Abstract
Post-transplantation therapy is commonly performed in patients with myeloma and can prolong progression-free survival (PFS). However, whether post-transplantation therapy contributes to achieving and continuing MRD-negativity remains controversial. This retrospective analysis aimed to evaluate the clinical impact of post-transplantation therapy, including tandem autologous stem cell transplantation (ASCT), in myeloma patients. The subjects were 79 patients (median age: 62 years) who received induction therapy, including bortezomib and/or lenalidomide, of whom 58 underwent post-transplantation therapy. At the median follow-up time of 50 months, the 4-year PFS rate was significantly higher in patients who underwent post-transplantation therapy than those who did not (60.6% vs. 28.6%, P = 0.012). Multivariate analysis revealed post-transplantation therapy to be a significant prognostic factor for long PFS. Tandem ASCT followed by consolidation and/or maintenance therapies improved PFS and OS. The minimal residual disease (MRD)-negative rate was significantly higher in patients who underwent post-transplantation therapy than those who did not (50.9% vs. 16.7%, P = 0.006). Post-transplantation therapy contributed to sustained MRD-negativity, which predicted long PFS and overall survival. Patients frequently discontinued post-transplantation therapy due to adverse events within 4 months. In conclusion, post-transplantation therapy improved PFS and contributed to sustained MRD-negativity in myeloma patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the development of novel agents has improved its prognosis over the last decade, multiple myeloma (MM) remains incurable [1]. Notably, upfront autologous hematopoietic stem cell transplantation (ASCT) is the standard treatment for young patients with myeloma [2,3,4,5,6]. Furthermore, post-transplant therapy has contributed to improving clinical outcomes [3, 4, 7,8,9,10] and is recommended in several guidelines for hematopoietic oncology practice [11,12,13]; specifically, it can be divided into consolidation and maintenance therapies. The former is a short-term chemotherapy regimen with a similar intensity to induction therapy and is designed to intensify the therapeutic effect after transplantation, while the latter aims to prolong the response to long-term therapy, is less intense than the pre-treatment induction regimen, and is administered as a single- or dual-drug therapy after ASCT [14, 15]. Additionally, tandem ASCT can be included in a post-transplantation therapy and improve clinical outcomes [16,17,18,19]. The efficacy and safety of post-transplant treatments, comprising lenalidomide (LEN) maintenance therapy [7], ixazomib (IXA) maintenance therapy [8], and bortezomib, LEN, plus dexamethasone (VRd) consolidation therapy [4], have been reported in large-scale clinical trials. Furthermore, MRD negativity could predict extended survival in the patients who received ASCT, according to a meta-analysis [20]; however, it remains controversial whether post-transplantation therapy contributed to achieving and continuing MRD-negativity [21,22,23]. Additionally, several patients discontinued post-transplantation therapy according to clinical trials and real-world evidence [24, 25], but it was not described clearly when the post-transplantation therapy was discontinued.
This retrospective study aimed to evaluate the efficacy and safety of post-autologous-transplantation therapy in patients with transplantation-eligible newly diagnosed MM (NDMM).
Materials and methods
We reviewed the medical records of NDMM patients who underwent upfront ASCT at the Jikei University Hospital and Jikei University Kashiwa Hospital between January 2012 and December 2020 and were followed up until October 2022. This study was approved by the independent ethics committee and the institutional review board of our institution (34-012(11,157)).
Patients
Patients aged ≥ 20 years, diagnosed with symptomatic MM, and who received high-dose melphalan followed by ASCT were included. Furthermore, patients with monoclonal gammopathy of undetermined significance and smoldering MM were excluded from the study. High-risk cytogenetic abnormality (HRCA) was defined as t(4;14), t(14;16), del17p, or 1q21 gain by fluorescence in situ hybridization [26]. Post-transplantation therapy was defined as all subsequent chemotherapy following a single ASCT, such as tandem ASCT, consolidation, and maintenance therapy.
Treatment strategy
Concrete treatment was chosen by the physicians’ choice according to the treatment strategy as follows. VRd was performed as induction therapy after 2017, while bortezomib plus dexamethasone therapy was performed as induction therapy before 2016. Tandem ASCT was conducted independently from cytogenetic risk in the Jikei University Kashiwa hospital and for the patients with HRCA in the Jikei University hospital when an adequate count of PBSC was harvested. We did not change from single to tandem ASCT depending on the treatment response after the first ASCT. Consolidation and maintenance therapy was performed if the patients were not willing to omit these therapies. LEN maintenance therapy was done before 2018, whereas 8 cycles of IXA, LEN plus dexamethasone (IRd) consolidation therapy followed by IXA maintenance therapy after 2019. IXA maintenance therapy was continued for 2 years in patients without HRCA and until progressive disease in those with HRCA, independently from the treatment response after post-transplantation therapy.
