Abstract
This study compared resting and exercise heat/hypoxic stress-induced levels of plasma extracellular heat shock protein 70 (eHSP70) in humans using two commercially available enzyme-linked immunosorbent assay (ELIS)A kits. EDTA plasma samples were collected from 21 males during two separate investigations. Participants in part A completed a 60-min treadmill run in the heat (HOT70; 33.0 ± 0.1 °C, 28.7 ± 0.8 %, n = 6) at 70 % V̇O2max. Participants in part B completed 60 min of cycling exercise at 50 % V̇O2max in either hot (HOT50; 40.5 °C, 25.4 relative humidity (RH)%, n = 7) or hypoxic (HYP50; fraction of inspired oxygen (FIO2) = 0.14, 21 °C, 35 % RH, n = 8) conditions. Samples were collected prior to and immediately upon termination of exercise and analysed for eHSP70 using EKS-715 high-sensitivity HSP70 ELISA and new ENZ-KIT-101 Amp’d™ HSP70 high-sensitivity ELISA. ENZ-KIT was superior in detecting resting eHSP70 (1.54 ± 3.27 ng·mL−1; range 0.08 to 14.01 ng·mL−1), with concentrations obtained from 100 % of samples compared to 19 % with EKS-715 assay. The ENZ-KIT requires optimisation prior to running samples in order to ensure participants fall within the standard curve, a step not required with EKS-715. Using ENZ-KIT, a 1:4 dilution allowed for quantification of resting HSP70 in 26/32 samples, with a 1:8 (n = 3) and 1:16 (n = 3) dilution required to determine the remaining samples. After exercise, eHSP70 was detected in 6/21 and 21/21 samples using EKS-715 and ENZ-KIT, respectively. eHSP70 was increased from rest after HOT70 (p < 0.05), but not HOT50 (p > 0.05) or HYP50 (p > 0.05) when analysed using ENZ-KIT. It is recommended that future studies requiring the precise determination of resting plasma eHSP70 use the ENZ-KIT (i.e. HSP70 Amp’d® ELISA) instead of the EKS-715 assay, despite additional assay development time and cost required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heat shock proteins (HSPs) are an evolutionarily conserved family of proteins, with individual members named according to their molecular weight. Intracellular HSPs are expressed both constitutively and accumulate after exposure to a wide array of physiological and psychological stressors (Moseley 1997; Kregel 2002; Horowitz 2007). The 70-kilodalton (kDa) HSP (HSP70/HSPA1A, Kampinga et al. 2009) remains the most widely studied member of the HSP family due to its multiple functions related to de novo protein folding (Fink 1999), refolding (Hartl 1996), degradation (Garrido et al. 2001) and intracellular anti-inflammatory action observed following induction (Ianaro et al. 2001)—all important factors in the maintenance of protein homeostasis.
In addition to its intracellular HSP70 (iHSP70) function, HSP70 has been detected in the circulation (i.e. plasma; Pockley et al. 1998), where it is described as an extracellular HSP (eHSP, Campisi et al. 2003). eHSP70 stimulates neutrophil microbiocidal activity (Ortega et al. 2006), chemotaxis (Ortega et al. 2009) and induces cytokine production via a CD14-mediated pathway (Asea 2006), thereby promoting innate immune activation (Krause et al. 2015). Elevated resting concentrations of eHSP70 has been positively correlated with insulin resistance (Krause et al. 2015) and disease progression in auto-immune (Luo et al. 2008) and inflammatory diseases (Schick et al. 2004; Najafizadeh et al. 2015). Therefore, eHSP70 may be a useful biomarker when monitoring the progression of diseases in which low-grade inflammation plays a role (Rodrigues-Krause et al. 2012), such as sarcopenia (Ogawa et al. 2012), rheumatoid arthritis (Najafizadeh et al. 2015), diabetes (Krause et al. 2015) and obesity (Chung et al. 2008). The balance between the anti-inflammatory action of iHSP70 and pro-inflammatory action eHSP70 may determine the outcome (induction or attenuation of inflammation) during disease progression, in response to a treatment, or following an exercise bout (Krause et al. 2015). Therefore, understanding the eHSP70 response and its actions is of importance in clinical situations.
In many studies, the determination of basal eHSP70 has proven to be problematic (Ogawa et al. 2012; Lee et al. 2014a, b; Najafizadeh et al. 2015) with no consensus normative data for resting eHSP70 reported to date. Sample handling considerations (e.g. blood collection tubes temporarily stored on ice versus room temperature, using heparin versus EDTA as an anticoagulant) have been suggested as explanations underlying the variability in eHSP70 values reported in the literature. For example, serum appears to yield lower basal values than those obtained by plasma (Whitham and Fortes 2006), which may be an artefact of eHSP70 binding to proteins involved in the clotting process, such as fibrin and fibrinogen (Whitham and Fortes 2006). Additionally, EDTA is recommended for use over heparin as higher resting concentrations are derived (Whitham and Fortes 2006). Despite the recommendation to use EDTA plasma for eHSP70 determination (Whitham and Fortes 2006), multiple studies have attempted to quantify eHSP70 in serum for a range of conditions, with varied results (Dulin et al. 2010; Najafizadeh et al. 2015).
