Abstract
This study aimed to identify the response of a salivary stress protein, extracellular heat shock protein (eHSP70), to intense exercise and to investigate the relationship between salivary eHSP70 and salivary immunoglobulin A (SIgA) levels in response to exercise. Sixteen healthy sedentary young males (means ± SD 23.8 ± 1.5 years, 172.2 ± 6.4 cm, 68.3 ± 7.4 kg) performed 59 min of cycling exercise at 75 % VO2max. Saliva and whole blood samples were collected before (Pre), immediately after (Post), and at 1, 2, 3, and 4 h after completion of the exercise (1, 2, 3, and 4 h). The salivary eHSP70 and SIgA levels were measured by enzyme-linked imunosorbent assay (ELISA), and the secretion rates were computed by multiplying the concentration by the saliva flow rate. White blood cells were analyzed using an automated cell counter with a direct-current detection system. The salivary eHSP70 secretion rates were 1.11 ± 0.86, 1.51 ± 1.47, 1.57 ± 1.32, 2.21 ± 2.04, 3.36 ± 2.72, and 6.89 ± 4.02 ng · min−1 at Pre, Post, and 1, 2, 3, and 4 h, respectively. The salivary eHSP70 secretion rate was significantly higher at 4 h than that at Pre, Post, 1, and 3 h (p < 0.05). The SIgA secretion rates were 26.9 ± 12.6, 20.3 ± 10.4, 19.6 ± 11.0, 21.8 ± 12.8, 21.5 ± 11.9, and 21.9 ± 11.7 μg · min−1 at Pre, Post, 1, 2, 3, and 4 h, respectively. The salivary SIgA secretion rate was significantly lower between 1 and 4 h than that at Pre (p < 0.05). There was a positive correlation between salivary eHSP70 and SIgA in both concentration and secretion rates before exercise (p < 0.05). The absolute number of white blood cells significantly increased after exercise, with a maximum at 2 h (p < 0.05). The neutrophil/lymphocyte ratio was significantly increased from 1 to 4 h when compared with that in the Pre samples (p < 0.05). The present study revealed that salivary eHSP70 significantly increased at 4 h after the 59 min of intense exercise in sedentary male subjects. Exercise stress can induce elevated salivary eHSP70 level and upregulate oral immune function partially.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heat shock protein 70 (HSP70) exists in cells throughout the body and in body fluids (blood, lymph fluid, spinal fluid, saliva, etc.) and are termed intracellular HSP70 (iHSP70) and extracellular HSP70 (eHSP70), respectively. eHSP70 induces the production of pro-inflammatory cytokines through binding to toll-like receptors (TLR)-2 and TLR-4, leading to inflammation (Asea et al. 2000, 2002). In the oral cavity, eHSP70 has a role of mucosal defense including entrapping, agglutinating, and opsonizing bacteria, and inhibiting pathogenic adhesion to the mucosal surface (Fabian et al. 2012). iHSP70 levels increase in response to exercise-induced stressors such as heat, reactive oxygen species (ROS), and hypoxia in cells (Fehrenbach and Northoff 2001). Actually, a single bout of exercise increases circulating eHSP70 level (Walsh et al. 2001; Febbraio et al. 2002; Fehrenbach et al. 2005) and heat stress further boosts this increase (Gibson et al. 2014). However, Fortes and Whitham (2011) reported that eHSP70 in the saliva unchanged during and until 20 min after the exercise (60 min of treadmill running at 50 − 70 % VO2max) with hot environment (30 °C). The change in salivary eHSP70 levels after exercise has not been adequately examined. Walsh et al. (2001) showed that increased serum eHSP70 levels returned to baseline at 2 to 8 h after exercise. It is, therefore, possible that exercise stress might continue to have an effect on salivary eHSP70 levels for a few hours. Demonstrating the salivary eHSP70 response to exercise might provide helpful information on evaluating both exercise-induced stress comprehensively and oral immune function.
Salivary secretory immunoglobulin A (SIgA) comprises 90 % of the immunoglobulin in the mucosal tissue and defends against pathogenic infections by binding to them. Low levels of SIgA are associated with a high risk of upper respiratory tract infection (URTI) (Peters and Bateman 1983; Nieman et al. 1990; Heath et al. 1991, 1992; Gleeson et al. 1999, 2012; Fahlman and Engels 2005; Yamauchi et al. 2011). Continuous intensive training reduces SIgA levels and increases the risk of URTI in athletes (Nieman 1994), whereas continuous moderate exercise training increases SIgA levels and reduces the risk of infections (Akimoto et al. 2003; Klentrou et al. 2002; Shimizu et al. 2007). Intense exercise reduces SIgA secretion (Tomasi et al. 1982; Mackinnon and Jenkins 1993), and the reduction of SIgA levels is sustained for 2 h after exercise (Usui et al. 2011).
Salivary eHSP70 and SIgA have important roles in preventing infections in the oral cavity. Measuring the change of both salivary eHSP70 and SIgA levels during and after exercise might clarify the immune response to exercise stress in the oral cavity. There are no studies investigating both the salivary eHSP70 and SIgA levels in response to exercise stress. Such a study may provide useful information for elucidating the effect of exercise stress on oral immune function.