Response assessment
Disease response was assessed according to the International Myeloma Working Group criteria [27]. The MRD status was analyzed using multicolor flow cytometry (SRL Inc., Tokyo, Japan) for the patients whose monoclonal protein band was not observed by two consecutive immunofixation electrophoresis (IFE) [28]. The cutoff value for MRD negativity was 1 × 10−5. MRD assessment was repeated once a year, generally when IFE status remained negative. Sustained MRD-negativity was defined as two or more consecutive MRD-negativity.
Statistical analysis
The primary endpoint of this study was the comparison of the progression-free survival (PFS) of patients with and without post-transplantation therapy. PFS was calculated from the date of the first ASCT to that of disease progression or death from any cause. The secondary endpoints were the overall survival (OS), treatment response, MRD status, and the cumulative incidence of post-transplantation therapy discontinuation due to adverse events. The OS was calculated from the first ASCT to the date of death due to any causes. The clinical outcome between the patients treated with and without post-transplantation therapy was analyzed by performing a matched-pair analysis using the Mantel–Haenszel test to match data from the two groups owing to significant differences in patient characteristics. Furthermore, the predictive value of post-transplantation therapy for MRD negativity and sustained MRD negativity were investigated. Actuarial survival analysis was performed using the Kaplan–Meier method, and the resulting curves were compared using the log-rank test. All prognostic variables were analyzed for survival and predictive factors for MRD status using Cox regression analysis and multiple logistic regression analysis, respectively. All reported P-values were two-sided, and P-values < 0.05 were considered statistically significant. Statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R (The R Foundation for Statistical Computing) [29]; specifically, it is a modified version of R Commander that incorporates frequently used biostatistical functions.
Results
Patient characteristics
Seventy-nine patients with a median age of 62 years (range, 45–71 years) at ASCT were included in this study. Forty-two patients received VRd as an induction treatment. The number of patients with and without HRCA was 23 and 29, respectively. Furthermore, 27 and 13 patients had no cytogenetic risk data available and achieved a complete response (CR) before ASCT, respectively; 58 patients received post-transplantation therapy, including 37 tandem ASCT, 37 consolidation, and 44 maintenance therapies. Consolidation therapy was as follows: IXA, LEN, plus dexamethasone (DEX) (IRd), 26 patients; elotuzumab and LEN plus DEX (Rd), 4; VRd, 3; Rd, 3; daratumumab plus Rd as consolidation therapy, 1. Maintenance therapy was as follows: LEN monotherapy, 23; and IXA monotherapy as maintenance therapy, 21. The percentage of patients who received VRd as induction therapy was significantly higher than that in the non-post-transplantation group (65.5% vs. 23.5%, P < 0.001). No significant difference was reported in other characteristics between patients treated with and without post-transplantation therapy. A summary is shown in Table 1.
Survival time
The median follow-up period for survival was 50.0 months (6.1–121.9 months). The median PFS and OS were 48.6 months and not reached; the 4-year PFS and OS rates were 50.1 and 83.3%, respectively. The 4-year PFS rate in the post-transplantation therapy group was significantly higher than that in the non-post-transplantation therapy group (60.6 and 28.6%, respectively; hazard ratio (HR) 0.455, 95% confidence interval (CI) 0.243–0.855, P = 0.012; Fig. 1a), although there was no significant difference in the 4-year OS rates between patients with and without post-transplantation therapy (84.6% and 80.7%, respectively; HR 0.941, 95% CI 0.361–2.455, P = 0.901; Fig. 1b). In univariate analysis, CR after the first ASCT tended to predict extended PFS in addition to post-transplantation therapy (P = 0.064). Other factors did not predict PFS. In multivariate analysis, post-transplantation therapy significantly predicted an extended PFS, and the HR of PFS for post-transplantation therapy and CR after ASCT were 0.458 (95% CI, 0.244–0.866, P = 0.015). A summary of univariate and multivariate analyses of PFS is presented in Table 2.
Progression-free survival and overall survival. a The median follow-up period for survival was 50.0 months (6.1–121.9 months). The 4-year PFS rate in the post-transplantation therapy group was significantly higher than those in the non-post-transplantation therapy group (60.6% and 28.6%; HR 0.455, 95% CI, 0.243–0.855, P = 0.012). b There was no significant difference in the 4-year OS rates between patients with and without post-transplantation therapy (87.6% and 80.7%; HR 0.941, 95% CI, 0.361–2.455, P = 0.901)
Additionally, we analyzed the clinical significance of post-transplantation therapy after matched-pair analysis using the VRd regimen as induction therapy. The significant difference in patient characteristics between these two groups was not observed after matched-pair analysis (Supplementary Table 1). The 4-year PFS rate in the post-transplantation therapy group was significantly higher than that in the non-post-transplantation therapy group after matched-pair analysis (60.3% and 28.6%, respectively; HR 0.436, 95% CI 0.203–0.936, P = 0.029).