Another factor affecting the detection of eHSP70 in the circulation at rest could be attributed to the sensitivity and detection capabilities of the most widely cited enzyme-linked immunosorbent assay (ELISA), ‘EKS-715 HSP70 high-sensitivity ELISA’ (Enzo life sciences, Lausen, Switzerland). This assay, which has been cited by many papers investigating eHSP70 in humans, has a working range of 0.20–12.5 ng·mL−1, a sensitivity of 0.09 ng·mL−1 and is recommended by Cell Stress Society International (http://www.cellstressresponses.org/sample-page/comments-and-suggestions-from-the-editor-in-chief/choosing-antibodies-that-recognize-extra-cellular-human-hsp70a1a/). In studies investigating eHSP70, resting values are highly variable, with many papers unable to detect basal eHSP70 in participants (Lee et al. 2014a, b; Gibson et al. 2014) or concentrations reported at the lower portion of a standard curve (<0.20 ng·mL−1, Ogawa et al. 2012; Rodrigues-Krause et al. 2012; Gibson et al. 2014; Lee et al. 2014a, b). This issue appears not to be uniform in the literature, however, as some studies report concentrations that are much higher (17.0 ± 2.6 ng·mL−1; Ruell et al. 2006).
A new ELISA has become available (ENZ-KIT-101-001 HSP70 Amp’d® ELISA), sensitive to 0.007 ng·mL−1 with a working range of 0.039–5.00 ng·mL−1. The increased sensitivity of this kit is mediated by an alkaline-phosphatase (AP) conjugate binding to a signal amplification substrate, which enhances colour production at lower analyte concentrations. The increased sensitivity therefore affords the potential to determine normative resting eHSP70 values for a range of individuals with a variety of conditions and could allow for a more sensitive determination of stress-induced changes in eHSP70.
Exercise stress can be used as a tool to study the eHSP70 response. Following exercise, both with and without a thermal component, eHSP70 is elevated in the circulation in a duration- and intensity-dependent manner (Whitham and Fortes 2008; Selkirk et al. 2009; Périard et al. 2012a, b; Gibson et al. 2014; Lee et al. 2014b). The magnitude of the postexercise eHSP70 response has been related to a minimum endogenous requirement, which suggests that thresholds of core temperature, rate of core temperature change and parasympathetic/sympathetic drive all play as of yet undetermined roles in the magnitude of this response (Gibson et al. 2014). A better understanding of the criteria required to increase or decrease circulating eHSP70 may provide researchers with a useful biomarker for assessing therapeutic approaches to inflammation-related diseases, as well as improve understanding regarding eHSP70 function following acute exercise, and repeated periods of exercise, adaptation and acclimation to extreme environments.
The aim of this investigation was to compare resting and exercise-induced levels of EDTA plasma eHSP70 in Humans using the EKS-715 high-sensitivity HSP70 ELISA and ENZ-KIT-101 Amp’d® HSP70 high-sensitivity ELISA methods. It was hypothesised that the use of the AP conjugate and signal amplification step would allow a more sensitive determination of basal eHSP70 within different cohorts of participants at rest while also showing greater sensitivity to different levels of stress induced by exercise undertaken in different environmental conditions.
Materials and methods
Participants
Twenty-one recreationally active healthy males provided signed informed consent prior to participation in this study, which was granted approval by the NHS South-West Research Ethics Committee (Reference ID. 14/SW/0098, part A) and Coventry University local ethics committee (part B). All procedures were conducted in accordance with the principles outlined in the Declaration of Helsinki. Data reported in this investigation were collected from two larger experimental trials (Lee et al. 2014a, b). The collected data represents a convenience sample of similarly characterised individuals providing EDTA-treated plasma before and after a 60-min bout of exercise under conditions of environmental stress.
All participants described themselves to be physically active and non-smokers with no prior history of cardiorespiratory illness. Participants were requested to abstain from caffeine (Luo et al. 2008) and alcohol consumption, as well as prolonged thermal exposures (baths, saunas, steam rooms and tanning devices) for 72 h prior to each laboratory visit, which were scheduled at similar times (08:30–09:30) between participants and trials. Participants adhered to an overnight fast prior to each trial and did not eat until after the final blood withdrawal.
Preliminary measurements
Participants in each part of the study were assessed for height, body mass and percentage body fat in accordance with the International Society for the Advancement of Kinathroprometry (ISAK) guidelines (Marfell-Jones et al. 2006).
Participants in part A (n = 6) of the investigation completed a continuous incremental running test to volitional exhaustion on a motorised treadmill (Woodway ELG70, Weiss, Germany). The test protocol was modified from that of Taylor et al. (1955) and was performed in thermoneutral conditions (19.7 ± 0.7 °C, 46.3 ± 4.0 % relative humidity (RH)). After a 5-min warm-up at 6 km·h−1, the test began at a speed of 10 km·h−1 on a 1 % inclination. Speed was then increased by 1 km·h−1 every 3 min until reaching 13 km·h−1, when inclination was increased by 2 % every 2 min. Participants were instructed to run for as long as possible and signal when they felt they could only complete 1 more minute to allow for a final set of recordings. Peak oxygen consumption was determined for participants in part B using an incremental exercise test to volitional exhaustion on calibrated SRM cycle ergometer (n = 15, Table 1) (Schoberer Rad Meßtechnik, Welldorf, Germany). Resting blood lactate (Biosen C-Line analyser, EKF Diagnostics, Germany) was determined from a finger capillary whole blood sample following a 10-min seated rest period. The test began at a workload of 70 W for 4 min and was then increased by 35 W every 4 min until a blood lactate value of >4 mmol·L−1 was reached. Thereafter, workload increased 35 W every 2 min until volitional exhaustion. A cadence of 70 rev·min−1 was maintained throughout. In both part A and part B, expired gases were collected using 200 L Douglas bags (Cranlea & Co, Birmingham, UK) during the final minute of each stage. Heart rate (Polar FT1, Polar Electro OY, Kempele, Finland) and perceived exertion (Borg 1976) were measured at the end of each gas collection. Respiratory gas analysis was completed as previously described (Lee et al. 2014a, b). Peak oxygen consumption was considered to be achieved if two of the following criteria were met: (i) a respiratory exchange ratio of >1.1, (ii) a heart rate greater than 95 % of age predicted maximum (220 − age) and (iii) a final blood lactate value in excess of 8 mmol·mL−1.