The aim of this study was to investigate salivary eHSP70 levels in response to intense exercise and to determine the relationship between salivary eHSP70 and SIgA levels within an expanded period of 4 h after exercise. We hypothesized that intense exercise increases salivary eHSP70 levels and that the response lasts for 4 h. Moreover, there might be a negative correlation between salivary eHSP70 and SIgA levels in response to exercise.
Materials and methods
Subjects
Sixteen healthy sedentary young males who exercise less than 60 min per week voluntarily participated in the study (Table 1). Before the exercise test, their height (Model AD-6227; A&D Co, Ltd, Tokyo, Japan) and body mass (HBF-354; Omron Corp, Kyoto, Japan) were measured. None of the participants had a history of either smoking or taking medications. Subjects were instructed to refrain from alcohol (Taylor et al. 2010b), caffeine (Lu et al. 2008), smoking (Anbarasi et al. 2006), and intense physical activity before and on the test day and until the 4-h post-exercise sampling was completed. On the experimental days, all subjects arrived at the laboratory at either 6:30 or 7:30 am after an overnight fast. Experimental exercise tests were conducted at room temperature (25 °C) to avoid environmental stress which might induce eHSP70, such as temperature changes (Selkirk et al. 2009), hypoxia (Taylor et al. 2010a), and hyperbaric changes (Taylor et al. 2012). All samples were collected between 7:00 and 8:00 am before exercise. All subjects were informed of the risks of the experiment before their informed voluntary written consent to participate was obtained. This study conformed to the principles outlined in the Declaration of Helsinki and was approved by the ethics committee of the University of Tsukuba.
Exercise protocol
The subjects performed two exercise tests on a bicycle ergometer (232CXL; Combi Wellness Co., Ltd, Tokyo, Japan) on separate days. In the first test, subjects performed an incremental cycling test for measurement of maximal oxygen consumption (VO2max). During the exercise, expired gases were collected by a facemask, and oxygen uptake and carbon dioxide were calculated by the breath-by-breath method (AE280S; Minato Medical Science Co., Ltd, Osaka, Japan). This test consisted of 2 min of unloaded cycling exercise and subsequent incremental loaded cycling exercise with the cadence maintained at 60 rpm until completion of the test. The workload was increased at 2 min intervals from 60 to 100 W. Thereafter, the workload was increased by 30 W every 3 min until the subject was exhausted. The submaximal exercise test was performed at least 4 days after the first test and within 1 month. Before the second exercise, the subjects were seated at rest for 30 min. After a 1 min of warming up at 50 % VO2max (21.3 ± 2.6 mL · kg−1 · min−1) workload, subjects cycled for 59 min at 75 % (32.3 ± 3.2 mL · kg−1 · min−1). During the cycling exercise, the workload was controlled at a maximum oxygen uptake of 75 %, and the heart rate (HR) was recorded every 5 min by a heart rate monitor (S610i, Polar Electro Oy, Kempele, Finland). Every 5 min during the exercise testing, the subjects were asked the rating of perceived exertion (RPE) using the Borg category scale (Borg 1982).
Sample collection
Saliva and whole venous blood samples were obtained before and after the submaximal exercise test and at every hour for 4 h after the exercise test (Pre, Post, 1, 2, 3, 4 h, respectively). To avoid the effect of diet, subjects were not allowed to eat 12 h before exercise and were given a uniform diet after the exercise. Peripheral venous blood was collected from the antecubital vein into a 2-mL EDTA-coated vacuum tube (VP-DK052K05, Terumo Co., Tokyo, Japan) using a butterfly needle (SV-21DLK, Terumo Co., Tokyo, Japan). Before saliva sample collection, the subjects rinsed their mouth with distilled water for 30 s and repeated the rinsing two more times. After 5 min of rest in a seated position, subjects swallowed all of the saliva in the oral cavity and placed cotton from a plastic container (Salivette; Sersted, Numbrecht, Germany) into their mouth, and chewed it once per second for 60 s. Saliva was separated from the cotton by centrifugation at 3000 rpm. After measurement of the sample volume, saliva samples were frozen at −80 °C and stored until the end of the study period. Total saliva volumes were calculated by saliva sample weight and density (1 mg · mL−1).
Saliva assay
The salivary eHSP70 concentration was measured using a commercial enzyme-linked imunosorbent assay (ELISA) kit (ESK-715, Assay Designs Inc., Ann Arbor, Michigan). Saliva samples were thawed and centrifuged for 1 min at 10,000 rpm prior to measurement as previously described (Fortes and Whitham 2008, 2011). Salivary SIgA concentration was measured by a sandwich ELISA created in-house, as previously described (Akimoto et al. 2003). Salivary eHSP70 and SIgA secretion rates (ng · min−1 and μg · min−1, respectively) were computed by multiplying the concentration (ng · mL−1 and μg · mL−1, respectively) by the saliva flow rate (mL · min−1).