Next, we analyzed the clinical impact of tandem ASCT followed by consolidation and/or maintenance therapy (tandem ASCT plus CONS/MT) because the PFS in the tandem ASCT plus CONS/MT group was significantly longer than that in not only the non-post-transplantation therapy group but also the single ASCT followed by CONS/MT and tandem ASCT without CONS/MT groups (P = 0.005 and 0.031). The frequencies of age over 65 years and VRd regimen as induction therapy in the tandem ASCT plus CONS/MT group were significantly higher than those in the non-tandem ASCT plus CONS/MT group (P = 0.006 and 0.011, respectively). Furthermore, the 4-year PFS rate in the tandem ASCT plus CONS/MT group was significantly longer than that in the non-tandem ASCT plus CONS/MT group (73.7% vs 34.0%, respectively; HR 0.567, 95% CI 0.391–0.824, P = 0.002, Fig. S1a). Moreover, the 4-year OS rate in the tandem ASCT plus CONS/MT group tended to be longer than that in the non-tandem ASCT plus CONS/MT group (97.0% vs 73.7%, HR 0.559, 95% CI 0.299–1.048, P = 0.056, Fig. S1b). After matched-pair analysis using age over 65 years and VRd regimen as induction therapy, the 4-year PFS rate in the tandem ASCT plus CONS/MT group was significantly longer than that in the non-tandem ASCT plus CONS/MT group (73.7% vs 44.8%, respectively; HR 0.629, 95% CI 0.420–0.940, P = 0.019). Additionally, the 4-year OS rate in the tandem ASCT plus CONS/MT group tended to be longer than that in the non-tandem ASCT plus CONS/MT group as well (97.0% vs 71.5%, respectively; HR 0.565, 95% CI 0.293–1.090, P = 0.073).
Finally, we analyzed the clinical impact of tandem ASCT followed by consolidation and/ or maintenance therapy in patients who achieved CR after the first and second ASCT. The 4-year PFS rate in the patients who achieved CR after the first ASCT and received tandem ASCT plus CONS/MT was significantly higher than that of those in another group (66.7% vs 46.4%, respectively; P = 0.046), while there was no significant difference of 4-year OS between these two groups (100% vs 81.0%, respectively; P = 0.158). However, tandem ASCT plus CONS/MT improved not only PFS, but also OS in patients who achieved CR after the second ASCT; the 4-year PFS and OS rates in those who achieved CR after the second ASCT and received tandem ASCT plus CONS/MT was significantly higher than that in another group (76.7% vs 40.0%, respectively; HR 0.510, 95% CI 0.303–0.858, P = 0006, Fig. S2a, and 100% vs 77.0%, HR not evaluated, P = 0.019, Fig. S2b, respectively).
Treatment response, including MRD status by post-transplantation therapy
Post-ASCT treatment responses were: CR, 39 patients; VGPR, 17; and partial response (PR), 23. Tandem ASCT improved the CR rate from 39.7 to 60.3% in the post-transplantation therapy group, and the CR rate after tandem ASCT in the post-transplantation therapy group was significantly higher than that after the first ASCT in the non-post-transplantation therapy group (60.3% and 23.8%, P = 0.005). For the best response, the CR rate in the post-transplantation therapy group was significantly higher than that of the non-post-transplantation therapy group (77.6% vs. 23.2%, respectively; P < 0.001). Additionally, the CR rate in the tandem ASCT plus CONS/MT group was significantly higher than that in the non-tandem ASCT plus CONS/MT group (78.8% vs 52.2%, P = 0.019).
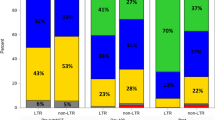
The MRD status was analyzed at least once in 38 patients and multiple times in 25 patients (twice, 7; 3 times, 12; 4 and 5 times, 3 each). The number of patients whose MRD assessment was done at least in the post-transplantation therapy and non-post-transplantation therapy groups was 34 and 4, respectively. The MRD-negative rate after ASCT and the best response rate in all patients were 26.6 and 38.0%, respectively. The MRD-negative rate was 81.6% in patients who achieved CR and underwent MRD assessments after ASCT. Furthermore, the MRD-negative rate in the post-transplantation therapy group was significantly higher than that of the non-post-transplantation therapy group as the best response (53.4% vs. 9.7%, respectively; P < 0.001). Additionally, the MRD-negativity and sustained MRD-negativity rates in the tandem ASCT plus CONS/MT group were significantly higher than those in the non-tandem ASCT plus CONS/MT group as well (60.6% vs 28.3%, P = 0.006; and 42.4% vs 19.6%, P = 0.044, respectively). The treatment responses following induction therapy, after the first ASCT, post-tandem ASCT, as well as the best response, are depicted in Fig. 2. Furthermore, the alteration in treatment response, including MRD status, between patients with and without post-transplantation therapies is presented in Fig. 3.
