Abstract
Diabetic retinopathy (DR) is the most severe microvascular complication of diabetes and a major cause of visual impairment and blindness. However, the treatment for DR is still limited. Our study aimed to explore the role of circular RNA_0002570 in DR. First, we predicted the potential microRNA and mRNA that could bind to circ_0002570 and identified the miR-1243 and angiomotin gene; then, we used RT-PCR and Western blot to measure their expression. Next, we evaluated the abilities of proliferation, migration, and angiogenesis in vitro in human retinal microvascular endothelial cells (hRMECs) by CCK-8, transwell assay, and tube formation assay, respectively. To analyze the relationship among miR-1243, circ_0002570, and angiomotin, RNA pull-down and luciferase assay were performed. Our results showed that, in DR patients and high-glucose–induced hRMECs, miR-1243, circ_0002570, and angiomotin were all abnormally expressed. MiR-1243 could directly and competitively bind to both circ_0002570 and angiomotin mRNA to inhibit their expression. Moreover, circ_0002570 suppressed the abilities of proliferation, migration, and angiogenesis in hRMECs induced by high glucose, which was dependent on miR-1243-angiomotin axis. Furthermore, circ_0002570 could upregulate angiomotin by targeting miR-1243 to mediate the dysfunction of hRMECs induced by high glucose. In conclusion, circ_0002570 might serve as a potential target for diagnosis and treatment for DR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a chronic metabolic disease whose morbidity has increased globally and dramatically in the last decades, threatening healthcare systems in advanced countries as well as in the developing world. Diabetes is characterized by high-glucose levels in the blood and is caused by insulin deficiency (type 1 diabetes mellitus, T1DM), insulin resistance (type 2 diabetes mellitus, T2DM), or others. Patients with diabetes of long duration can develop vascular complications, including macrovascular complications with a mortality of up to 70% (stroke, heart disease, and peripheral artery disease) and microvascular complications which affect the kidney (nephropathy) and the eyes (retinopathy) (Jourdan et al. 2017).
Diabetic retinopathy (DR) is one of the most frequently occurring microvascular complications of diabetes and is a major cause of visual impairment and blindness in all diabetic patients. The rapid spread and development of diabetic mellitus has led to a dramatic increase in the number of patients with diabetic retinopathy. Diabetic retinopathy is thus the leading cause of preventable blindness affecting more than 2.5 million people with an overall prevalence of one-third of all diabetes patients worldwide (Pascolini and Mariotti 2012). Early-stage diabetic retinopathy is characterized by pericyte loss, destruction of the blood-retinal barrier (BRB), and proliferation of capillary cells, which is mostly driven by chronic hyperglycemia (Stitt et al. 2016). High blood glucose levels cause microvascular dysfunction in the retina by destroying the tight function of the inner endothelial blood-retinal barrier (Erickson et al. 2007; Klaassen et al. 2013). The pathology and pathogenesis of diabetic retinopathy are associated with the interaction of multi-gene and multiple signaling pathways. Although it is believed that the pathogenesis of DR is involved with angiogenesis, oxidative stress, chronic inflammation, and other factors (Chen et al. 2018), its detailed molecular mechanism remains unknown. The fragility of the blood vessel wall leads to an accelerated proliferation of endothelial cells and the formation of microaneurysms, resulting in vascular leakage and insufficiency. Vascular remodeling in the retina and capillary perfusion break down the balance in the blood vessels, leading to the abnormal formation of new blood vessels. Previous studies indicate that high blood glucose levels improved the proliferation and migration of endothelial cells as well as angiogenesis, which is consistent with the pathogenesis of new vessels formation in DR (Gao et al. 2008; Huang and Sheibani 2008; Mei et al. 2018; Yang et al. 2017). Therefore, it might provide a novel therapeutic approach to treat diabetic retinopathy by regulating the function of endothelial cells in the retina.
Circular RNA (circRNA) is a special type of non-coding RNAs, and the latest studies have discovered that circRNAs are sometimes expressed in vivo, which become the hotspot in the field of RNA research (Yu et al. 2017). Unlike traditional linear RNA that contains 3′ and 5′ ends, the circRNAs have a closed-loop structure, which cannot be affected by RNA exonucleases, leading to its stable expression and non-degradation. Recent studies showed that circRNAs were rich in functional microRNA (miRNA) binding sites and acted as a miRNA sponge in cells, which in turn reversed the inhibitory effects of miRNA on its target genes and also increased the expression of target genes. This mechanism is known as the competitive endogenous RNA (ceRNA). CircRNAs play an important regulatory role in varieties of diseases through direct interactions with disease-related miRNAs (Han et al. 2018; Nicolet et al. 2018; Salzman 2016). Recently, numerous studies have focused on the role of circRNAs in the development of DR (Gu et al. 2017; He et al. 2019; Zhang et al. 2017a; Zhu et al. 2019); however, more investigation is needed. Specifically, hsa_circ_0002570 was reported to be abnormally expressed in DR (He et al. 2019), but its role in DR is still not clear. Therefore, our study further explored the modulation of this circRNA in the development of DR.