Blood assay
Total white blood cells (WBC) counts were analyzed using an automated cell counter (Sysmex SE9000, Sysmex, Japan) with direct-current detection. Monocytes, lymphocytes, and neutrophils were counted using a microscopic test and adjusted for changes in blood volume. The neutrophil/lymphocyte ratio (NLR) was computed by dividing neutrophil counts by lymphocyte counts.
Statistical analysis
Data were reported as mean ± SD. Changes over time were evaluated using ANOVA for repeated measurements, followed by the Bonferroni post hoc test. The relation between concentrations and secretion rates of salivary eHSP70 and SIgA levels, NLR and salivary eHSP70 levels and secretion rate, and NLR and SIgA levels and secretion rate were investigated by Pearson’s correlation coefficient test. All calculations were performed using software (SPSS ver.21), and a p value of 0.05 or less was used to define statistical significance.
Results
VO2, HR, and RPE
The subjects’ VO2max and HRmax in the incremental exercise test are shown in Table 1. During the submaximal exercise test, the peak HR was 182 ± 10 bpm (93.3 ± 4.5 %HRmax) and the average HR was 173 ± 9 bpm (88.7 ± 4.0 %HRmax). The peak and average RPE in the submaximal exercise test were 18 ± 1 and 16 ± 1 respectively.
Saliva flow rate
The saliva flow rates were 1.19 ± 0.32, 1.07 ± 0.33, 1.35 ± 0.41, 1.34 ± 0.40, 1.29 ± 0.37, and 1.35 ± 0.38 mL at Pre, Post, 1, 2, 3, and 4 h, respectively. The sequential changes in the saliva flow rate were statistically significant (F = 3.661, p < 0.05). The saliva flow rate at Post was significantly lower than that at 4 h (p < 0.05), and the saliva flow rate at 1 h tended to be higher than that at Post (p < 0.10).
Salivary eHSP70
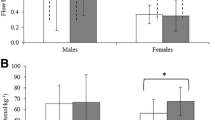
The salivary eHSP70 concentrations were 0.91 ± 0.53, 1.44 ± 1.46, 1.32 ± 1.50, 1.58 ± 1.26, 2.70 ± 2.21, and 5.19 ± 2.48 ng · mL−1 at Pre, Post, 1, 2, 3, and 4 h, respectively. The sequential changes in the salivary eHSP70 concentration were statistically significant (F = 18.864, p < 0.05, Fig. 1a). The salivary eHSP70 concentration at 4 h was higher than that at Pre and at 1 h (p < 0.05). The salivary eHSP70 concentration at 4 h tended to be higher than that at 2 and 3 h (p < 0.10). The salivary eHSP70 secretion rates were 1.11 ± 0.86, 1.51 ± 1.47, 1.57 ± 1.32, 2.21 ± 2.04, 3.36 ± 2.72, and 6.89 ± 4.02 ng · min−1 at Pre, Post, 1, 2, 3, and 4 h, respectively. The sequential changes in the salivary eHSP70 secretion rates were statistically significant (p < 0.05, Fig. 1b). The salivary eHSP70 secretion rate at 4 h was higher than that at Pre, Post, 1, and 3 h (p < 0.05). The salivary eHSP70 secretion rate at 4 h tended to be higher than that at Post 2 h (p < 0.10).
a The sequential changes of salivary eHSP70 and SIgA concentration were statistically significant (p < 0.05). Salivary eHSP70 concentration at 4 h was significantly higher than that at Pre and 1 h after exercise (p < 0.05). SIgA concentration after exercise was significantly lower than that at Pre (p < 0.05), and the lowest concentration was identified at 1 h. b The sequential changes of salivary eHSP70 and SIgA secretion rate were statistically significant (p < 0.05). Salivary eHSP70 at 4 h were significantly higher than that at Pre, immediately Post, 1 h, and 3 h after exercise (p < 0.05). Results are presented as mean ± SD. *p < 0.05 vs. Pre; #p < 0.05 vs. Post; †p < 0.05 vs. 1 h; §p < 0.05 vs. 3 h
Saliva SIgA
The SIgA concentrations were 22.8 ± 8.5, 18.5 ± 7.6, 13.9 ± 5.8, 15.7 ± 6.7, 16.5 ± 7.4, and 15.9 ± 6.0 μg · mL−1 at Pre, Post, 1, 2, 3, and 4 h, respectively. The sequential changes in the SIgA concentration were statistically significant (F = 14.897, p < 0.05, Fig. 1a). The lowest concentration was identified at 1 h. The SIgA concentration at Post, 1, 2, 3, and 4 h decreased from that at the Pre concentration (p < 0.05), and at 1 h was lower than that at Post (p < 0.05). The SIgA secretion rates were 26.9 ± 12.6, 20.3 ± 10.4, 19.6 ± 11.0, 21.8 ± 12.8, 21.5 ± 11.9, and 21.9 ± 11.7 μg · min−1 at Pre, Post, 1, 2, 3, and 4 h, respectively. The sequential changes in the SIgA secretion rates were statistically significant (F = 3.898, p < 0.05, Fig. 1b). The SIgA secretion rate at 1 h tended to be decreased compared with the Pre secretion rate (p < 0.10).