Change of treatment response between the patients with and without post-transplantation therapy. The treatment response was improved by post-transplantation therapy in the post-transplantation therapy group although the treatment response was not changed in the post-transplantation therapy group. The CR rate after the second ASCT in the post-transplantation therapy group was significantly higher than those after the first ASCT in the non-post-transplantation therapy group (60.3% vs 23.8%, P = 0.005). The CR rate as best response in the post-transplantation therapy group was significantly higher than those in the non-post-transplantation therapy group (77.6% vs 23.8%, P < 0.001). The MRD-negative rate in the post-transplantation therapy group was significantly higher than that of the non-post-transplantation therapy group as best response (53.4% vs. 9.7%, P < 0.001). a Treatment response in the post-transplantation therapy group. b Non-post-transplantation therapy group. ASCT1 the first autologous stem cell transplantation, ASCT2 the second autologous stem cell transplantation, MRD minimal residual disease, CR complete response, VGPR very good partial response, PR partial response, SD stable disease, PD progressive disease, −ve negative, + ve positive, w/o without
Change of MRD status after ASCT. MRD status was analyzed at least once in 38 patients and multiple times in 25 patients among them; 9 of twice, 12 of three times, 3 of four times, and 3 of five times, respectively. The number of patients, who MRD assessment was done at least, in the post-transplantation therapy and non- post-transplantation therapy groups were 34 and 4, respectively. White round,. black round, black triangle, and cross signs show MRD-negativity, MRD-positivity, progressive disease and death, respectively. MRD minimal residual disease, ASCT autologous stem cell transplantation, CR complete response, VGPR very good partial response, PR partial response, PD progressive disease, post-Tx post-transplantation therapy, TFI treatment-free interval
The MRD status as the best response predicted PFS and OS; the 4-year PFS and OS rates between the patients with MRD-negativity and MRD-positivity, including the patients without MRD assessment, were 84.3 and 34.7% (P < 0.001; Fig. 4a) and 96.8 and 76.2% (P = 0.050; Fig. 4b), respectively. The sustained MRD-negativity predicted a PFS and OS; the 4-year PFS and OS rates between the patients with sustained MRD-negativity and other groups were 93.8% and 36.3% (P < 0.001; Fig. 4c), and 100.0% and 77.2% (P = 0.014; Fig. 4d), respectively.
Clinical impact of MRD-negativity and sustained MRD-negativity for survival time. a The 4-year PFS rates between the patients with MRD-negativity and non-MRD-negativity groups, including the patients without MRD assessment, were 84.3% and 34.7% (P < 0.001). b The 4-year OS rates between the patients with MRD-negativity and non-MRD-negativity groups were 96.8% and 76.2% (P = 0.050). c The 4-year PFS rates between the patients with sustained MRD-negativity group and another were 93.8% and 36.3% (P < 0.001). d The 4-year OS rates between the patients with sustained MRD-negativity group and another were 100.0% and 77.2% (P = 0.014). PFS progression free survival, OS overall survival, MRD minimal residual disease, sus-MRD sustained MRD, −ve negativity, + ve positivity
Discontinuation of consolidation and maintenance therapy
Thirty patients discontinued consolidation and maintenance therapy. The causes for discontinuation of consolidation and maintenance therapy were as follows: adverse events in 13 patients (skin rash in five; one each for secondary myelodysplastic syndrome, pneumonia, interstitial pneumonia, sepsis, influenza virus infection, renal insufficiency, cytopenia, and rheumatoid arthritis); progressive disease in 10; planned discontinuation in 6; and one due to economic issues. Importantly, the 4-month, 1-year, 2-year, and 4-year cumulative incidence rates of discontinuation of consolidation and maintenance therapy due to adverse events were 13.3, 15.7, 28.9, and 33.3%, respectively (Fig. S3). Early onset adverse events, defined as those occurring within 4 months of initiation of consolidation and maintenance therapy, included three instances of skin rash during IRd consolidation therapy and one instance each of skin rash, interstitial pneumonia, and renal insufficiency during LEN maintenance therapy. Late-onset adverse events numbered six; one during IXA maintenance therapy and five during LEN maintenance therapy. Three patients succumbed during consolidation and maintenance therapy due to sepsis, influenza virus infection, and secondary myelodysplastic syndrome.
Discussion
We demonstrated that the patients treated with post-transplantation therapy demonstrated significantly higher PFS than did those without such therapy, even after harmonizing patient characteristics through matched-pair analysis for the VRd regimen as induction therapy. Furthermore, tandem ASCT plus CONS/MT significantly improved PFS and tended to extend OS, particularly in patients who achieved CR after the second ASCT. MRD-negativity was observed in 81.6% of those who achieved CR, and the incidence of loss of MRD-negativity and PD stood at 5.5% during post-ASCT treatment. Thus, post-transplantation therapy facilitated the achievement and maintenance of MRD-negativity, which predicted prolonged PFS and OS. Importantly, the cumulative incidence of discontinuation due to adverse events was comparatively high in clinical practice relative to clinical trials, and such occurrences were frequently observed within the first 4 months.
