of high molecular weight von Willebrand factor multimers.13,35 As a result, both thrombosis and major bleeding rates encountered in patients with VADs, ECMO, or CPB are high. For instance, rates of oxygenator thrombosis, arterial thrombosis, and major bleeding in ECMO approach 16, 10, and 30%, respectively.6,28 Here we found that inhibiting the contact activation system by blocking FXIIa activity has an anti-platelet effect in a model of vascular device-initiated thrombus formation. This suggests that when blood comes in contact with foreign surfaces such as an ECMO circuit, inhibiting the initial events that trigger activation of the coagulation cascade that lead to thrombin generation may be an effective route for dampening deleterious platelet activation.
Vascular-device associated thrombosis or outright device failure remain important complications of these medical technologies, and systemic anticoagulation during their use has traditionally been mandated. Although numerous potent anticoagulant drugs that can reduce the incidence of these complications if given at effective doses are widely available, all of them have dose-limiting hemorrhagic toxicities.7 This has inspired researchers to develop vascular technologies with improved biocompatible surfaces, alternative dosing protocols for anticoagulants, and entirely new therapeutic agents aimed at blunting the pathologic processes implicated in bleeding and thrombosis.6,28 FXII, of the CAS, has emerged as a promising target due to an abundance of pre-clinical and early clinical data supporting the efficacy and safety of agents targeting the protein. Murine and NHP models have demonstrated that pharmacologic inhibition or genetic knockout of FXII substantially reduces collagen- and FeCl3-induced experimental venous and arterial thrombosis, respectively.1,21 Perhaps more compelling is the demonstration that inhibiting FXII activation or activity is useful in reducing device failure in animal models where membrane oxygenators were perfused with blood to induce clot formation.16,33 Herein, we validate this hypothesis by demonstrating that the anti-FXII monoclonal antibody 1B2 inhibits platelet deposition and fibrin formation in an extracorporeal membrane oxygenator deployed in non-human primates. These studies, and our prior work with the anti-FXII mAb 5C12, suggest that targeting the catalytic domain of FXII may be a useful approach for limiting vascular-device associated thrombosis.
In addition to inciting thrombosis, vascular devices including ECMO and hemodialysis circuits induce a pro-inflammatory process similar in some aspects to that observed in sepsis. Indeed, in patients with extracorporeal devices or sepsis, excessive cytokine release as part of the overwhelming inflammatory response may result in organ damage and contribute to morbidity. We observed that the CAS, and specifically crosstalk between FXII and FXI, serves as a nexus between thrombosis and inflammation in animal models of severe infection and bacterial challenge.26,32 In these models, selective inhibition of FXII activation significantly blunted the inflammatory response, exemplified by nearly a sixfold reduction in interleukin (IL)-6 levels. Since increased IL-6 levels are associated with poorer survival in patients undergoing ECMO,5,23,25 the CAS may prove an effective target to reduce vascular device-associated inflammation, in addition to its anticoagulant properties.
Translational approaches for reducing vascular device-associated thrombosis by targeting and inhibiting the CAS must take into account the effects of anti-FXII antibodies on FXII activation and activity in the absence of a surface. In the absence of surface, some antibodies directed at the FXII heavy chain accelerate the reciprocal activation of PK and FXII in plasma. While this does not lead to clotting, it could potentiate the release of bradykinin as a consequence of kallikrein cleavage of HK. An example of this effect was observed for the anti-FXII antibody, 15H8, which interferes with contact activation by preventing FXII from binding to surfaces. Previously, we demonstrated that 15H8 prevented thrombus formation in vitro on collagen as well as in vivo in an AV shunt thrombosis model.17 Yet, addition of 15H8 to human plasma was found to potentiate FXII-mediated activation of PK leading to the complete cleavage of HK. In the absence of a surface, the FXII heavy chain serves an important regulatory function that prevents FXII from activating. 15H8 interferes with that function, setting off brisk reciprocal activation with PK. 15H8 binds to the N-terminal fibronectin type 2 domain of FXII, which has been shown to be important in preventing FXII activation in the absence of a surface.2 Thus, caution is advised when developing therapies directed at the FXII heavy chain, as they may promote activities with a detrimental effect on patients.
In summary, we have generated a panel of anti-FXII antibodies with varying affinity and specificity for FXII; the functional effects of these antibodies were screened and evaluated for their anticoagulant and antithrombotic properties in in vitro, ex vivo, and in vivo models. Our data provide rationale for targeting the contact activation system to reduce or prevent vascular-device associated thrombosis in a variety of clinical settings, including ECMO.