Materials and methods
Ethical statement
We collected the retinal proliferative fibrovascular membranes from 20 patients with diabetic retinopathy for the DR group and epiretinal membranes from 20 patients with idiopathic epiretinal membrane for the non-DR group. All patients were informed and signed the consent. This study was approved by the Ethical Review Committee of the Fourth Affiliated Hospital of Harbin Medical University.
Cell culture
Human retinal microvascular endothelial cells (hRMECs) (ATCC, Manassas, VA) were cultured in endothelial cell medium (ECM) with 10% fetal bovine serum (Gibco, Grand Island, NY) and 100 U/ml penicillin and streptomycin at 37 °C. To investigate the effect of high glucose (HG) or osmotic pressure in hRMECs, we used 25 mM high glucose or high concentration of mannose (5.5 mM glucose and 19.5 mM mannose; OS) to stimulate the cells for 48 h as previously described (Liu et al. 2019).
Western blot
Protein were extracted from lysed cells after washing with phosphate-buffered saline (PBS) three times, and its concentration was measured using Pierce BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA). Equal amount of protein with loading buffer was loaded into sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels and transferred onto polyvinylidene difluoride membrane. After blocking with 5% milk in tris-buffered saline and Tween-20, membranes were incubated with primary antibody overnight at 4 °C, followed by the incubation of peroxidase-conjugated secondary antibodies, and the bands were visualized by ECL detection reagents (Sigma, St. Louis, MO). All antibodies including monoclonal Rabbit Angiomotin (#43130), GADPH (#5174), and mouse anti-rabbit IgG (HRP Conjugate) (#93702) were purchased from Cell Signaling Technology (CST, Danvers, MA) (Zhang et al. 2019).
qRT-PCR
Total RNA was extracted from lysed retina derived from patients and also from cultured cells respectively with RNeasy mini kit according to the manufacturer’s instructions (Qiagen, Valencia, CA). RNA was reversely transcripted into cDNA, and RT-PCR was performed as previously described (Huang et al. 2017; Zhang et al. 2019). The circRNA and mRNA levels were normalized by β-actin. The miRNA level was normalized by small nuclear U6.
The sequences of primers were listed as below:
-
circ_0002570: F: CGTGGAAAGAAGAAAGGAAA; R: CTGTCTATTGCGAAAGGTCA, angiomotin: F: AGGCAAGAGTTGGAAGGATGC; R: AGGATGACTTCACGAGGTTCT, β-actin: F: AAATCTGGCACCACACCTTC; R: GGGGTGTTGAAGGTCTCAAA.
-
miR-1243 RT primer:GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACTCC; miR-1243 qRT-PCR primer: F: GTCAACTGGATCAATTATAGG, R: GTGCAGGGTCCGAGGT.
-
U6 RT primer: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATATGGAA.
-
U6 qRT-PCR primer: F: CTCGCTTCGGCAGCACA, R: AACG CTTCACGAATTTGCGT.
Luciferase assay
To assess the relationship among miR-1243, circ_0002570, four vectors containing the sequences of predicted binding sites /mutated binding sites of circ_0002570, the sequence of predicted binding sites/mutate binding sites of 3′-UTR of angiomotin were cloned into psiCHECK2 vectors respectively (Promega, Madison, WI). These constructed vectors (hsa_circ_0002570-WT, hsa_circ_0002570-MT, AMOT 3′UTR-WT, and AMOT 3′UTR-MT) were co-transfected into HEK293T cells or hRMECs with miR-1243 mimic or negative control (miR-nc) respectively for 48 h, then luciferase activities were measured by the Dual-Luciferase Reporter Assay System (Promega) which was normalized to firefly luciferase activity (Fu and Ou 2020).
The sequence of primers as followed:
-
Circ_0002570-WT: F: CTTTCTCGAGATCAAATTTTCTCCGGTGGCTA, R: CTTT GCGGCC GCCAATATCATCAGCAAGGGAATTAGGATCAGA.
-
Circ_0002570-MT: F: AAAGGTTCAAGTTGTTGCCGGAG, R: CTCCGGCAACAACTTGA ACCTTT.
-
AMOT 3′UTR-WT: F: CTTT CTCGAG ACGGCCAAATCAAGAGCTGCAG, R: CTTT GCGGCCGC AAAACTGATGAATTGGCATCAGAA.
-
AMOT 3′UTR-MT: F: GTTTGTTCAGTTTTTTACCGCAG, R: CTGCGGTAAAAAACTGA ACAAAC.