The registry analyses revealed that a planned post-transplantation therapy prolonged the PFS and OS [9, 10, 30]. The median PFS was 3.0–3.4 years when the percentage of patients treated with VRd induction therapy was 9.0–39.0% [9, 10]. In the STaMINA trial in which approximately 60% of participants received VRd induction therapy followed by ASCT and LEN maintenance therapy, the 38-month PFS rates among the single ASCT, single ASCT plus VRd consolidation therapy, and tandem ASCT groups were 53.9, 57.8, and 58.5%, respectively [16] In the IFM2009 trial, which investigated a 1-year fixed duration of LEN maintenance therapy, and the DETERMINATION trial, which evaluated continuous LEN maintenance therapy, the median PFS in the ASCT group was 50 months and 67.5 months, respectively, among patients treated with VRd induction therapy [5, 6]. In this study, post-transplantation therapy also enhanced the PFS, yielding a 4-year post-transplant PFS rate of 64.9%. This rate may be marginally higher than the outcomes of prior studies, likely due to a greater proportion of patients receiving VRd induction and long-term post-transplantation therapy. Furthermore, evidence is limited regarding post-transplantation therapy after tandem ASCT. In this study, tandem ASCT plus CONS/MT could improve PFS compared with single ASCT followed by CONS/MT and tandem without CONS/MT, similar to the results of the EMN02/HO95 trial [4]. Therefore, post-transplantation therapy can improve clinical outcomes, and consolidation and/or maintenance therapy remains the standard of care for transplantation-eligible NDMM patients, even if the patients received tandem ASCT.
Here, post-transplantation therapy improved PFS even in the patients who achieved CR after ASCT; notably, tandem ASCT followed by CONS/MT improved PFS and OS in the patients who achieved CR after the second ASCT. According to previous large-scale clinical trials for post-transplantation therapy, the clinical impact of post-transplantation therapy for the patients who achieved CR after ASCT was various. In a meta-analysis and the MRC Myeloma-XI trial, LEN maintenance therapy improved PFS but did not improve OS significantly [7, 31]. In the TOURMALINE-MM3 trial, IXA maintenance improved PFS for patients achieving VGPR after ASCT but did not statistically demonstrate a significant PFS improvement for those with CR after ASCT [8]. However, deepening treatment response was associated with prolonged PFS in the same trial [32]. Data on the significance of consolidation therapy are limited. The EMN02/HO95 and prospective Nordic Myeloma Study Group trials indicated prolonged PFS with consolidation therapy, and the benefit was notably observed in patients without CR prior to consolidation therapy [33, 34]. Thus, post-transplantation therapy could potentially enhance clinical outcomes in patients lacking CR after ASCT through deepening response. Importantly, CR as the best response emerged as a significant prognostic factor for both OS and PFS, irrespective of post-transplantation regimens, according to the Mayo Clinic dataset [35]. This suggests that the best response, rather than treatment response post-ASCT, serves as a key surrogate marker for extended PFS. Tandem ASCT significantly improved PFS; yet, its impact on OS remains uncertain [16,17,18,19]. Developed before the novel agent era, tandem ASCT improved not just PFS, but also OS [17]; however, a meta-analysis showed that it did not enhance OS [18]. In contrast, the HOVON-65/GMMG-HD4 trial indicated that tandem ASCT improved OS, compared with single ASCT, when either bortezomib or thalidomide maintenance therapy was administered [19]. Evidence concerning the clinical significance of tandem ASCT in the novel agent era, particularly focusing on OS, is limited. We posit that our findings may bolster the potential role of tandem ASCT followed by CONS/MT in this novel agent era.
According to a meta-analysis, MRD-negativity has been reported to predict the long PFS and OS even in CR patients [20]. In this study, tandem ASCT followed by CONS/MT improved OS in the patients who achieved CR after the second ASCT, suggesting that this intensive treatment strategy might contribute to deepening treatment response of MRD status even in CR patients. Notably, maintenance therapy contributes to sustaining MRD negativity even in patients with MRD negativity after ASCT [21]. According to the MRC Myeloma XI trial, changes in MRD status were rarely observed after ASCT and LEN maintenance therapy, contributing to conversion from MRD-positivity to negativity [23]. In this study, patients whose MRD status changed from positivity to negativity during post-transplantation therapy were not identified, suggesting that post-transplantation therapy might not be enough to eradicate minimal residual myeloma diseases, although a majority of patients whose MRD status was negative by the first MRD assessment achieved sustained MRD-negativity during post-transplantation therapy. Furthermore, none of the patients suddenly relapsed without conversion of the MRD status to positivity, and two patients who tested positive for MRD by the first MRD assessment and had PD during post-transplant therapy were able to quickly achieve MRD-negativity again with the administration of daratumumab. These results suggest that post-transplant therapy does not result in aggressive relapse and that selecting a new class of drugs not previously used is important after relapse. Finally, consolidation therapy improved PFS in patients with MRD-positive status as determined by NGS and PET/CT, compared to MRD double-negative patients without consolidation therapy [36]. Additionally, intensified therapy enhanced PFS in MRD-positive patients while discontinuing therapy did not adversely affect PFS in MRD-negative patients, according to real-world data [25]. These findings suggest that achieving MRD-negativity may be crucial for sustained deep response prior to maintenance therapy. Therefore, in clinical practice, we plan to administer consolidation therapy to MRD-positive patients post-ASCT and to forgo consolidation therapy for MRD-negative patients after ASCT.