White blood cells
The sequential changes in WBC counts were statistically significant (F = 38.947, p < 0.05, Table 2). WBC counts at Post were not significantly higher than that at Pre (p < 0.05), and the maximum was at 2 h. At 3 and 4 h, WBC counts significantly decreased from that at 2 h (p < 0.05). There were no significant sequential changes in monocyte counts (Table 2). The sequential changes in lymphocyte counts were statistically significant (F = 54.904, p < 0.05, Table 2). Lymphocyte counts at Post were significantly increased from that at Pre (p < 0.05). At 1, 2, 3, and 4 h, lymphocytes significantly decreased from Post (p < 0.05), and at 4 h tended to be higher than that at both 1 and 2 h (p <0.10). The sequential changes in neutrophil counts were statistically significant (F = 16.239, p < 0.05, Table 2). The neutrophil counts at Post were significantly increased from that at Pre (p < 0.05), and the maximum was at 2 h. At 3 and 4 h, neutrophils were significantly decreased from that at 2 h (p < 0.05). The NLR were 0.96 ± 0.29, 1.15 ± 0.32, 4.23 ± 1.92, 5.41 ± 3.22, 4.22 ± 3.18, and 3.20 ± 2.15 at Pre, Post, 1, 2, 3, and 4 h, respectively (Fig. 2). The sequential changes in the NLR were statistically significant (F = 18.749, p < 0.05). The NLR at 1 h was significantly increased from that at Pre (p < 0.05), and the maximum was at 2 h. The NLR at 4 h tended to be lower than that at 2 h (p < 0.10).
The sequential changes of the neutrophil/lymphocyte ratio (NLR) were statistically significant (p < 0.05). NLR 1 h after exercise was significantly higher than that at Pre and Post (p < 0.05), and the highest NLR was identified at 2 h. Results are presented as mean ± SD. *p < 0.05 vs. Pre; #p < 0.05 vs. Post
Correlation between salivary eHSP70 vs. SIgA levels and white blood cells
The correlation coefficients of concentrations and secretion rates between salivary eHSP70 and SIgA and white blood cells are reported in Table 3. There is a significant positive correlation between salivary eHSP70 concentration and SIgA concentration (p < 0.05), and a negative correlation between salivary eHSP70 concentration and neutrophil counts (p < 0.05) at Pre. However, there was no correlation in these parameters after exercise.
Discussion
The present study investigated the influence of intense exercise on eHSP70 levels in healthy sedentary male subjects. The primary finding of the present study was that salivary eHSP70 levels were significantly elevated at 4 h after completion of the 59 min of cycling exercise at 75 %VO2max. In addition, a positive correlation was observed between salivary eHSP70 and SIgA levels at rest, but no correlation was observed between these parameters after completion of the exercise.
In the oral cavity, salivary eHSP70 prevents pathogenic infections. Salivary eHSP70 derives from the salivary glands, oral mucosa, periodontal tissue, and bacteria (Fabian et al. 2007). In the present study, the saliva samples may have been slightly contaminated with exogenous HSP70 from bacteria because of the mouth rinsing performed prior to collection of the saliva samples. A previous study reported that there was little eHSP70 in gingival crevicular fluid (GCF) except in the presence of braces for orthodontic correction (Kuroki et al. 2013). Although eHSP70 derived from periodontal tissue may co-mix into the saliva samples via GCF, no subject had orthodontic appliances in this study. In addition, Lancaster and Febbraio (2005) showed that exosomes contributed to circulating eHSP70 levels, and Ogawa et al. (2011) reported that exosomes exists in saliva. Therefore, most eHSP70 in the collected saliva samples was likely derived from either the salivary glands, oral mucosa, or salivary exosomes.