Experimental design
Samples for eHSP70 analysis were obtained from two separate experiments which both involved a resting measure of eHSP70 and a measurement collected immediately after a 60-min bout of exercise.
Part A
Samples were obtained before and immediately after a 60-min treadmill run at a speed equivalent of 70 % V̇O2max (HOT70) from six healthy males (mean ± SD; age 20 ± 2 years; height 1.79 ± 0.04 m; body mass 71.8 ± 2.7 kg; % body fat, 11.8 ± 3.3 %; V̇O2max 57.9 ± 9.7 mL·kg−1·min−1). All trials were performed in an environmental chamber that was regulated at a dry bulb temperature of 33.0 ± 0.1 °C and RH of 28.7 ± 0.8 %, with blood samples obtained at rest, upon termination of exercise. Participants (n = 6) returned to the lab on two more occasions each separated by 14 days, to provide two further resting samples, which formed part of a larger experiment. These resting samples were included in the present analysis of resting data.
Part B
Samples were obtained before and immediately after 60 min of cycling at a power output equivalent of 50 % V̇O2max in either hot (HOT50; 40.5 °C, 25.4 RH%) or hypoxic (HYP50; fraction of inspired oxygen (FIO2) of ~0.14, 21 °C, 35 % RH) conditions. The hypoxic environment was generated by an oxygen filtration device (Hypoxico HYP-123 hypoxicator, New York, NY, USA) set to produce the desired FIO2. Participant characteristics for HOT50 (n = 7) were (mean ± SD) as follows: age = 22 ± 5 years; height 1.76 ± 0.05 m; body mass 70.9 ± 5.7 kg; % body fat 13.2 ± 4.0 %, V̇O2max 54.9 ± 3.2 mL·kg−1·min−1. Participant characteristics for HYP50 were as follows: 23.4 ± 4 years; height 1.80 ± 0.08 m; body mass 70.0 ± 9.1 kg; % body fat 12.6 ± 3.7 %; V̇O2max 52.2 ± 3.3 mL·kg−1·min−1.
Participant preparation
Participant preparation and physiological measurements were completed in the same manner and at the same time intervals for both parts A and B. Prior to each visit, participants adhered to an overnight fast (Febbraio et al. 2002) and consumed 500 mL of plain water 1 h before in accordance with the American College of Sports Medicine position stance on hydration (Sawka et al. 2007). Upon arrival, participants began by voiding their bladder to provide a sample for hydration assessment via urine specific gravity (USG; Atago Refractomer, Jencons Pls, Leighton Buzzard, UK) and urine osmolarity (Uosmo; Advanced 3300 Micro-Osmometer, Advanced Inc, Massachusetts, USA). Euhydration was assumed for urine specific gravity values of ≤1.020 g·mL−1 and osmolarity values of ≤700 mOsm·kg−1 (Armstrong et al. 1994). This control was not violated by any participant during any trial. Following this, participants measured their own nude body mass (Seca 880, Seca, Hamburg, Germany), inserted a calibrated rectal thermistor probe (Grant Squirrel 2020, Grant Instruments, Shepreth, UK) to a depth of 10 cm and fitted a telemetric heart rate monitor around their chest (Polar FT1, Polar Electro OY, Kempele, Finland). An indwelling cannula (BD Insyte-W, Becton Dickinson, UT, USA) was then inserted 2.5 cm into an antecubital vein of the participants’ left arm. After a 20-min stabilisation period with the participant lying supine, a baseline 10-mL blood sample was then drawn, with patency of the cannula being maintained with saline (0.9 % sodium chloride, Braun, Melsungen, Germany).
Physiological measurements
Participants entered the regulated environmental chamber at 09:30. The exercise bout began with a standardised 5-min warm-up, running on a motorised treadmill at a speed calculated to elicit a work rate of 50 % V̇O2max on a fixed 1 % inclination (Jones and Doust 1996) in part A or a 15-min seated wash-in for the hypoxic gas in group B. Upon completion of the warm-up/wash-in period, participants in part A began a 60-min run at a work rate of 70 % V̇O2max, and participants in part B began 60 min of cycling exercise at 50 % V̇O2max in the prescribed environmental conditions (HOT50 or HYP50).
During exercise, heart rate (HR), rectal temperature (T rectal), ratings of perceived exertion (RPE) and thermal sensation (ISO, 1995, part A, part B) were all recorded at 10-min intervals. The physiological strain index (PSI) was subsequently calculated at each time point using heart rate and rectal temperature data as described by Moran et al. (1998). The T rectal area under the curve was calculated using a modification of the trapezium rule (Hubbard et al. 1977) when T rectal exceeded 38.5 °C (Cheuvront et al. 2008) and 39.0 °C. A T rectal of 38.5 °C was selected as a possible threshold for eHSP70 appearance (Gibson et al. 2014). In instances where participants did not complete the full 60-min run/cycle, termination time was recorded and all aforementioned measures taken in the final minute before cessation.
Determination of extracellular HSP70
Circulating eHSP70 was assessed using two commercially available ELISAs, EKS-715 high-sensitivity HSP70 kit (http://static.enzolifesciences.com/fileadmin/files/manual/ADI-EKS-715_insert.pdf; hereafter referred to as EKS-715) and ENZ-KIT-101-001 Amp’d® HSP70 high-sensitivity ELISA kit (http://static.enzolifesciences.com/fileadmin/files/manual/ENZ-KIT-101_insert.pdf, hereafter referred to as ENZ-KIT) according to the manufacturer’s instructions (Enzo Life Sciences, Lausen, Switzerland).