Cell transfection
When cells were 70–80% confluent, siRNA/miRNA mimic/miRNA inhibitor (1 nM) (RiboBio, Guangzhou, China) were transfected into cells with lipofectamine RNAimax (Life Technologies, Carlsbad, CA) in the medium of opti-MEM as previously described (Liu et al. 2012). For overexpression of circ_0002570, vectors (0.1 μg/ml) were transfected into cells of 70%–80% confluence with lipofectamine 2000 (Life Technologies). After 4–6 h, cells were changed with new medium.
The sequences of siRNA are shown as below:
-
si-circ_0002570-1:GAACCAGAGCACCTCATACTT; si-circ_0002570-2:GTCCAGAGTGTCAATTCTTGT; si-NC:TTCTCCGAACGTGTCACGT.
Vector construction
The vector of overexpressed circ_0002570 was constructed as previously described (Cheng et al. 2019). Briefly, circ_0002570 was cloned using the designated primers (F: CTTT GAATTCTGAAATATGCT AT CTTAC AG ATCAAATTTTCTCCGGTGGCTA, R: CTTTGGATCCTCAAGAAAAAATATA TTCACCAATATCATCAGCAAGGGAATTAGGATCAGA), then inserted into PLCDH-ciR which was reconstructed by inserting front and back circular frame to promote RNA circularization.
Pull-down assay of targeted mRNA with biotinylated miR-1243
hRMECs were collected in the extraction buffer (25 mM pH 7.5Tris-HCl, 70 mM KCl, 2.5 mM EDTA, 0.05% NP-40) with 80 U/mL RNase, and protease inhibitor cocktail for 20 min on ice after washing with pre-cold PBS, and centrifuged at 12,000g for 15 min at 4 °C. The supernatant was treated with biotinylated miR-1243 and miR-NC at 4 °C for 30 min followed by 1 h incubation at 30 °C with shaking. A Streptavidin Mutein Matrix was pretreated with extraction buffer with 250 g BSA and 100 g yeast tRNA for 3 h, washed three times with extraction buffer, and added into the extract. The streptavidin/biotin–miRNA/mRNA complex was harvested after spin and wash. The biotin-miRNA/mRNA complex was eluted in the elution buffer (20 mM pH 7.4 Tris-HCl, 400 mM KCl, 0.5% NP-40, 5 mM biotin, and 80 U/mL RNase inhibitor) at 42 °C for 5 min (Yamamoto et al. 2015).
Cell proliferation assay
hRMECs were seeded in a 96-well plate and incubated with 10 μ Cell Counting Kit-8 (CCK-8, Dojindo Laboratories, Mashikimachi, Japan) solution for 4 h at 37 °C. The absorbance values were read at 450 nm (Zhu et al. 2019).
Cell migration assay
hRMECs were added into the transwell chamber placed on top of a 24-well plate containing ECM in the bottom. After 20 h incubation, hRMECs migrated across the transwell were fixed and stained with 0.1% crystal violet. Attached cells were counted under a microscope in 6-random fields (Zhu et al. 2019).
Tube formation assay in vitro
hRMECs were plated in a 24-well plate precoated with 300 μL growth factor reduced Matrigel matrix. After 20 h incubation, we took photos in 6 random fields, then measured meshes and branches and the branching length of the capillary-like structures were measured by the ImageJ software (NIH, Bethesda, MD).
Enzyme-linked immunosorbent assay
Media were collected after treatment, and the expression of all pro-inflammatory cytokines were measured by the commercially available ELISA kits (R&D System, Minneapolis, MN) according to the manufacturer’s instructions.
Statistical analysis
All data of the study were expressed as means ± standard deviation (SD). Biochemical assays were repeated at least in triplicate. Data were analyzed by statistical product and service solutions (SPSS 16.0, SPSS Inc., Chicago, IL, USA). Groups were compared by Student’s t test or one-way ANOVA analysis with a Tukey’s post hoc test. P values < 0.05 were considered to be statistically significant.
Results
The expression of hsa_circ_0002570, miR-1243, and angiomotin were regulated in DR patients and high-glucose–induced hRMECs
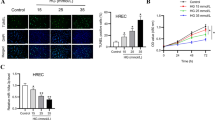
As consistent with a previous report, we also observed that hsa_circ_0002570 was significantly increased in the DR group compared with the non-DR group (Fig. 1a). Numerous studies indicated that circRNAs could function in a competing endogenous RNA (ceRNA) manner; therefore, we predicted that microRNA-1243 might interact with circ_0002570 through circular RNA Interactome (https://circinteractome.nia.nih.gov). Of note, we found that miR-1243 was remarkably decreased in the DR group compared with the non-DR group, which further confirmed the potential relationship between miR-1243 and hsa_circ_0002570 in DR (Fig. 1b). Furthermore, we also predicted the potential target gene of miR-1243, namely angiomotin by Targetscan (http://www.targetscan.org/vert_71/). Angiomotin (AMOT) is a protein that belongs to the motin family of angiostatin-binding proteins and contributes to cell motility and angiogenesis. DR patients had a higher mRNA expression of AMOT than non-DR patients (Fig. 1c).