Fabian et al. (2007) suggested the possibility that salivary glands release eHSP70 into the saliva. The salivary glands consist of both acinar and ductal cells. A large proportion of salivary proteins, such as SIgA, α-amylase, and mucin, are secreted from the acinar cells of the salivary glands by sympathetic nerve stimuli. In the present study, an increase in the NLR continued until 4 h after exercise, suggesting that sympathetic nerve activity may be promoted by exercise. However, Fortes and Whitham (2011) presumptively suggested another mechanism involving acinar cells release of salivary eHSP70 via exosomes, not via sympathetic nerve stimuli but rather via parasympathetic nerve stimuli. Acceleration of parasympathetic nerve activity induces a decrease in the NLR (Nozaki et al. 2000) and an increase in the saliva flow rate. In the present study, an increase in salivary eHSP70 was observed 4 h after exercise, whereas there was neither a decrease in the NLR nor an increase in the saliva flow rate. Further research is required to identify the influence of the autonomic nervous system on eHSP70 secretion. Vanmuylder et al. (2000) reported that eHSP70 is strongly expressed in epithelial cells of the striated ducts and weakly expressed in the acinar cells of the salivary glands. Therefore, it can be inferred that constitutive salivary eHSP70 is secreted from the ductal cells. However, it is unclear whether eHSP70 is secreted from either the acinar cells and/or ductal cells of salivary glands under stress conditions. There is another mechanism possible, in which the oral mucosa secretes eHSP70. Some previous studies advocate the hypothesis that eHSP70 is secreted from the oral mucosa (Kaur and Ralhan 1995; Sugerman et al. 1995). In animal and in vitro studies, mucosal cells synthesize and secrete eHSP70 in stress conditions. Matsuo et al. (2009) reported that eHSP70 is secreted from the mouse gut lamina propria by physical stress, including restraint for 2 h in a 50-mL centrifuge tube. Broquet et al. (2003) reported that epithelial cells (Caco-2) undergoing heat stress synthesized and secreted eHSP70 via detergent-resistant microdomains. In addition, they showed that eHSP70 expression increased with the rise in heat stress, and that the strongest eHSP70 expression was observed 6 h after 30 min of heat stress at 44 °C. Therefore, it appears that mucosal cells constitutionally have an eHSP70 synthesizing and secreting function in response to some types of stress, such as physical (restraint stress) and heat stress. The mechanisms of eHSP70 secretion from human oral mucosa in exercise-stressed conditions remain unclear. Lancaster and Febbraio (2005) reported that exosomes contribute to eHSP70 release from PBMCs in both unheated (37 °C) and heated (40 or 43 °C for 1 h) conditions in vitro. Accordingly, they suggested that exosomal eHSP70 was increased in heat-shocked cells. It was reported that acute stress (i.e., stress due to restraining of the animal and electronic tail shocks) induced exosomal eHSP70 in rat plasma (Beninson et al. 2014). However, it is still unclear whether exercise stress induced exosomal eHSP70 in both the circulation and saliva. Further molecular, biological, and biochemical research might provide useful information on the mechanisms underlying salivary eHSP70 secretion from the salivary glands, oral mucosa, and salivary exosomes.
To the best of our knowledge, there is only one study examining the effect of exercise on salivary eHSP70 (Fortes and Whitham 2011). Fortes and Whitham (2011) reported a lack of change in salivary eHSP70 in response to multiple stresses, such as exercise stress, caffeine intake, and hyperthermia exposure, in athletes. In that study, healthy endurance-trained males performed intense exercise, consisting of 30 min of treadmill running at 50 % VO2max (first bout) and 30 min at 70 % (second bout) in hot conditions (30 °C). The intervention was considered sufficiently stressful based on the subjects’ mean heart rate (181 ± 7 bpm) and core temperature (39.0 ± 0.3 °C) at the end of the second exercise interval. In the present study, healthy sedentary males performed exercise comprised of 59 min of cycling at 75 % VO2max at 25 °C. In the first 30 min of exercise, the exercise intensity was higher in the present study compared to that in the previous study. The subjects’ mean heart rate throughout the exercise was 173 ± 9 bpm (88.7 ± 4.0 % HRmax). In addition, the exercise workload may be sufficient to impose stress on the subjects because the NLR increased and the SIgA decreased after the exercise. The NLR is recognized as an index to assess exercise stress (Gleeson and Bishop 2000). Several previous studies have reported that the NLR increases after intense exercise (Nieman et al. 1995, 1999; Makras et al. 2005; Davison and Diment 2010; Lancaster et al. 2003). In the present study, the NLR increased after exercise compared with that at Pre, and the increase continued until 4 h after exercise. SIgA is well known as an index of defensive function against pathogens in the oral cavity. SIgA levels decreased after high intensity exercise (more than 80 % VO2max) (McDowell et al. 1992). Therefore, it is clear that the exercise stress of the present study was sufficient to evoke an immune response in the peripheral blood and to reduce the defense function of the oral cavity. These responses continued until the end of the present study.
It is generally believed that training status affects immune function (Walsh et al. 2011). It is likely that training status might affect the salivary eHSP70 level. Reportedly, HSP70-positive leukocytes in the peripheral blood were lower in trained athletes compared with that in untrained people (Fehrenbach et al. 2000). It is therefore possible that the resting salivary eHSP70 level might reflect training adaptation in active people (i.e., recreational and competitive athletes). Training adaptation includes exercise tolerance. It is possible that exercise-induced eHSP70 might increase inflammation, thereby inducing fatigue sensation (Heck et al. 2011). Therefore, the change of salivary eHSP70 might describe the inflammatory status and relative fatigue sensation. In the present study, inflammation-related markers (e.g., WBC and neutrophil) temporarily increased after exercise, while each marker did not show the positive correlations with salivary eHSP70 at any time point. However, the effect of training status on the salivary eHSP70 level at rest and response to exercise has not been elucidated. Further investigation is required to determine the effect of training status and regular exercise on salivary eHSP70 levels. These studies might provide useful information for evaluating the training adaptation and physical condition of active people.