The ENZ-KIT is designed to replace traditional alkaline phosphatase substrates, such as p-nitrophenyl phosphate (pNPP), with a combination substrate and amplifier system that results in greater sensitivity when compared to a classic substrate ELISA. In the ENZ-KIT, bound AP converts a substrate that is utilised in a second enzyme reaction system which is initiated by addition of the amplifier reagent. Figure 1 shows typical standard curves (n = 4) prepared on the same 96-well plate (HSP70 Clear Mirotiter plate, catalogue number: 80-1581), using the same HSP70 high-sensitivity standard (Cat no: 80-1776). HRP conjugate (Cat no: 80-1778) was added to HSP70 high-sensitivity antibody (Cat no: 80-1777) and a TMB substrate (Cat no: 80-0350) used to develop the EKS-715. For ENZ-KIT, an AP conjugate (Cat no: 80-2600) was added to the HSP70 high-sensitivity antibody (Cat no: 80-1777) and incubated with signal amplification substrate (Cat no: 80-2596) containing NADPH prior to a final amplification step (Cat no: 80-2598). The amplification step allows for greater (amplified) colour production at lower analyte concentrations resulting in an increased assay sensitivity (Fig. 1).
Standard curves (mean ± SD; n = 4) generated in duplicate from the same pre-prepared standards analysed on the same 96-well plate. The amplification steps produce a clear increase in the assay sensitivity when compared to the EKS-715 kit reagents allowing for determination of low levels of eHSP70 in plasma samples
The EKS-715 kit has a sensitivity of 0.090 ng·mL−1 and a working range of 0.20 to 12.5 ng·mL−1. The ENZ-KIT assay is sensitive to 0.007 ng·mL−1 with a working range of 0.039–5.00 ng·mL−1. Following an initial analysis of samples (one ENZ-KIT assay), it became apparent that the minimum recommended dilution of 1:4 was not sufficient in all cases, with some samples containing more HSP70 than the top standard. Thus, a further analysis using 1:4, 1:8 and 1:16 dilution steps with assay diluent (sodium carbonate) was necessary to determine the optimal dilution for each sample, with results multiplied by the this dilution factor in order to give eHSP70 values in nanograms per millilitre. Once the optimal sample dilution was determined for each participant on the ENZ-KIT, pre- and postexercise samples were analysed in duplicate.
Statistical analysis
A total of 32 resting blood samples from 21 individuals (11 repeat samples) were analysed for basal eHSP70. The between-assay coefficient of variation was determined using standard concentration curves of four separate kits run using both EKS-715 and ENZ-KIT. The reliability of the ENZ-KIT assay was further assessed by comparing resting data obtained during a serial dilution test to the data obtained from a further assay on these samples measuring both pre- and postexercise data. Where each ELISA method provided eHSP70 for paired samples, or samples assayed on separate occasions, Pearson correlations determined the relationship between each measurement.
Mean and peak physiological, thermoregulatory and eHSP70 responses were analysed between groups using a one-way analysis of variance (ANOVA) and Tukey’s honestly different test to explore main effects. All data analyses were performed using PASW software version 20.0 for Mac (SPSS, Chicago, IL, USA).
Stepwise multiple regression analysis was performed using the three dependent variables (time spent above T rectal 38.5 °C, rate of change in T rectal and area under the curve (AUC) for Trectal 38.5 °C) that were significantly correlated to postexercise eHSP70 concentrations. The significance level was set at p < 0.05 for all analysis. Data are reported as means ± SD unless otherwise stated and individual data shown where possible.
Results
pNPP conjugate (EKS-715) compared with AP conjugate and amplifier substrate (ENZ-KIT)
Figure 1 illustrates a typical standard curve when the pNPP conjugate and TMB substrate is used (closed circles) in comparison to the increased sensitivity obtained from the AP conjugate and amplifier solution (open circles). The intra-assay precision, obtained by determining the coefficient of variation between duplicate samples obtained from the standard curves on four separate plates, was 2.6 and 4.1 % for EKS-715 and ENZ-KIT, respectively. These data are lower than the manufacturer-reported intra-assay precision of between 3.9 and 11.4 % for EKS-715, and 7 and 15 % for ENZ-KIT. Inter-assay precision was also determined from the standard curves of four separate assays performed on four separate occasions and was 4.9 and 6.2 % for EKS-715 and ENZ-KIT, respectively. These values were also lower than manufacturer-reported inter-assay variation (EKS-715 = 12.8–19.1 %; ENZ-KIT = 7.7–9.7 %).
Extracellular HSP70 at rest
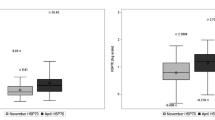
The ENZ-KIT was able to detect basal eHSP70 in all 32 resting samples (Fig. 2, 1.54 ± 3.27 ng·mL−1). In contrast, the EKS-715 assay did not detect eHSP70 in 26 out of 32 resting samples analysed (81 %). When results were available from both kits (n = 6), there was a good correlation between values (r = 0.86, p = 0.0004, Fig. 2), with values not significantly different between kits (t = 0.35, p = 0.72). In 15 of the samples measured with ENZ-KIT (47 %), eHSP70 was below the 0.20 ng·mL−1 limit of EKS-715 standard curve (0.15 ± 0.04 ng·mL−1; 95 % CI = 0.13 to 0.17 ng·mL−1).
a, b The optical density and eHSP70 values obtained from each assay. EKS-715 was able to detect eHSP70 in 6 of the 32 resting observations (3.57 ± 2.68 ng·mL−1), whereas ENZ-KIT measured eHSP70 in all 32 resting observations (1.54 ± 3.19 ng·mL−1). When data for an individual was available from both assays (n = 12, c, d) the ENZ-KIT tended to indicate lower values, though this was not statistically significant (p = 0.501; R 2 = 0.73). Between test reliability for samples assayed on two different occasions was high (e), with the between test CV 7.86 % and a correlation coefficient of 0.99 (f)
A minimum dilution of 1:4 (sample to assay diluent) is recommended to remove matrix interference during the ENZ-KIT assay. In the present investigation, we found the 1:4 dilution allowed for determination of basal HSP70 in 26/32 samples studied. For samples with resting concentrations of eHSP70 above the top standard concentration (5.00 ng·mL−1), 1:8 (n = 3) and 1:16 (n = 3) dilutions were required to locate data on the standard curve. No participants exhibited eHSP70 values below the detection limit of 0.039 ng·mL−1 using the ENZ-KIT.