The expression of hsa_circ_0002570, miR-1243, and angiomotin were regulated in DR patients and high-glucose–induced hRMECs. a–c The RNA levels of hsa_circ_0002570, miR-1243, and angiomotin were analyzed by qRT-PCR, in proliferative fibrovascular membranes or epiretinal membranes collected from patients with or without DR (n = 20). d The RNA levels of hsa_circ_0002570, miR-1243, and angiomotin in hRMECs cultured under the normal glucose (NG), high-mannose (high osmotic pressure, OS), or high-glucose (HG) conditions for 48 h were measured by qRT-PCR. n = 3. (E) The protein level of angiomotin in hRMECs cultured under the normal glucose (NG), high-mannose (5.5 mM glucose and 19.5 mM mannose) (high osmotic pressure, OS), or high-glucose (HG) condition for 48 h were determined by Western blot. *P < 0.05; **P < 0.01
Next, in hRMECs, we discovered that high glucose upregulated the expression of circ_0002570 (Fig. 1d) as well as the mRNA/protein expression of AMOT (Fig. 1d and e) and downregulated the expression of miR-1243 (Fig. 1d), which were all consistent with our results in DR patients. To avoid the effect of osmotic pressure in the hRMECs, we used high levels of mannose (5.5 mM glucose and 19.5 mM mannose; OS) to stimulate cells and found that there was no significant difference between the OS group and the NG group (Fig. 1d and e).
Taken together, circ_0002570 and AMOT were significantly increased with a decreased expression of miR-1243 both in DR patients and in high-glucose-stimulated hRMECs.
Circ_0002570 bound to miR-1243 and inhibited its expression
To investigate the relationship between circ_0002570 and miR-1243, we first predicted their potential binding sites by circular RNA interactome (Fig. 2a). To further validate the predicted binding sites, we made mutations on three of six binding sites as shown in Fig. 2a and constructed two luciferase reporter vectors containing a normal sequence of circ0002570 (hsa_circ_0002570-WT) and a mutated sequence of circ_0002570 (hsa_circ_0002570-MT) respectively. Luciferase assay showed that miR-1243 could bind to circ_0002570-WT, but not to the mutated circ_0002570-MT both in hRMECs and HEK293T cells (Fig. 2b and c). Moreover, knocking down of circ_0002570 by siRNA-cric_0002570 significantly increased the expression of miR-1243 in hRMECs (Fig. 2d). In contrast, overexpressing circ_0002570 remarkably inhibited the expression of miR-1243 (Fig. 2e). All these data suggested that circ_0002570 directly bound to miR-1243 and inhibited its expression.
Circ_0002570 bound to miR-1243 and inhibited its expression. a Luciferase reporter vectors containing wild-type or mutant hsa_circ_0002570 sequence were constructed. The predicted binding sites of miR-1243 on hsa_circ_0002570 sequence were shown. b, c Luciferase activity was detected in hRMECs and HEK-293T cells transfected with vectors containing wild-type (hsa_circ_0002570-WT) or mutated hsa_circ_0002570 (hsa_circ_0002570-MT) in response to the transfection of miR-1243 mimics or negative control mimics (miR-NC). n = 3. d The RNA levels of hsa_circ_0002570 and miR-1243 in hRMECs transfected with two specific hsa_circ_0002570 siRNAs (si-circ_0002570-1 and si-circ_0002570-2) or negative control siRNA (si-NC) were measured by qRT-PCR. n = 3. (E) The RNA levels of hsa_circ_0002570 and miR-1243 in hRMECs transfected with hsa_circ_0002570 overexpressed plasmid (circ_0002570-OE) or empty vector (Vector) were measured by qRT-PCR. n = 3. The data were represented as means ± SD. *P < 0.05; **P < 0.01, ns = no significance
MiR-1243 bound to both circ_0002570 and angiomotin in hRMECs
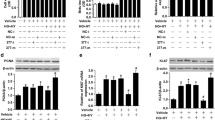
Next, we predicted the potential binding sites on miR-1243 and 3′UTR of angiomotin mRNA through Targetscan as shown in Fig. 3a. To further validate the predicted binding sites, we constructed two vectors containing sequences of normal 3′UTR of AMOT (AMOT 3′UTR-WT) and mutated 3′UTR of AMOT (AMOT 3; UTR-MT) respectively (Fig. 3a). Co-transfection AMOT 3′UTR-WT with miR-1243 could significantly diminish the luciferase activity compared with miR-NC transfected ones both in HEK293T cells and hRMECs, while this inhibitory effect could be totally blocked in AMOT 3′UTR-MT-transfected cells (Fig. 3b and c). Moreover, both the mRNA and protein expression of AMOT could be remarkably suppressed by miR-1243 mimic and promoted by miR-1243 inhibitor (Fig. 3d and e). Biotinylated miR-1243 captured more circ_0002570 and AMOT mRNA in hRMECs than biotinylated negative control (Fig. 3f); thus, this RNA pull-down assay further elucidated that miR-1243 directly targeted both circ_0002570 and AMOT mRNA. To further explore the relationship between circ_0002570 and AMOT, we knocked down and overexpressed circ_0002570 respectively in hRMECs (Fig. 3g and h). AMOT expression was remarkably downregulated by circ_0002570 knockdown and upregulated with circ_0002570 over-expression (Fig. 3i). Taken together, circ_0002570 and AMOT mRNA competitively bound to miR-1243 and regulated their expression.