Abbreviations
- ACT:
-
Activated clotting time
- aPTT:
-
Activated partial thromboplastin time
- BK:
-
Bradykinin
- BT:
-
Bleeding time
- CAS:
-
Contact activation system
- CBC:
-
Complete blood count
- cDNA:
-
Complementary DNA
- CFT:
-
Clot formation time
- CT:
-
Clotting time
- ECMO:
-
Extracorporeal membrane oxygenation
- ECOS:
-
Extracorporeal organ support
- F:
-
Factor
- HGFA:
-
Hepatocyte growth factor activator
- HK:
-
High molecular weight kininogen
- IgG:
-
Immunoglobulin G
- IP:
-
Intraperitoneal
- mAb:
-
Monoclonal antibody
- MCF:
-
Maximum clot firmness
- NATEM:
-
Non-activated thromboelastometry
- NHP:
-
Non-human primate
- PCR:
-
Polymerase chain reaction
- PK:
-
Prekallikrein
- polyP:
-
Polyphosphate
- PPP:
-
Platelet-poor plasma
- PT:
-
Prothrombin time
- TAT:
-
Thrombin-antithrombin
- VAD:
-
Ventricular assist device
References
Cheng, Q., E. I. Tucker, M. S. Pine, I. Sisler, A. Matafonov, M. F. Sun, T. C. White-Adams, S. A. Smith, S. R. Hanson, O. J. McCarty, T. Renne, A. Gruber, and D. Gailani. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood. 116:3981–3989, 2010.
Clark, C. C., Z. L. M. Hofman, W. Sanrattana, L. den Braven, S. de Maat, and C. Maas. The fibronectin type II Domain Of Factor XII ensures zymogen quiescence. Thromb. Haemost. 120:400–411, 2020.
Colman, R. W., and A. H. Schmaier. Contact system: a vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood. 90:3819–3843, 1997.
Crosby, J. R., U. Marzec, A. S. Revenko, C. Zhao, D. Gao, A. Matafonov, D. Gailani, A. R. MacLeod, E. I. Tucker, A. Gruber, S. R. Hanson, and B. P. Monia. Antithrombotic effect of antisense factor XI oligonucleotide treatment in primates. Arterioscler. Thromb. Vasc. Biol. 33:1670–1678, 2013.
Datzmann, T., and K. Trager. Extracorporeal membrane oxygenation and cytokine adsorption. J. Thorac. Dis. 10:S653–S660, 2018.
Doyle, A. J., and B. J. Hunt. Current understanding of how extracorporeal membrane oxygenators activate haemostasis and other blood components. Front. Med. 5:352, 2018.
Granja, T., K. Hohenstein, P. Schussel, C. Fischer, T. Prufer, D. Schibilsky, H. P. Wendel, K. Jaschonek, L. Serna-Higuita, C. Schlensak, H. Haberle, P. Rosenberger, and A. Straub. Multi-modal characterization of the coagulopathy associated with extracorporeal membrane oxygenation. Crit. Care Med. 48:e400–e408, 2020.
Gruber, A., S. Carlsson, H. F. Kotze, U. Marzec, T. C. Sarich, and S. R. Hanson. Hemostatic effect of activated factor VII without promotion of thrombus growth in melagatran-anticoagulated primates. Thromb. Res. 119:121–127, 2007.
Gruber, A., and S. R. Hanson. Factor XI-dependence of surface- and tissue factor-initiated thrombus propagation in primates. Blood 102:953–955, 2003.
Gruber, A., U. M. Marzec, L. Bush, E. Di Cera, J. A. Fernandez, M. A. Berny, E. I. Tucker, O. J. McCarty, J. H. Griffin, and S. R. Hanson. Relative antithrombotic and antihemostatic effects of protein C activator versus low-molecular-weight heparin in primates. Blood 109:3733–3740, 2007.
Hanson, S. R., J. H. Griffin, L. A. Harker, A. B. Kelly, C. T. Esmon, and A. Gruber. Antithrombotic effects of thrombin-induced activation of endogenous protein C in primates. J. Clin. Invest. 92:2003–2012, 1993.
Harker, L. A., U. M. Marzec, A. B. Kelly, N. R. Chronos, I. B. Sundell, S. R. Hanson, and J. M. Herbert. Clopidogrel inhibition of stent, graft, and vascular thrombogenesis with antithrombotic enhancement by aspirin in nonhuman primates. Circulation 98:2461–2469, 1998.
Hastings, S. M., D. N. Ku, S. Wagoner, K. O. Maher, and S. Deshpande. Sources of circuit thrombosis in pediatric extracorporeal membrane oxygenation. ASAIO J. 63:86–92, 2017.