The incidence of discontinuation due to adverse events might be relatively high compared to that in previous reports, although those data were various; specifically, during LEN monotherapy after ASCT, it was 8.0–29.7% [5, 7, 31, 37, 38]. In the IFM2009 trial, including 1-year fixed duration LEN maintenance therapy, the incidence of discontinuation due to adverse events was 11% from induction to maintenance therapy [5], suggesting that short-interval LEN maintenance therapy might reduce the incidence of discontinuation due to adverse events. Meanwhile, the incidence of discontinuation due to adverse events during PI-containing post-transplantation therapy was 7–14% [8, 21, 39]. Moreover, in TOURMALINE-MM3 and 4 trials, the initial dose of IXA was 3 mg, considering the clinical and model-based analysis for relapsed and/or refractory myeloma [40]. Meanwhile, in the IFM2013-06 trial, when the initial dose of IXA as consolidation therapy was 4 mg, the incidence of grade 3 to 4 adverse events during IRd consolidation therapy was high compared to that during IRd induction therapy [39]. In this trial, discontinuation due to adverse events occurred within 4 months frequently compared with 4 months or later. Thus, adverse events during post-transplantation therapy could be more frequent and severe compared to pre-transplantation therapy; dose adjustment of therapeutics in the post-transplantation phase may depend not only on adverse events, but also on the initial administration dose, particularly for patients treated with PI-containing post-transplantation therapy.
The study has few limitations. First, MRD measurements were not always performed regularly, and the interval of regular MRD measurements was once a year, which might not be adequate to assess MRD in detail. Second, the follow-up time might not be long enough to investigate the clinical impact of MRD-negativity for PFS and OS. We will continue to follow these datasets to understand them well. Finally, this was a two-center, retrospective study with a small number of cases. We plan to accumulate more cases and continue to study the usefulness of post-transplantation therapy and changes in MRD status.
In conclusion, post-transplantation therapy could improve PFS even in the patients who achieved CR after the first ASCT and contribute to sustained MRD negativity, similar to previous reports. Tandem ASCT followed by CONS/MT improved both OFS and OS in patients who achieved CR after the second ASCT. Moreover, MRD negativity is key to improving clinical outcomes, and the conversion of MRD status during post-transplantation therapy was rare but sometimes observed during treatment-free intervals, even if MRD negativity was achieved. Finally, the optimal dose adjustment of therapeutics as post-transplantation therapy can prevent discontinuation of post-transplantation therapy. Thus, post-transplantation therapy is the standard of care for myeloma patients receiving PI and/or immunomodulator induction therapy. However, this was a small-scale retrospective study, and the patient characteristics that indicated discontinuation of post-transplantation therapy remained unclear. The results of a prospective study on MRD-conducted post-transplantation therapy are essential to resolve this clinical question.
Data availability
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–20.
Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371:895–905.
Gay F, Oliva S, Petrucci MT, Conticello C, Catalano L, Corradini P, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015;16:1617–29.
Cavo M, Gay F, Beksac M, Pantani L, Petrucci MT, Dimopoulos MA, et al. Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study. Lancet Haematol. 2020;7:e456–68.
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–20.
Richardson PG, Jacobus SJ, Weller EA, Hassoun H, Lonial S, Raje NS, et al. Triplet therapy, transplantation, and maintenance until progression in myeloma. N Engl J Med. 2022;387:132–47.
McCarthy PL, Holstein SA, Petrucci MT, Richardson PG, Hulin C, Tosi P, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35:3279–89.
Dimopoulos MA, Gay F, Schjesvold F, Beksac M, Hajek R, Weisel KC, et al. Oral ixazomib maintenance following autologous stem cell transplantation (TOURMALINE-MM3): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2019;393:253–64.
Ozaki S, Handa H, Koiso H, Saitoh T, Sunami K, Ishida T, et al. Propensity-score matched analysis of the efficacy of maintenance/continuous therapy in newly diagnosed patients with multiple myeloma: a multicenter retrospective collaborative study of the Japanese Society of Myeloma. J Cancer Res Clin Oncol. 2022;148:191–203.