MiR-1243 targeted both hsa_circ_0002570 and angiomotin in hRMECs. a Luciferase reporter vectors containing the wild-type (AMOT 3′UTR-WT) or mutant (AMOT 3′UTR-MT) 3′UTR of AMOT were constructed according to the predicted binding sites of miR-1243. b, c Luciferase activity was detected in hRMECs and HEK-293T cells transfected with constructs containing wild-type (AMOT 3′UTR-WT) or mutant (AMOT 3′UTR-MT) 3′UTR of AMOT plasmid in response to the transfection of miR-1243 mimics or negative control mimics (miR-NC). n = 3. d, e The expression of angiomotin in hRMECs transfected with miR-1243 mimics and miR-1243 inhibitor or their respective negative controls were detected by qRT-PCR and western blot. n = 3. (F) Detection of circ_0002570 and angiomotin RNA levels in biotinylated miRNA/target mRNA complex by qRT-PCR. The relative RNA levels of circ_0002570 and angiomotin in the complex pulled down using biotinylated miR-1243 were compared to that of the complex pulled down using the biotinylated control random RNA. n = 3. g, i The expression of angiomotin in hRMECs transfected with two specific hsa_circ_0002570 siRNAs (si-circ_0002570-1 and si-circ_0002570–2) or negative control siRNA (si-NC) were measured by qRT-PCR and western blot. n = 3. h, i The expression of angiomotin in hRMECs transfected with hsa_circ_0002570 overexpressed plasmid (circ_0002570-OE) or empty vector (Vector) were measured by qRT-PCR and western blott. n = 3. The data were represented as means ± SD. *P < 0.05; **P < 0.01, ns = no significance
Knocking down circ_0002570 inhibited proliferation, migration, tube formation, and the release of pro-inflammatory cytokines induced by high glucose in hRMECs
We observed that a 48-h treatment of high glucose stimulated the expression of circ_0002570 in hRMECs (Fig. 4a). Furthermore, high glucose also promoted cell viability (Fig. 4b), cell migration (Fig. 4c and d), and tube formation (Fig. 4e and f) in hRMCEs, which were all inhibited by siRNA circ-0002570. Moreover, the protein expression of vascular endothelial growth factor (VEGF) and pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-8 (IL-8), interleukin-6 (IL-6), and monocyte chemoattractant protein −1 (MCP-1), all induced by high glucose, were reversed after knocking down circ_0002570 (Fig. 4g–k). All these data revealed that knocking down circ_0002570 inhibited proliferation, migration, tube formation, and the release of pro-inflammatory cytokines induced by high glucose in hRMECs.
Knocking down circ_0002570 inhibited proliferation, migration, tube formation, and pro-inflammatory cytokines release induced by high glucose in hRMECs. hRMECs were treated with normal glucose (NG) or high-glucose (HG) for 48 h after transfection with two specific hsa_circ_0002570 siRNAs (si-circ_0002570-1 and si-circ_0002570-2) or negative control siRNA (si-NC). a The RNA levels of hsa_circ_0002570 in hRMECs were measured by qRT-PCR. n = 3. b cell viability was measured by CCK-8 assay. n = 6. c, d Cell migration was assessed using transwell assay and representative images were shown. n = 6. e, f Angiogenesis was evaluated by tube formation assay and representative images of tube-like structures were shown. n = 6. g The protein concentrations of VEGF in the conditional medium of hRMECs were measured by ELISA. n = 6. h–k The protein levels of pro-inflammatory cytokines (TNF-α, IL-8, IL-6, and MCP-1) were evaluated by ELISA assay. n = 6. The data were represented as means ± SD. *P < 0.05; **P < 0.01
Dysfunctions induced by high glucose in hRMECs were regulated by circ_0002570-miR-1243-AMOT axis
To evaluate the effect of circ_0002570-miR-1243-AMOT axis in high-glucose–stimulated hRMECs, we did three different groups of co-transfections: si-NC + NC-inhibitor; si-circ_0002570 + NC-inhibitor and si-circ_0002570 + miR-1243-inhibitor. Under the stimulation of high glucose in hRMECs, miR-1243 expression upregulated by knocking down cric_0002570 was suppressed by additionally supplementing with miR-1243 inhibitor (Fig. 5a). In contrast, miR-1243 inhibitor along with siRNA circ_0002570 significantly increased both the mRNA and protein expression of AMOT, which was suppressed by siRNA-circ_0002570 alone in the presence of high glucose (Fig. 5a and b). We subsequently evaluated the effect of this circ_0002570-miR-1243-AMOT axis in the high-glucose–induced dysfunction of hRMECs and found that cell viability (Fig. 5c), cell migration (Fig. 5d), tube formation (Fig. 5e), VEGF secretion (Fig. 5,f), and the release of pro-inflammatory cytokines (Fig. 5g–j) inhibited by si-circ_0002570 were all restored by additionally supplementing with miR-1243 inhibitor after high-glucose stimulation. Therefore, circ_0002570-miR-1243-AMOT could effectively regulate the dysfunction induced by high glucose in hRMECs.