To the best of our knowledge, there are no other studies evaluating the correlation between eHSP70 and SIgA levels. In the present study, there was a positive correlation between resting eHSP70 levels and SIgA levels and no correlation after exercise. Salivary eHSP70 and SIgA levels may synchronously play an important role in defense against pathogenic infections. Based on the functions of salivary eHSP70, such as entrapping, agglutinating, and opsonizing bacteria, salivary eHSP70 levels may be an index of infection risk. Further studies are required to investigate the correlation between eHSP70 and the risk of infections, such as URTI. On the other hand, there was no correlation after exercise. Individual variability may affect the responses of salivary eHSP70 and SIgA levels to exercise. Because the increase of salivary eHSP70 levels after exercise remains poorly understood, molecular, biological, and biochemical research into the mechanism of salivary eHSP70 secretion is required. In addition, the time to peak salivary eHSP70 level is still unclear. In order to clarify the salivary eHSP70 peak level, it is necessary to study the changes of salivary eHSP70 levels during the recovery process. Those studies may provide useful information for assessing the physical condition of athletes by saliva. There is negative correlation at rest between salivary eHSP70 and neutrophil levels, while no correlation is found after exercise. It is suggested that exercise-induced circulating eHSP70 stimulates neutrophil phagocytic and fungicidal capacities (Giraldo et al. 2010). Neutrophils also exist in saliva. Davison and Jones (2015) reported that salivary neutrophil numbers and functions (oxidative burst) were unchanged immediately after prolonged exercise. eHSP70 activates neutrophils via TLR-4 (Wheeler et al. 2009). Increased salivary eHSP70 might enhance innate immune function via salivary neutrophils after exercise.
This study has the following study limitations. First, the time of return to baseline value for salivary eHSP70 levels is still unclear. Previous studies showed that circulating eHSP70 increased temporarily (Fehrenbach et al. 2005; Whitham et al. 2006) and returned to baseline within 8 or 24 h, after intense exercise (Walsh et al. 2001; Suzuki et al. 2006). Although the reaction times to respond to exercise stress in circulating eHSP70 and salivary eHSP70 were different, it was assumed that exercise-induced salivary eHSP70 levels might return to baseline within 24 h. Changes in eHSP70 levels should be observed for more than 4 h or until 1 or 2 days after exercise to completely determine the changes in levels at various times until return to baseline. Second, sedentary healthy males participated in the present study. Given the effect of the subject’s exercise history and physical fitness, it is impossible to compare the results of our study with those of a previous study involving endurance-trained males (Fortes and Whitham 2011). Further studies are required to investigate the effects of exercise stress on salivary eHSP70, with respect to the subject’s exercise habits, intensity and duration of exercise intervention, ambient temperature during exercise, and times of sample collection. Those studies may also be very helpful in evaluating the physical condition of athletes under continuous stress.
In conclusion, our data show that salivary eHSP70 increased after the 59 min of cycling exercise at 75 %VO2max in sedentary male subjects. Salivary eHSP70 increased at 4 h after intense exercise. Exercise stress can induce elevated salivary eHSP70 level and upregulate oral immune function partially.
References
Akimoto T, Kumai Y, Akama T, Hayashi E, Murakami H, Soma R, Kuno S, Kono I (2003) Effects of 12 months of exercise training on salivary secretory IgA levels in elderly subjects. Br J Sports Med 37:76–79
Anbarasi K, Kathirvel G, Vani G, Jayaramanb G, Shyamala Devi CS (2006) Cigarette smoking induces heat shock protein 70 kDa expression and apoptosis in rat brain: modulation by bacoside A. Neuroscience 138:1127–1135
Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK (2000) HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 6:435–442
Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK (2002) Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277:15028–15034
Beninson LA, Brown PN, Loughridge AB et al (2014) Acute stressor exposure modifies plasma exosome-associated heat shock protein 72 (Hsp72) and microRNA (miR-142-5p and miR-203). PLoS ONE 9(9):e108748
Borg G (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–381
Broquet AH, Thomas G, Masliah J, Trugnan G, Bachelet M (2003) Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J Biol Chem 278:21601–21606
Davison G, Diment BC (2010) Bovine colostrum supplementation attenuates the decrease of salivary lysozyme and enhances the recovery of neutrophil function after prolonged exercise. Br J Nutr 103:1425–1432