A further determination of ENZ-KIT assay reliability was made by comparing resting eHSP70 data obtained from the serial dilution plate to the resting data obtained during the test run on a separate plate (part A: n = 6, part B: n = 15; r = 0.998, p < 0.001, Fig. 2e, f), indicating good inter-assay reliability (CV = 7.9 %).
Physiological and thermoregulatory responses to each stressor
The duration of exercise undertaken at 70 % V̇O2max (i.e. HOT70 54.0 ± 9.4 min) was shorter than the duration of exercise undertaken at 50 % V̇O2max (i.e. HOT50, HYP50, 60 ± 0.0 min; F (2, 18) = 4.25, p = 0.032). Physiological and thermoregulatory data are shown in Table 1.
Although some thermoregulatory responses (peak, delta and rate of T rectal change) were greater in HOT50 compared to HYP50 (Table 1), no other differences in physiological response (e.g. mean and peak HR and PSI) were observed between these conditions. In contrast, greater mean and peak exercising HR and T rectal responses were observed in HOT70 compared to HOT50 and HYP50. Similarly, the delta change in T rectal, the rate of T rectal change, the duration of the exercise bout spent above both 38.5 and 39.0 °C and AUC for these temperatures were all greater in HOT70 compared to HOT50 and HYP50 (p < 0.05; Table 1). The data therefore indicate two different levels of physiological strain achieved (HOT70 versus HOT50 and HYP50).
The exercise-induced eHSP70 response
In accordance with the resting data, EKS-715 only detected postexercise eHSP70 in the six samples that had detectable eHSP70 at rest, with five samples obtained in HYP50 and one sample from the HOT50. Postexercise eHSP70 obtained from the EKS-715 kit (n = 6, 3.92 ± 4.34 ng·mL−1) had a good relationship to those obtained with the ENZ-KIT (n = 6, 3.37 ± 5.38 ng·mL−1, r = 0.84).
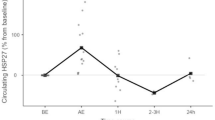
There was a significant group × time interaction (F (2, 17) = 4.235, p = 0.03) for eHSP70 when analysed using ENZ-KIT. Resting HSP70 was higher in HOT70 than HOT50 or HYP50 group (p < 0.05; Fig. 3).
Exercise results in an increase in eHSP70 are from 2.79 ± 2.59 ng·mL−1 (95 % CI = 0.074 to 5.51 ng·mL−1) at rest to 3.51 ± 2.90 ng·mL−1 (0.47 to 6.56 ng·mL−1) in the HOT70 group (t = 3.82, p = 0.012). However, with the HOT50 trial, eHSP70 was unchanged from 0.22 ± 0.13 ng·mL−1 (95 % CI = 0.084 to 0.362 ng·mL−1) at rest to 0.20 ± 0.16 ng·mL−1 (95 % CI = 0.034 to 0.370 ng·mL−1) following exercise (t = 0.886 p = 0.410). In addition, in the HYP50 trial, eHSP70 was unchanged from 2.82 ± 5.51 ng·mL−1 (95 % CI = 2.96 to 8.60 ng·mL−1) at rest to 2.85 ± 5.56 ng·mL−1 (95 % CI = 2.98 to 8.69 ng·mL−1) following exercise (t = 0.635, p = 0.545). Thus, only HOT70 induced changes of eHSP70 above resting values (Fig. 3).
Relationship between eHSP70 and thermo-physiological measures
Time spent above 38.5 °C (r = 0.54), rate of change in T rectal (r = 0.52) and AUC for T rectal 38.5 °C (r = 0.47) were entered into a stepwise multiple regression analysis to assess the association of these variables in postexercise eHSP70 concentration. The only predictor variable was the duration of exercise above 38.5 °C. The adjusted R 2 for this model was 0.26 with a large standard error of 55.1.
Discussion
The aim of this study was to compare two commercially available high-sensitivity ELISAs for the determination of eHSP70 in plasma. The results illustrate that the ENZ-KIT (Enzo Life Sciences, Lausen, Switzerland) is more sensitive than the EKS-715 (Enzo Life Sciences, Lausen, Switzerland) when quantifying both resting and postexercise eHSP70 values in a sample of healthy, moderately trained males. The increased sensitivity and lower working range, facilitated by the use of amplifier reagents, significantly improves the ability of the ENZ-KIT assay to detect resting eHSP70 in plasma thereby supporting our hypothesis.
In the present investigation, only 6 of the 21 samples analysed using the EKS-715 kit allowed for the quantification of eHSP70 at rest and after exercise, whereas ENZ-KIT provided data for all resting and all postexercise samples (Fig. 2b). The increased sensitivity of ENZ-KIT introduces the potential requirement for serial dilution of samples to ensure results are not above the standard curve, thereby reducing the reliance on extrapolation. In the current analysis, the manufacturer’s recommended minimum sample dilution of 1:4 was sufficient to detect eHSP70 in 26/32 samples. Additional dilutions of 1:8 (n = 3) and 1:16 (n = 3) were necessary in the instances when data fell above the standard curve. It may be prudent for researchers using the ENZ-KIT to conduct 1:4 and 1:8 serial dilutions of all resting samples prior to full analysis to ensure that all samples can be analysed together, potentially saving both time and the additional cost of running more assays. The ENZ-KIT demonstrated excellent reproducibility between individual assay kits, with duplicate measurements of resting values highly correlated between assays (R 2 = 0.99, Fig. 2f).