Dysfunctions induced by high glucose in hRMECs were regulated by circ_0002570-miR-1243-AMOT axis. hRMECs were treated with high glucose (HG) for 48 h after co-transfection with negative control siRNAs and negative control of miRNA inhibitor (si-NC + NC-inhibitor), a specific hsa_circ_0002570 siRNAs and negative control of miRNA inhibitor (si-circ_0002570-1 + NC-inhibitor) or a specific hsa_circ_0002570 siRNAs and miR-1243 inhibitor (si-circ_0002570-1 + miR-1243-inhibitor). a The RNA levels of miR-1243 and angiomotin in hRMECs were measured by qRT-PCR. n = 3. b The protein levels of angiomotin in hRMECs were determined by western blot. n = 6. c Cell viability was measured by CCK-8 assay. d Cell migration was assessed using transwell assay. n = 6. e Angiogenesis was evaluated by tube formation assay. n = 6. f The protein concentrations of VEGF in conditional medium of hRMECs were measured by ELISA. n = 6. g–j The protein levels of pro-inflammatory cytokines (TNF-α, IL-8, IL-6, and MCP-1) were evaluated by ELISA assay. n = 6. The data were represented as means ± SD. *P < 0.05; **P < 0.01
Discussion
Diabetes mellitus is a chronic metabolic disease characterized by high-glucose levels in the blood and long-term hyperglycemia causing life-threatening macrovascular and microvascular complications. Diabetic retinopathy is the most severe microvascular complication of diabetes, which can cause irreversible blindness, because retinal vasculature is the early and major target of hyperglycemia. Diabetic retinopathy is one of the major causes of vision loss all over the world and is also the leading cause of vision impairment in patients aged 25–71. The visual loss caused by DR might be secondary to macular edema (ME, which is involved in thickening of the retina and edema of macula), neovascular hemorrhage, retinal detachment, or neovascular glaucoma. Long-term hyperglycemia causes a wide range of morphological changes in retinal vasculature, including thickened vascular wall, increased vascular permeability, and vascular occlusion. All of these changes contribute to the release of pro-proliferative factors by hypoxia retinal tissue, which induces the proliferation of capillary endothelial cells to initiate neovascularization leading to hemorrhage, retinal embolism, and eventually to the disorder of diabetic retinopathy. Retinal vascular cells are mostly composed of pericytes and endothelial cells. The early pathological feature of diabetic patients and animals with diabetic retinopathy is pericyte loss. The phenotype of endothelial cells could change from a normal quiescent phenotype to an apoptotic and active one, and this dysfunction of retinal endothelial cells causes increased vascular permeability, macular edema, and angiogenesis. Although both pericytes or the crosstalk between pericytes and endothelial cells in the retina are important to the development of DR as previously reported (Zhang et al. 2017b), endothelial cells of retina play a central role in the late stage of diabetic retinopathy, which is the reason our study is focused on this particular cell.
Numerous studies indicate that some circRNAs are associated with the development and progression of diabetic retinopathy (He et al. 2019; Zhang et al. 2017a). The profile of circular RNAs in vitreous humor between DR and non-DR patients reveals twelve circRNAs including circ_0002570 that are involved in DR pathogenesis; circRNAs might serve as potential biomarkers of DR diagnosis as well as therapeutic targets for treatment, although the mechanism remains unclear. Based on previous reports, we further explored the detailed molecular mechanism of circ_0002570 in the development of diabetic retinopathy and identified its target miRNA (miR-1243) as well as miR-1243-targeted gene angiomotin, which provided evidence for circ_0002570 to serve as a novel strategy and therapeutic target to treat diabetic retinopathy clinically.
MiR-1243 is a poorly understood microRNA with one exception that miR-1243 along with miR-509-5p promoted the sensitivity to gemcitabine to improve pancreatic cancer by suppressing the transition from epithelial cells to mesenchymal cells (Cui et al. 2019). To date, there is no publication related to miR-1243 and diabetes or complications of diabetes. Our study investigates for the first time the role of miR1243 in DR pathogenesis and identifies its target gene angiomotin.