Davison G, Jones AW (2015) Oral neutrophil responses to acute prolonged exercise may not be representative of blood neutrophil responses. Appl Physiol Nutr Metab 40:298–301
Fabian TK, Fejerdy P, Nguyen MT, Soti C, Csermely P (2007) Potential immunological functions of salivary Hsp70 in mucosal and periodontal defense mechanisms. Arch Immunol Ther Exp 55:91–98
Fabian TK, Hermann P, Beck A, Fejerdy P, Fabian G (2012) Salivary defense proteins: their network and role in innate and acquired oral immunity. Int J Mol Sci 13:4295–4320
Fahlman MM, Engels HJ (2005) Mucosal IgA and URTI in American college football players: a year longitudinal study. Med Sci Sports Exerc 37:374–380
Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, Secher NH, Pedersen BK (2002) Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol 544:957–962
Fehrenbach E, Northoff H (2001) Free radicals, exercise, apoptosis, and heat shock proteins. Exerc Immunol Rev 7:66–89
Fehrenbach E, Niess AM, Schlotz E, Passek F, Dickhuth HH, Northoff H (2000) Transcriptional and translational regulation of heat shock proteins in leukocytes of endurance runners. J Appl Physiol 89:704–710
Fehrenbach E, Niess AM, Voelker K, Northoff H, Mooren FC (2005) Exercise intensity and duration affect blood soluble HSP72. Int J Sports Med 26:552–557
Fortes MB, Whitham M (2008) No endogenous circadian rhythm in resting plasma Hsp72 concentration in humans. Cell Stress Chaperones 14:273–280
Fortes MB, Whitham M (2011) Salivary Hsp72 does not track exercise stress and caffeine stimulated plasma Hsp72 responses in humans. Cell Stress Chaperones 16:345–352
Gibson OR, Dennis A, Parfitt T, Taylor L, Watt PW, Maxwell NS (2014) Extracellular Hsp72 concentration relates to a minimum endogenous criteria during acute exercise-heat exposure. Cell Stress Chaperones 19:389–400
Giraldo E, Martin-Cordero L, Garcia JJ, Gehrmann M, Gerhmann M, Multhoff G et al (2010) Exercise-induced extracellular 72 kDa heat shock protein (Hsp72) stimulates neutrophil phagocytic and fungicidal capacities via TLR-2. Eur J Appl Physiol 108(2):217–225
Gleeson M, Bishop NC (2000) Special feature for the Olympics: effects of exercise on the immune system: modification of immune responses to exercise by carbohydrate, glutamine and anti-oxidant supplements. Immunol Cell Biol 78:554–561
Gleeson M, McDonald WA, Pyne DB, Cripps AW, Francis JL, Fricker PA, Clancy RL (1999) Salivary IgA levels and infection risk in elite swimmers. Med Sci Sports Exerc 31:67–73
Gleeson M, Bishop N, Oliveira M, McCauley T, Tauler P, Muhamad AS (2012) Respiratory infection risk in athletes: association with antigen-stimulated IL-10 production and salivary IgA secretion. Scand J Med Sci Sports 22:410–417
Heath GW, Ford ES, Craven TE, Macera CA, Jackson KL, Pate RR (1991) Exercise and the incidence of upper respiratory tract infections. Med Sci Sports Exerc 23:152–157
Heath GW, Macera CA, Nieman DC (1992) Exercise and upper respiratory tract infections. Is there a relationship? Sports Med 14:353–365
Heck TG, Schoeler CM, Homem de Bittencourt PI (2011) HSP70 expression: does it a novel fatigue signalling factor from immune system to the brain? Cell Biochem Funct 29:215–226
Kaur J, Ralhan R (1995) Differential expression of 70-kDa heat shock-protein in human oral tumorigenesis. Int J Cancer 63:774–779
Klentrou P, Cieslak T, MacNeil M, Vintinner A, Plyley M (2002) Effect of moderate exercise on salivary immunoglobulin A and infection risk in humans. Eur J Appl Physiol 87:153–158
Kuroki H, Miyagawa Y, Shimomura-Kuroki J, Endo T, Shimomura H (2013) Identification of marker proteins by orthodontic treatment: relationship of RANKL in the gingival crevicular fluid and of amylase in whole saliva with orthodontic treatment. Odontology 102:303–309
Lancaster GI, Febbraio MA (2005) Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem 280:23349–23355
Lancaster GI, Jentjens RLPG, Moseley L, Jeukendrup AE, Gleeson M (2003) Effect of pre-exercise carbohydrate ingestion on plasma cytokine, stress hormone, and neutrophil degranulation responses to continuous, high-intensity exercise. Int J Sport Nutr Exerc Metab 13:436–453
Lu P-Z, Lai C-Y, Chan W-H (2008) Caffeine induces cell death via activation of apoptotic signal and inactivation of survival signal in human osteoblasts. Int J Mol Sci 9:698–718
Mackinnon LA, Jenkins DG (1993) Decreased salivary immunoglobulins after intense interval exercise before and after training. Med Sci Sports Exerc 25:678–683
Makras P, Koukoulis GN, Bourikas G, Papatheodorou G, Bedevis K, Menounos P, Pappas D, Kartalis G (2005) Effect of 4 weeks of basic military training on peripheral blood leucocytes and urinary excretion of catecholamines and cortisol. J Sports Sci 23:825–834
Matsuo K, Zhang X, Ono Y, Nagatomi R (2009) Acute stress-induced colonic tissue HSP70 expression requires commensal bacterial components and intrinsic glucocorticoid. Brain Behav Immun 23:108–115