Heat shock proteins play an important role in maintaining cellular protein homeostasis, with HSP dysfunction implicated in the pathology of Alzheimer’s disease, Parkinson’s disease, cardiovascular disease and sarcopenia (Krause et al. 2015). It is therefore surprising that there is currently no substantial normative data regarding resting eHSP70 for either healthy individuals or clinical cohorts. Indeed, a characteristic of eHSP70 research is the large variability both between studies (Ruell et al. 2014; Whitham and Fortes 2006; Gibson et al. 2014; Lee et al. 2014b) and within studies, likely exacerbated by small sample sizes typically used in exercise studies (Gibson et al. 2014; Lee et al. 2014b). Between-participant variation is evident in the present investigation, in which three groups of seemingly physiologically matched males present significantly different eHSP70 values at rest (Fig. 3). Such a large disparity in resting data will limit the ability to detect changes in eHSP70 concentrations between two or more matched groups. When a repeated measures design is not feasible, it may be appropriate for experimenters to match individuals based on resting eHSP70 concentrations rather than more common physiological and anthropometric features (providing that eHSP70 is an important outcome). Doing so may facilitate a clearer understanding regarding responders, such as the participant with high post HOT50 eHSP70 concentration (Fig. 3b; rest = 0.67 ng·mL−1, postexercise = 2.29 ng·mL−1) and non-responders to thermal or hypoxic stress.
The reasons for the observed disparity between similarly matched groups are unclear and could not be determined in the present work. In order to elucidate the role this biochemical marker plays in health and disease, future studies should aim to thoroughly characterise an individual’s lifestyle factors, normal weekly physical activity levels and anthropometric and physiological characteristics. The increased sensitivity of the ENZ-KIT compared to other commercially available kits allow for a sensitive quantification of resting eHSP70 across a wide range of populations. Only once these data have been collected in a sufficiently large sample can eHSP70 be investigated as a potential biomarker of, for example, sarcopenia (Ogawa et al. 2012). Some studies have attempted to determine the efficacy of eHSP70 in clinical scenarios using the EKS-715 assay. Based on the present work, it is likely that prior results have been influenced by sensitivity. For example, Ogawa et al. (2012) conducted a detailed study in which 652 elderly Japanese males and females were screened for a range of biochemical and physiological markers related to sarcopenia, such as TNF-α, interleukin-6 (IL-6) and C-reactive protein (CRP). However, the majority of the eHSP70 data reported (436/652) was near to (n = 207; 0.13–0.22 ng·mL−1) or below (n = 229 < 0.13 ng·mL−1) the EKS-715 assay standard curve (0.20 ng·mL−1). Thus, although these results are important, the present work highlights that the data should be interpreted with caution. The data in the present investigation has demonstrated that, even where eHSP70 values are theoretically within the measurable range of the EKS-715 assay (e.g. concentrations of over 0.20 ng·mL−1), they are not always detectable by EKS-715, but were all detected with ENZ-KIT (Fig. 2). Thus, when resting eHSP70 concentrations are required for clinical observations or for studying the role of eHSP70 in health and disease, the use of the ENZ-KIT is recommended above EKS-715.
Exercise is known to increase concentrations of eHSP70 in an intensity- and duration-dependent manner, with a 60-min run at 75 % V̇O2max leading to a 175 % increase in eHSP70, compared to a 140 % increase when running for 120 min at 60 % V̇O2max (Fehrenbach et al. 2005). The sum exercise stress of cycling is less that of running due to its low impact and less muscle-damaging nature; thus, cycling-induced elevations in eHSP70 concentration are much lower than those observed following running (Febbraio et al. 2002; Febbraio et al. 2004; Lancaster et al. 2004). Postexercise increases in eHSP70 have been hypothesised to relate to a minimum endogenous level of thermal strain, corresponding to a T rectal of >38.5 °C (Amorim et al. 2008; Gibson et al. 2014). Both the rate of Trectal increase and change in T rectal are also thought to be important factors affecting eHSP70 concentrations after a stressor (Périard et al. 2012a, b; Gibson et al. 2014).
In addition to the ability of the ENZ-KIT to quantify resting eHSP70, we therefore examined the postexercise response at three levels of physiological strain using two distinctly different modes (running and cycling) of hyperthermic exercise as well as hypoxic stress (Table 1). The data presented in Table 1 indicate two levels of physiological strain were achieved, with HOT70 eliciting significantly greater mean and peak HR, T rectal and PSI compared to HOT50 and HYP50. No significant differences between the HOT50 and HYP50 groups were observed, agreeing with previous work showing exercise at 50 % V̇O2max in either 40 °C heat or an FIO2 of 0.14 to be of a comparable physiological stress up to 40 min of exercise (Lee et al. 2014a, b). The 60-min duration of exercise used herein was therefore insufficient for significant differences in physiological strain between HOT50 and HYP50 to become apparent (Girard and Racinais 2014; Lee et al. 2014a, b).
As with the resting data, the EKS-715 kit only detected eHSP70 in the six samples that also provided resting data (n = 5 from HYP50 and n = 1 from HOT50). In contrast, a 52 % increase (pre 2.79 ± 2.58; post 3.51 ± 2.90 ng·mL−1) in eHSP70 was observed immediately after HOT70 when samples were analysed with ENZ-KIT. This is comparable with other studies using a similar level of external heat stress at a lower exercise intensity (e.g. Périard et al. 2012a, b) but for a longer duration (90 min, Gibson et al. 2014), and further illustrates the utility of the ENZ-KIT over EKS-715.