Angiomotin (AMOT) is a protein that belongs to the motif family of angiostatin-binding proteins, including angiomotin, angiomotin-like 1 (AMOL1), and angiomotin-like 2 (AMOTL2) characterized by coiled-coil domains of N-terminus and consensus PDZ-binding domain at the C-terminus. Angiomotin predominantly expresses in endothelial cells of capillaries as well as in angiogenic tissues such as the placenta and solid tumors. Angiomotin has two different splice isoforms, angiomotin p80 and angiomotin p130, which are tissue-specific expressed. Numerous investigations demonstrate that angiomotin p80 improves the migration of endothelial cells as well as the migration of endothelial cells toward growth factors, such as VEGF, basic fibroblast growth factor (bFGF), and lysophosphatidic acid (LPA). Angiomotin also mediates tube formation of endothelial cells and promotes angiogenesis by both stimulating cell spreading and stabilizing established tubes. Unlike angiomotin p80, angiomotin 130 mediates paracellular permeability, which does not promote cell migration or improve angiogenesis. Therefore, in our study, we only analyzed the expression of angiomotin p80, and all references to angiomotin in this manuscript are to angiomotin p80. Furthermore, our study establishes for the first time the relationship between miR-1243 and angiomotin and the target gene of miR-1243, which is angiomotin. We used biotinylated RNA pull-down assay and luciferase assay to clarify that the mRNA of angiomotin directly binds to miR-1243.
However, with the exception of the tissues from DR patients, the cell model of DR we used is to stimulate hRMECs with high glucose for 48 h, which might not be a perfect fit for DR, considering that the progression of DR involves long-term hyperglycemia with chronic inflammation. Thus, it would be necessary to stimulate cells with high levels (fluctuating levels) of glucose over long periods, probably more than 10 years. This is not realistic, and therefore, this cell model of DR has its limitations, but has nonetheless been widely used and accepted (Cui et al. 2019), (Liu et al. 2019). Moreover, the development of DR involves multi-genes, multi-targets, and multi-pathways, so our study merely provides a novel mechanism of DR, and gives us a hint of potential treatments for DR.
In summary, our study demonstrates for the first time the molecular mechanism of circ_0002570 in DR pathogenesis. Circ_0002570 regulates DR progression by a circ_0002570-miR-1243-angiomotin axis in a ceRNA pattern; therefore, circ_0002570 could serve as a potential therapeutic target for treating DR clinically.
Conclusion
Our study demonstrates the molecular mechanism of circ_0002570 in DR pathogenesis. Circ_0002570 regulates DR progression by a circ_0002570-miR-1243-angiomotin axis in a ceRNA pattern, which could serve as a potential therapeutic target for treating DR clinically.
References
Chen Q et al (2018) Characteristics of retinal structural and microvascular alterations in early type 2 diabetic patients. Invest Ophthalmol Vis Sci 59:2110–2118. https://doi.org/10.1167/iovs.17-23193
Cheng Z et al (2019) circTP63 functions as a ceRNA to promote lung squamous cell carcinoma progression by upregulating FOXM1. Nat Commun 10:3200. https://doi.org/10.1038/s41467-019-11162-4
Cui C, Li Y, Liu Y (2019) Down-regulation of miR-377 suppresses high glucose and hypoxia-induced angiogenesis and inflammation in human retinal endothelial cells by direct up-regulation of target gene SIRT1. Hum Cell 32:260–274. https://doi.org/10.1007/s13577-019-00240-w
Erickson KK, Sundstrom JM, Antonetti DA (2007) Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis 10:103–117. https://doi.org/10.1007/s10456-007-9067-z
Fu X, Ou B (2020) miR-152/LIN28B axis modulates high-glucose-induced angiogenesis in human retinal endothelial cells via VEGF signaling. J Cell Biochem 121:954–962. https://doi.org/10.1002/jcb.28978
Gao R, Zhu BH, Tang SB, Wang JF, Ren J (2008) Scutellarein inhibits hypoxia- and moderately-high glucose-induced proliferation and VEGF expression in human retinal endothelial cells. Acta Pharmacol Sin 29:707–712. https://doi.org/10.1111/j.1745-7254.2008.00797.x
Gu Y, Ke G, Wang L, Zhou E, Zhu K, Wei Y (2017) Altered Expression Profile of Circular RNAs in the Serum of Patients with Diabetic Retinopathy Revealed by Microarray. Ophthalmic Res 58:176–184. https://doi.org/10.1159/000479156
Han B, Chao J, Yao H (2018) Circular RNA and its mechanisms in disease: From the bench to the clinic. Pharmacol Ther 187:31–44. https://doi.org/10.1016/j.pharmthera.2018.01.010