Cornell RF, D’Souza A, Kassim AA, Costa LJ, Innis-Shelton RD, Zhang MJ, et al. Maintenance versus induction therapy choice on outcomes after autologous transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2017;23:269–77.
Dimopoulos MA, Moreau P, Terpos E, Mateos MV, Zweegman S, Cook G, et al. Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:309–22.
Gonsalves WI, Buadi FK, Ailawadhi S, Bergsagel PL, Chanan Khan AA, Dingli D, et al. Utilization of hematopoietic stem cell transplantation for the treatment of multiple myeloma: a mayo stratification of myeloma and risk-adapted therapy (mSMART) consensus statement. Bone Marrow Transplant. 2019;54:353–67.
Iida S, Ishida T, Murakami H, Ozaki S, Abe M, Hata H, Shimazaki C. JSH practical guidelines for hematological malignancies, 2018: III. Myeloma-1 Multiple myeloma (MM). Int J Hematol. 2019;109:509–38.
Ludwig H, Durie BG, McCarthy P, Palumbo A, San Miguel J, Barlogie B, et al. IMWG consensus on maintenance therapy in multiple myeloma. Blood. 2012;119:3003–15.
Dimopoulos MA, Jakubowiak AJ, McCarthy PL, Orlowski RZ, Attal M, Bladé J, et al. Developments in continuous therapy and maintenance treatment approaches for patients with newly diagnosed multiple myeloma. Blood Cancer J. 2020;10:17.
Stadtmauer EA, Pasquini MC, Blackwell B, Hari P, Bashey A, Devine S, et al. Autologous transplantation, consolidation, and maintenance therapy in multiple myeloma: results of the BMT CTN 0702 Trial. J Clin Oncol. 2019;37:589–97.
Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–502.
Kumar A, Kharfan-Dabaja MA, Glasmacher A, Djulbegovic B. Tandem versus single autologous hematopoietic cell transplantation for the treatment of multiple myeloma: a systematic review and meta-analysis. J Natl Cancer Inst. 2009;101:100–6.
Sonneveld P, Schmidt-Wolf IG, van der Holt B, El Jarari L, Bertsch U, Salwender H. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol. 2012;30:2946–55.
Munshi NC, Avet-Loiseau H, Anderson KC, Neri P, Paiva B, Samur M, et al. A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma. Blood Adv. 2020;4:5988–99.
Paiva B, Manrique I, Dimopoulos MA, Gay F, Min CK, Zweegman S, et al. MRD dynamics during maintenance for improved prognostication of 1280 myeloma patients in TOURMALINE-MM3 and -MM4 trials. Blood. 2023;141:579–91.
Diamond B, Korde N, Lesokhin AM, Smith EL, Shah U, Mailankody S, et al. Dynamics of minimal residual disease in patients with multiple myeloma on continuous lenalidomide maintenance: a single-arm, single-centre, phase 2 trial. Lancet Haematol. 2021;8:e422–32.
de Tute RM, Pawlyn C, Cairns DA, Davies FE, Menzies T, Rawstron A, et al. Minimal residual disease after autologous stem-cell transplant for patients with myeloma: prognostic significance and the impact of lenalidomide maintenance and molecular risk. J Clin Oncol. 2022;40:2889–900.
Costa LJ, Chhabra S, Medvedova E, Dholaria BR, Schmidt TM, Godby KN, et al. Daratumumab, carfilzomib, lenalidomide, and dexamethasone with minimal residual disease response-adapted therapy in newly diagnosed multiple myeloma. J Clin Oncol. 2022;40:2901–12.
Martinez-Lopez J, Alonso R, Wong SW, Rios R, Shah N, Ruiz-Heredia Y, et al. Making clinical decisions based on measurable residual disease improves the outcome in multiple myeloma. J Hematol Oncol. 2021;14:126.
Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127:2955–62.
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–46.
Takamatsu H, Yoroidaka T, Fujisawa M, Kobori K, Hanawa M, Yamashita T, et al. Comparison of minimal residual disease detection in multiple myeloma by SRL 8-color single-tube and EuroFlow 8-color 2-tube multiparameter flow cytometry. Int J Hematol. 2019;109:377–81.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Karam D, Gertz M, Lacy M, Dispenzieri A, Hayman S, Dingli D, et al. Impact of maintenance therapy post autologous stem cell transplantation for multiple myeloma in early and delayed transplant. Bone Marrow Transplant. 2022;57:803–9.
Jackson GH, Davies FE, Pawlyn C, Cairns DA, Striha A, Collett C, et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019;20:57–73.
Goldschmidt H, Dimopoulos MA, Rajkumar SV, Weisel KC, Moreau P, Chng WJ, et al. Deepening responses associated with improved progression-free survival with ixazomib versus placebo as posttransplant maintenance in multiple myeloma. Leukemia. 2020;34:3019–27.