Ivanov, I., A. Matafonov, M. F. Sun, B. M. Mohammed, Q. Cheng, S. K. Dickeson, S. Kundu, I. M. Verhamme, A. Gruber, K. McCrae, and D. Gailani. A mechanism for hereditary angioedema with normal C1 inhibitor: an inhibitory regulatory role for the factor XII heavy chain. Blood 133:1152–1163, 2019.
Juang, L. J., N. Mazinani, S. K. Novakowski, E. N. P. Prowse, M. Haulena, D. Gailani, L. M. Lavkulich, and C. J. Kastrup. Coagulation factor XII contributes to hemostasis when activated by soil in wounds. Blood Adv. 4:1737–1745, 2020.
Larsson, M., V. Rayzman, M. W. Nolte, K. F. Nickel, J. Bjorkqvist, A. Jamsa, M. P. Hardy, M. Fries, S. Schmidbauer, P. Hedenqvist, M. Broome, I. Pragst, G. Dickneite, M. J. Wilson, A. D. Nash, C. Panousis, and T. Renne. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci. Transl. Med. 6:222ra17, 2014.
Matafonov, A., P. Y. Leung, A. E. Gailani, S. L. Grach, C. Puy, Q. Cheng, M. F. Sun, O. J. McCarty, E. I. Tucker, H. Kataoka, T. Renne, J. H. Morrissey, A. Gruber, and D. Gailani. Factor XII inhibition reduces thrombus formation in a primate thrombosis model. Blood 123:1739–1746, 2014.
Ponczek, M. B., D. Gailani, and R. F. Doolittle. Evolution of the contact phase of vertebrate blood coagulation. J. Thromb. Haemost. 6:1876–1883, 2008.
Puy, C., A. T. P. Ngo, J. Pang, R. S. Keshari, M. W. Hagen, M. T. Hinds, D. Gailani, A. Gruber, F. Lupu, and O. J. T. McCarty. Endothelial PAI-1 (Plasminogen Activator Inhibitor-1) blocks the intrinsic pathway of coagulation, inducing the clearance and degradation of FXIa (Activated Factor XI). Arterioscler. Thromb. Vasc. Biol. 39:1390–1401, 2019.
Raffini, L. Anticoagulation with VADs and ECMO: walking the tightrope. Hematol. Am. Soc. Hematol. Educ. Program. 674–80:2017, 2017.
Renne, T., A. H. Schmaier, K. F. Nickel, M. Blomback, and C. Maas. In vivo roles of factor XII. Blood 120:4296–4303, 2012.
Revenko, A. S., D. Gao, J. R. Crosby, G. Bhattacharjee, C. Zhao, C. May, D. Gailani, B. P. Monia, and A. R. MacLeod. Selective depletion of plasma prekallikrein or coagulation factor XII inhibits thrombosis in mice without increased risk of bleeding. Blood 118:5302–5311, 2011.
Risnes, I., K. Wagner, T. Ueland, T. Mollnes, P. Aukrust, and J. Svennevig. Interleukin-6 may predict survival in extracorporeal membrane oxygenation treatment. Perfusion 23:173–178, 2008.
Schmaier, A. H. Coagulation Factor XIIa. Handbook of Proteolytic Enzymes, Vol. 3. New York: Academic Press, pp. 2881–2885, 2013.
Schopka, S., A. Philipp, T. Muller, M. Lubnow, D. Lunz, C. Unterbuchner, L. Rupprecht, A. Keyser, and C. Schmid. The impact of interleukin serum levels on the prognosis of patients undergoing venoarterial extracorporeal membrane oxygenation. Artif. Org., 2020.
Silasi, R., R. S. Keshari, C. Lupu, W. J. Van Rensburg, H. Chaaban, G. Regmi, A. Shamanaev, J. J. Shatzel, C. Puy, C. U. Lorentz, E. I. Tucker, D. Gailani, A. Gruber, O. J. T. McCarty, and F. Lupu. Inhibition of contact-mediated activation of factor XI protects baboons against S aureus-induced organ damage and death. Blood Adv. 3:658–669, 2019.
Smith, S. A., S. H. Choi, R. Davis-Harrison, J. Huyck, J. Boettcher, C. M. Rienstra, and J. H. Morrissey. Polyphosphate exerts differential effects on blood clotting, depending on polymer size. Blood. 116:4353–4359, 2010.
Sniderman, J., P. Monagle, and G. M. Annich. Hematologic concerns in extracorporeal membrane oxygenation. Res. Pract. Thromb. Haemost. 4(4):455–468, 2020.