The higher postexercise eHSP70 concentrations reported in the present investigation were observed despite the shorter duration of exposure (54.0 ± 9.3 min compared to 90.0 ± 0.0 min in Gibson et al. 2014) and are likely due to the increased exercise intensity (70 vs. 50 % V̇O2max in Gibson et al. 2014), and different exercise modes (running vs. cycling) used between investigations. Additionally, thermoregulatory stress, evidenced by the AUC for a T rectal of >38.5 °C (9.21 ± 1.95 °C·min−1), the duration spent above 38.5 °C (17.5 ± 10.4 min) and duration spent above 39 °C (8.9 ± 1.6 min), mean and peak T rectal, change in T rectal and rate of change in HOT70 were all higher than those reported after cycling exercise at 50 % V̇O2max for 90 min at 30.2 °C, 51 % RH (Gibson et al. 2014). Thus, the total level of endogenous strain was greater in the present investigation and is reflected in the eHSP70 results.
In contrast, no postexercise increase was observed following HOT50 or HYP50 (Fig. 3). It is likely that while the thermal component of HOT50 was sufficient for increased eHSP70, the duration of exercise, and therefore overall level of exogenous strain, was not sufficient to increase eHSP70. Of the suggested endogenous requirement for postexercise increases in eHSP70 (peak T rectal of >39.2 °C, a mean T rectal of 38.6 °C for a period of ~57 min, a core temperature change of 2.2 °C from baseline at a rate of 1.6 °C·h−1 and a mean heart rate of 153 beats·min−1), the HOT50 trial only achieved the required heart rate, and the HYP50 group failed to reach any of these potential eHSP70 inducing thresholds. Our data therefore lend support to the notion of a minimum endogenous criteria required for eHSP70 induction (Amorim et al. 2008; Gibson et al. 2014). The increased sensitivity afforded by the ENZ-KIT may allow for a more detailed and nuanced description of minimum eHSP70 inducing criteria during and after exercise stress in future studies.
In summary, this investigation presents preliminary data showing the effectiveness of the ENZ-KIT assay for detecting and quantifying resting eHSP70 at the low end of the measurable range in young healthy males. Secondly, our results support the notion that a minimum endogenous strain threshold needs surpassing in order to increase systemic HSP70. As a result, it is recommended that all future investigations requiring accurate resting eHSP70 quantification use the ENZ-KIT assay in place of EKS-715. The increased sensitivity afforded by this assay could provide a more in-depth understanding of normal and abnormal levels of systemic eHSP70 and provide novel information regarding the use of eHSP70 as a biomarker of disease.
AUC area under the curve, AP alkaline phosphatase, ENZ-KIT-101 Amp’d® HSP70 high-sensitivity ELISA kit, eHSP70 extracellular heat shock protein 70, EKS-715 HSP70 high-sensitivity ELISA kit, ELISA enzyme-linked immunosorbent assay, F I O 2 fraction of inspired oxygen, HEAT70 heat running trial, HEAT50 heat cycling trial, HSP70 heat shock protein 70, HR heart rate, HYP hypoxic trial, IL-10 interleukin-10, kDa kilodalton, pNPP p-nitrophenyl phosphate, PSI physiological strain index, RPE rating of perceived exertion, T rectal rectal temperature, RH relative humidity, SpO 2 arterial oxygen saturation, TS thermal sensation, TNF-α tumour necrosis factor alpha, V̇O 2max maximal oxygen consumption
References
Amorim FT, Yamada PM, Robergs RA et al (2008) The effect of the rate of heat storage on serum heatshock protein 72 in humans. Eur J Appl Physiol 104:965–972
Armstrong LE, Maresh CM, Castellani JW, Bergeron MF, Kenefick RW, LaGasse KE, Riebe D (1994) Urinary indices of hydration status. Int J Sport Nutr 4(3):265–279
Asea A (2006) Initiation of the immune response by extracellular Hsp72: chaperokine activity of Hsp72. Curr Immunol Rev 2:209
Borg G (1976) Simple rating methods for estimation of perceived exertion. Phys Work Effort 39–46
Campisi J, Leem TH, Fleshner M (2003) Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system. Cell Stress Chaperones 8:272
Cheuvront SN, Chinevere TD, Ely BR et al. (2008) Serum S-100 beta response to exercise-heat strain before and after acclimation. In:DTIC Document
Chung J, Nguyen A-K, Henstridge DC et al (2008) HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci 105:1739–1744
Dulin E, García-Barreno P, Guisasola MC (2010) Extracellular heat shock protein 70 (HSPA1A) and classical vascular risk factors in a general population. Cell Stress Chaperones 15:929–937
Febbraio MA, Steensberg A, Walsh R et al (2002) Reduced glycogen availability is associated with an elevation in HSP72 in contracting human skeletal muscle. J Physiol 538:911–917
Febbraio MA, Mesa JL, Chung J et al (2004) Glucose ingestion attenuates the exercise-induced increase in circulating heat shock protein 72 and heat shock protein 60 in humans. Cell Stress Chaperones 9:390
Fehrenbach E, Niess A, Voelker K et al (2005) Exercise intensity and duration affect blood soluble HSP72. Int J Sports Med 26:552–557
Fink AL (1999) Chaperone-mediated protein folding. Physiol Rev 79:425–449
Garrido C, Gurbuxani S, Ravagnan L et al (2001) Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun 286:433–442
Gibson OR, Dennis A, Parfitt T et al (2014) Extracellular Hsp72 concentration relates to a minimum endogenous criteria during acute exercise-heat exposure. Cell Stress Chaperones 19:389–400
Girard O, Racinais S (2014) Combining heat stress and moderate hypoxia reduces cycling time to exhaustion without modifying neuromuscular fatigue characteristics. Eur J Appl Physiol 114:1521–1532
Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381:571–579
Horowitz M (2007) Heat acclimation and cross-tolerance against novel stressors: genomic–physiological linkage. Prog Brain Res 162:373–392
Hubbard R, Bowers W, Matthew W et al (1977) Rat model of acute heatstroke mortality. J Appl Physiol 42:809–816
Ianaro A, Ialenti A, Maffia P et al (2001) HSF1/hsp72 pathway as an endogenous anti-inflammatory system. FEBS Lett 499:239–244
Jones AM, Doust JH (1996) A 1% treadmill grade most accurately reflects the energetic cost of outdoor running. J Sports Sci 14:321–327
Kampinga HH, Hageman J, Vos MJ et al (2009) Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 14:105–111
Krause M, Heck TG, Bittencourt A et al. (2015) The chaperone balance hypothesis: the importance of the extracellular to intracellular HSP70 ratio to inflammation-driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediat Inflamm 2015
Kregel KC (2002) Invited review: heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 92:2177–2186
Lancaster G, Møller K, Nielsen B et al (2004) Exercise induces the release of heat shock protein 72 from the human brain in vivo. Cell Stress Chaperones 9:276
Lee BJ, Emery-Sinclair EL, Mackenzie RW et al (2014a) The impact of submaximal exercise during heat and/or hypoxia on the cardiovascular and monocyte HSP72 responses to subsequent (post 24 h) exercise in hypoxia. Extreme Physiol Med 3:15
Lee BJ, Mackenzie RW, Cox V et al. (2014b) Human monocyte heat shock protein 72 responses to acute hypoxic exercise after 3 days of exercise heat acclimation. BioMed Research International, Article ID 849809
Luo X, Zuo X, Zhou Y et al (2008) Extracellular heat shock protein 70 inhibits tumour necrosis factor-alpha induced proinflammatory mediator production in fibroblast-like synoviocytes. Arthritis Res Ther 10:R41
Marfell-Jones M, Olds T, Stewart A et al. (2006) ISAK: Potchefstroom: International Standards for Anthropometric Assessment
Moran DS, Shitzer A, Pandolf KB (1998) A physiological strain index to evaluate heat stress. Am J Physiol-Regul Integr Comp Physiol 275:R129–R134
Moseley PL (1997) Heat shock proteins and heat adaptation of the whole organism. J Appl Physiol 83:1413–1417
Najafizadeh SR, Ghazizadeh Z, Nargesi AA et al. (2015) Analysis of serum heat shock protein 70 (HSPA1A) concentrations for diagnosis and disease activity monitoring in patients with rheumatoid arthritis. Cell Stress Chaperones:1–7
Ogawa K, Kim H-K, Shimizu T et al (2012) Plasma heat shock protein 72 as a biomarker of sarcopenia in elderly people. Cell Stress Chaperones 17:349–359
Ortega E, Giraldo E, Hinchado M et al (2006) Role of Hsp72 and norepinephrine in the moderate exercise-induced stimulation of neutrophils’ microbicide capacity. Eur J Appl Physiol 98:250–255
Ortega E, Hinchado M, Martin-Cordero L et al (2009) The effect of stress-inducible extracellular Hsp72 on human neutrophil chemotaxis: a role during acute intense exercise. Stress 12:240–249
Périard J, Patricia R, Caillaud C et al. (2012a) Plasma Hsp72 and Hsp27 during moderate and intense exercise to exhaustion in the heat. In: Proceedings of The Physiological Society. The Physiological Society
Périard JD, Ruell P, Caillaud C et al (2012b) Plasma Hsp72 (HSPA1A) and Hsp27 (HSPB1) expression under heat stress: influence of exercise intensity. Cell Stress Chaperones 17:375–383
Pockley A, Shepherd J, Corton J (1998) Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Investig 27:367–377
Rodrigues-Krause J, Krause M, O’hagan C et al (2012) Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperones 17:293–302
Ruell PA, Simar D, Périard JD et al (2014) Plasma and lymphocyte Hsp72 responses to exercise in athletes with prior exertional heat illness. Amino Acids 46:1491–1499
Ruell PA, Thompson MW, Hoffman KM et al (2006) Plasma Hsp72 is higher in runners with more serious symptoms of exertional heat illness. Eur J Appl Physiol 97:732–736
Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NSl (2007) American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports 39(2):377–390
Schick C, Arbogast M, Lowka K et al (2004) Continuous enhanced expression of Hsc70 but not Hsp70 in rheumatoid arthritis synovial tissue. Arthritis Rheum 50:88–93
Selkirk GA, Mclellan TM, Wright HE et al (2009) Expression of intracellular cytokines, HSP72, and apoptosis in monocyte subsets during exertional heat stress in trained and untrained individuals. Am J Physiol-Regul Integr Comp Physiol 296:R575–R586
Taylor HL, Buskirk E, and Henschel A (1955) Maximal oxygen intake as an objective measure of cardio-respiratory performance. J Appl Physiol 8:73–80
Whitham M, Fortes M (2006) Effect of blood handling on extracellular Hsp72 concentration after high-intensity exercise in humans. Cell Stress Chaperones 11:304
Whitham M, Fortes MB (2008) Heat shock protein 72: release and biological significance during exercise. Front Biosci 13:1328–1339
Acknowledgments
The authors would like to thank Morgan Mathieu, Ph.D., for his technical advice during the optimization steps of the ELISA assays. We would also like to thank all the volunteers for their time during the study.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, B.J., Sukri, N.M., Ogden, H. et al. A comparison of two commercially available ELISA methods for the quantification of human plasma heat shock protein 70 during rest and exercise stress. Cell Stress and Chaperones 20, 917–926 (2015). https://doi.org/10.1007/s12192-015-0610-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-015-0610-3