He M et al (2019) Comparison of expression profiling of circular RNAs in vitreous humour between diabetic retinopathy and non-diabetes mellitus patients. Acta Diabetol. https://doi.org/10.1007/s00592-019-01448-w
Huang Q, Sheibani N (2008) High glucose promotes retinal endothelial cell migration through activation of Src, PI3K/Akt1/eNOS, and ERKs. Am J Phys Cell Phys 295:C1647–C1657. https://doi.org/10.1152/ajpcell.00322.2008
Huang JK et al (2017) LncRNA-MALAT1 promotes angiogenesis of thyroid cancer by modulating tumor-associated macrophage FGF2 protein secretion. J Cell Biochem 118:4821–4830. https://doi.org/10.1002/jcb.26153
Jourdan T et al (2017) Developmental role of macrophage Cannabinoid-1 receptor signaling in type 2 diabetes. Diabetes 66:994–1007. https://doi.org/10.2337/db16-1199
Klaassen I, Van Noorden CJ, Schlingemann RO (2013) Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res 34:19–48. https://doi.org/10.1016/j.preteyeres.2013.02.001
Liu Z et al (2012) Hyperhomocysteinemia exaggerates adventitial inflammation and angiotensin II-induced abdominal aortic aneurysm in mice. Circ Res 111:1261–1273. https://doi.org/10.1161/circresaha.112.270520
Liu P, Jia SB, Shi JM, Li WJ, Tang LS, Zhu XH, Tong P (2019) LncRNA-MALAT1 promotes neovascularization in diabetic retinopathy through regulating miR-125b/VE-cadherin axis. Biosci Rep 39. https://doi.org/10.1042/bsr20181469
Mei X, Zhou L, Zhang T, Lu B, Sheng Y, Ji L (2018) Chlorogenic acid attenuates diabetic retinopathy by reducing VEGF expression and inhibiting VEGF-mediated retinal neoangiogenesis. Vasc Pharmacol 101:29–37. https://doi.org/10.1016/j.vph.2017.11.002
Nicolet BP, Engels S, Aglialoro F, van den Akker E, von Lindern M, Wolkers MC (2018) Circular RNA expression in human hematopoietic cells is widespread and cell-type specific. Nucleic Acids Res 46:8168–8180. https://doi.org/10.1093/nar/gky721
Pascolini D, Mariotti SP (2012) Global estimates of visual impairment: 2010. Br J Ophthalmol 96:614–618. https://doi.org/10.1136/bjophthalmol-2011-300539
Salzman J (2016) Circular RNA Expression: Its Potential Regulation and Function. Trends Genet 32:309–316. https://doi.org/10.1016/j.tig.2016.03.002
Stitt AW et al (2016) The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res 51:156–186. https://doi.org/10.1016/j.preteyeres.2015.08.001
Yamamoto K, Ito S, Hanafusa H, Shimizu K, Ouchida M (2015) Uncovering direct targets of MiR-19a involved in lung cancer progression. PLoS One 10:e0137887. https://doi.org/10.1371/journal.pone.0137887
Yang WZ, Yang J, Xue LP, Xiao LB, Li Y (2017) MiR-126 overexpression inhibits high glucose-induced migration and tube formation of rhesus macaque choroid-retinal endothelial cells by obstructing VEGFA and PIK3R2. J Diabetes Complicat 31:653–663. https://doi.org/10.1016/j.jdiacomp.2016.12.004
Yu B et al (2017) CYLD deubiquitinates nicotinamide adenine dinucleotide phosphate oxidase 4 contributing to adventitial remodeling. Arterioscler Thromb Vasc Biol 37:1698–1709. https://doi.org/10.1161/atvbaha.117.309859
Zhang SJ, Chen X, Li CP, Li XM, Liu C, Liu BH, Shan K, Jiang Q, Zhao C, Yan B (2017a) Identification and characterization of circular RNAs as a new class of putative biomarkers in diabetes retinopathy. Invest Ophthalmol Vis Sci 58:6500–6509. https://doi.org/10.1167/iovs.17-22698
Zhang Y et al (2017b) Nogo-B promotes angiogenesis in proliferative diabetic retinopathy via VEGF/PI3K/Akt pathway in an autocrine manner. Cell Physiol Biochem 43:1742–1754. https://doi.org/10.1159/000484061
Zhang H et al (2019) Apatinib suppresses breast cancer cells proliferation and invasion via angiomotin inhibition. Am J Transl Res 11:4460–4469
Zhu K et al (2019) Downregulation of circRNA DMNT3B contributes to diabetic retinal vascular dysfunction through targeting miR-20b-5p and BAMBI. EBioMedicine 49:341–353. https://doi.org/10.1016/j.ebiom.2019.10.004
Funding
This study was supported by the Special Fund of the Fourth Affiliated Hospital of Harbin Medical University (HYDSYTB201913).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All patients were informed and signed the consent. This study was approved by the Ethical Review Committee of the Fourth Affiliated Hospital of Harbin Medical University.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, G., Zhou, S., Li, X. et al. Inhibition of hsa_circ_0002570 suppresses high-glucose–induced angiogenesis and inflammation in retinal microvascular endothelial cells through miR-1243/angiomotin axis. Cell Stress and Chaperones 25, 767–777 (2020). https://doi.org/10.1007/s12192-020-01111-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-020-01111-2