Stavrou, E., and A. H. Schmaier. Factor XII: what does it contribute to our understanding of the physiology and pathophysiology of hemostasis & thrombosis. Thromb. Res. 125:210–215, 2010.
Tillman, B., and D. Gailani. Inhibition of factors XI and XII for prevention of thrombosis induced by artificial surfaces. Semin. Thromb. Hemost. 44:60–69, 2018.
Tucker, E. I., U. M. Marzec, T. C. White, S. Hurst, S. Rugonyi, O. J. McCarty, D. Gailani, A. Gruber, and S. R. Hanson. Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI. Blood 113:936–944, 2009.
Tucker, E. I., N. G. Verbout, P. Y. Leung, S. Hurst, O. J. McCarty, D. Gailani, and A. Gruber. Inhibition of factor XI activation attenuates inflammation and coagulopathy while improving the survival of mouse polymicrobial sepsis. Blood 119:4762–4768, 2012.
Wallisch, M., C. U. Lorentz, H. H. S. Lakshmanan, J. Johnson, M. R. Carris, C. Puy, D. Gailani, M. T. Hinds, O. J. T. McCarty, A. Gruber, and E. I. Tucker. Antibody inhibition of contact factor XII reduces platelet deposition in a model of extracorporeal membrane oxygenator perfusion in nonhuman primates. Res. Pract. Thromb. Haemost. 4:205–216, 2020.
White-Adams, T. C., M. A. Berny, I. A. Patel, E. I. Tucker, D. Gailani, A. Gruber, and O. J. McCarty. Laminin promotes coagulation and thrombus formation in a factor XII-dependent manner. J. Thromb. Haemost. 8:1295–1301, 2010.
Yaw, H. P., S. Van Den Helm, G. MacLaren, M. Linden, P. Monagle, and V. Ignjatovic. Platelet phenotype and function in the setting of pediatric extracorporeal membrane oxygenation (ECMO): a systematic review. Front. Cardiovasc. Med. 6:137, 2019.
Zilberman-Rudenko, J., S. E. Reitsma, C. Puy, R. A. Rigg, S. A. Smith, E. I. Tucker, R. Silasi, A. Merkulova, K. R. McCrae, C. Maas, R. T. Urbanus, D. Gailani, J. H. Morrissey, A. Gruber, F. Lupu, A. H. Schmaier, and O. J. T. McCarty. Factor XII activation promotes platelet consumption in the presence of bacterial-type long-chain polyphosphate in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 38:1748–1760, 2018.
Acknowledgments
We thank H. Lakshmanan, M. Carris, and J. Pang for technical assistance and valuable discussions. We also thank Dr. Sally Nofs (Nashville Zoo at Grassmere, Nashville, TN) for mammalian plasma samples. This study was supported in part by the National Heart, Lung, and Blood Institute Grants: HL126235 to E.I. Tucker and M. Wallisch, R35HL140025 to D. Gailani, and HL144113 to O.J.T. McCarty and M.T. Hinds; and the Oregon National Primate Research Center NIH Grant Award P51OD011092.
Conflict of interest
M. Wallisch, C.U. Lorentz, E.I. Tucker, and A. Gruber are employees of Aronora, Inc., and they as well as Oregon Health & Science University may have a financial interest in the results of this study. J.J. Shatzel reports receiving consulting fees from Aronora, Inc. T.C.L. Kohs, J. Johnson, C. Puy, S.R. Olson, D. Gailani, M. T. Hinds, and O.J.T. McCarty state that they have no conflicts of interest.
Research Involving Human Rights
All human subjects research was carried out in accordance with institutional guidelines approved by the Oregon Health & Science University Institutional Review Board.
Research Involving Animal Rights
All animal studies were approved by the Oregon Health & Science University Institutional Animal Care and Use Committee. All institutional and national guidelines for the care and use of laboratory animals were followed and approved by our institutional committee.
Author information
Authors and Affiliations
Contributions
MW, AG, EIT were responsible for the project concept and design. MW, JJ, and MTH executed and led the primate studies. CUL, CP, TK, and DG contributed in vitro data acquisition and analysis. MW performed data analysis and statistics for in vivo primate data. TK, MW, OJTM, and AG drafted the manuscript. CUL, JJS, SO, DG, and EIT critically reviewed and revised the manuscript.
Corresponding author
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kohs, T.C.L., Lorentz, C.U., Johnson, J. et al. Development of Coagulation Factor XII Antibodies for Inhibiting Vascular Device-Related Thrombosis. Cel. Mol. Bioeng. 14, 161–175 (2021). https://doi.org/10.1007/s12195-020-00657-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-020-00657-6