McDowell SL, Hughes RA, Hughes RJ, Housh TJ, Johnson GO (1992) The effect of exercise training on salivary immunoglobulin-a and cortisol responses to maximal exercise. Int J Sports Med 13:577–580
Nieman DC (1994) Exercise, infection, and immunity. Int J Sports Med 15(Suppl 3):S131–S141
Nieman DC, Johanssen LM, Lee JW, Arabatzis K (1990) Infectious episodes in runners before and after the Los Angeles Marathon. J Sports Med Phys Fitness 30:316–328
Nieman DC, Simandle S, Henson DA, Warren BJ, Suttles J, Davis JM, Buckley KS, Ahle JC, But- terworth DE, Fagoaga OR (1995) Lymphocyte proliferative response to 2.5 hours of running. Int J Sports Med 16:404–409
Nieman DC, Nehlsen-Cannarella SL, Fagoaga OR, Henson DA, Shannon M, Davis JM, Austin MD, Hisey CL, Holbeck JC, Hjertman JM, Bolton MR, Schilling BK (1999) Immune response to two hours of rowing in elite female rowers. Int J Sports Med 20:476–481
Nozaki T, Komaki G, Takii M, Kawai K, Matsumoto Y, Murakami S, Kubo C (2000) The relation between the ratio of neutrophils to lymphocytes in leukocytesand and the function of the autonomic nervous system in patients with anorexia nervosa. Jpn J Psychosom Med 40:611–616
Ogawa Y, Miura Y, Harazono A, Kanai-Azuma M, Akimoto Y, Kawakami H, Yamaguchi T, Toda T, Endo T, Tsubuki M, Yanoshita R (2011) Proteomic analysis of two types of exosomes in human whole saliva. Biol Pharm Bull 34:13–23
Peters EM, Bateman ED (1983) Ultramarathon running and upper respiratory tract infections. An epidemiological survey. S Afr Med J 64:582–584
Selkirk GA, McLellan TM, Wright HE, Rhind SG (2009) Expression of intracellular cytokines, HSP72, and apoptosis in monocyte subsets during exertional heat stress in trained and untrained individuals. Am J Physiol Regul Integr Comp Physiol 296:R575–R586
Shimizu K, Kimura F, Akimoto T, Akama T, Otsuki T, Nishijima T, Kuno S, Kono I (2007) Effects of exercise, age and gender on salivary secretory immunoglobulin A in elderly individuals. Exerc Immunol Rev 13:55–66
Sugerman PB, Savage NW, Xu LJ, Walsh LJ, Seymour GJ (1995) Heat shock protein expression in oral epithelial dysplasia and squamous cell carcinoma. Eur J Cancer B Oral Oncol 31:63–67
Suzuki K, Peake J, Nosaka K, Okutsu M, Abbiss CR, Surriano R, Bishop D, Quod MJ, Lee H, Martin DT, Laursen PB (2006) Changes in markers of muscle damage, inflammation and HSP70 after an Ironman Triathlon race. Eur J Appl Physiol 98:525–534
Taylor L, Midgley A, Chrismas B (2010a) The effect of acute hypoxia on heat shock protein 72 expression and oxidative stress in vivo. Eur J Appl Physiol 109:849–855
Taylor L, Midgley AW, Chrismas B et al (2010b) Daily quadratic trend in basal monocyte expressed HSP72 in healthy human subjects. Amino Acids 38:1483–1488
Taylor L, Midgley AW, Sandstrom ME et al (2012) The effect of the hyperbaric environment on heat shock protein 72 expression in vivo. Res Sports Med 20:142–153
Tomasi TB, Trudeau FB, Czerwinski D, Erredge S (1982) Immune parameters in athletes before and after strenuous exercise. J Clin Immunol 2:173–178
Usui T, Yoshikawa T, Orita K, Ueda SY, Katsura Y, Fujimoto S, Yoshimura M (2011) Changes in salivary antimicrobial peptides, immunoglobulin A and cortisol after prolonged strenuous exercise. Eur J Appl Physiol 111:2005–2014
Vanmuylder N, Evrard L, Daelemans P, Dourov N (2000) Chaperones in the parotid gland: localization of heat shock proteins in human adult salivary glands. Cells Tissues Organs 167(2–3):199–205
Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA (2001) Exercise increases serum Hsp72 in humans. Cell Stress Chaperones 6:386–393
Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P (2011) Position statement. Part one: immune function and exercise. Exerc Immunol Rev 17:6–63
Wheeler DS, Chase MA, Senft AP, Poynter SE, Wong HR, Page K (2009) Extracellular Hsp72, an endogenous DAMP, is released by virally infected airway epithelial cells and activates neutrophils via Toll-like receptor (TLR)-4. Respir Res 10(1):31
Whitham M, Walker GJ, Bishop NC (2006) Effect of caffeine supplementation on the extracellular heat shock protein 72 response to exercise. J Appl Physiol 101:1222–1227
Yamauchi R, Shimizu K, Kimura F, Takemura M, Suzuki K, Akama T, Kono I, Akimoto T (2011) Virus activation and immune function during intense training in rugby football players. Int J Sports Med 32:393–398
Acknowledgments
We acknowledge the advice of Dr. Fuminori Kimura (Graduate School of Comprehensive Human Sciences, University of Tsukuba). This study was supported by a Grant-in-Aid for Science Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (22300235 to I. K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Murase, Y., Shimizu, K., Tanimura, Y. et al. Salivary extracellular heat shock protein 70 (eHSP70) levels increase after 59 min of intense exercise and correlate with resting salivary secretory immunoglobulin A (SIgA) levels at rest. Cell Stress and Chaperones 21, 261–269 (2016). https://doi.org/10.1007/s12192-015-0656-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-015-0656-2