Sonneveld P, Dimopoulos MA, Beksac M, van der Holt B, Aquino S, Ludwig H, et al. Consolidation and maintenance in newly diagnosed multiple myeloma. J Clin Oncol. 2021;39:3613–22.
Mellqvist UH, Gimsing P, Hjertner O, Lenhoff S, Laane E, Remes K, et al. Bortezomib consolidation after autologous stem cell transplantation in multiple myeloma: a Nordic Myeloma Study Group randomized phase 3 trial. Blood. 2013;121:4647–54.
Sidiqi MH, Aljama MA, Bin Riaz I, Dispenzieri A, Muchtar E, Buadi FK. Bortezomib, lenalidomide, and dexamethasone (VRd) followed by autologous stem cell transplant for multiple myeloma. Blood Cancer J. 2018;8:106.
Böckle D, Tabares P, Zhou X, Schimanski S, Steinhardt MJ, Bittrich M, et al. Minimal residual disease and imaging-guided consolidation strategies in newly diagnosed and relapsed refractory multiple myeloma. Br J Haematol. 2022;198:515–22.
Alonso R, Cedena MT, Wong S, Shah N, Ríos-Tamayo R, Moraleda JM, et al. Prolonged lenalidomide maintenance therapy improves the depth of response in multiple myeloma. Blood Cancer J. 2020;4:2163–71.
Gay F, Musto P, Rota-Scalabrini D, Bertamini L, Belotti A, Galli M, et al. Carfilzomib with cyclophosphamide and dexamethasone or lenalidomide and dexamethasone plus autologous transplantation or carfilzomib plus lenalidomide and dexamethasone, followed by maintenance with carfilzomib plus lenalidomide or lenalidomide alone for patients with newly diagnosed multiple myeloma (FORTE): a randomised, open-label, phase 2 trial. Lancet Oncol. 2021;22:1705–20.
Touzeau C, Perrot A, Roussel M, Karlin L, Benboubker L, Jacquet C, et al. All-oral triplet combination of ixazomib, lenalidomide, and dexamethasone in newly diagnosed transplant-eligible multiple myeloma patients: final results of the phase II IFM 2013–06 study. Haematologica. 2022;107:1693–7.
Gupta N, Yang H, Hanley MJ, Zhang S, Liu R, Kumar S. Dose and schedule selection of the oral proteasome inhibitor ixazomib in relapsed/refractory multiple myeloma: clinical and model-based analyses. Target Oncol. 2017;12:643–54.
Acknowledgements
We would like to thank the attending doctors and nurses at the Jikei University Hospital and the Jikei University Kashiwa Hospital. We would also like to extend gratitude to the myeloma patients and their families for consenting to participate in our study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K. Suzuki received personal fees from Takeda Pharmaceutical Company, Janssen Pharmaceutical K.K., Sanofi, Bristol Myers Squibb, Ono Pharmaceutical Co., Ltd., outside the submitted work; H. Uryu received personal fees from Janssen Pharmaceutical K.K., Ono Pharmaceutical Co., Ltd., outside the submitted work; K. Nishiwaki reports personal fees from Pfizer and Alexion Pharmaceuticals Inc. and grants from Kyowa Kirin Co, Ltd, outside the submitted work; Dr. Yano reports grants from Kyowa Kirin, grants from Lilly Phrama, and grants from Otsuka Pharmaceutical, outside the submitted work;.the other authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12185_2023_3682_MOESM1_ESM.jpeg
Supplementary file1 Fig. S1. Tandem ASCT followed by consolidation and/or maintenance therapy improved progression free survival and overall survival. a The 4year-PFS rate in the tandem ASCT plus CONS/MT group was significantly longer than those in the non-tandem ASCT plus CONS/MT group (73.7% vs 34.0%, HR 0.567, 95% CI 0.391–0.824, P = 0.002) (JPEG 125 KB). b the 4year-OS rate in the tandem ASCT plus CONS/MT group tended to be longer that those in the non-tandem ASCT plus CONS/MT group (97.0% vs 73.7%, HR 0.559, 95% CI 0.299–1.048, P = 0.056). PFS progression free survival, OS overall survival, ASCT autologous stem cell transplantation, CONS/MT consolidation and/or maintenance therapy
12185_2023_3682_MOESM2_ESM.jpeg
Supplementary file2 Fig. S2. Tandem ASCT followed by consolidation and/or maintenance therapy improved progression free survival and overall survival in the patients who achieved CR after the second ASCT. a The 4year-PFS rate in the patients who achieved CR after the second ASCT and received tandem ASCT plus CONS/MT was significantly higher than those in another group (76.7% vs 40.0%, HR 0.510, 95% CI 0.303–0.858, P = 0006). b The 4year-OS rate in the patients who achieved CR after the second ASCT and received tandem ASCT plus CONS/MT was significantly higher than those in another group (100% vs 77.0%, HR not evaluated, P = 0.019). PFS progression free survival, OS overall survival, ASCT autologous stem cell transplantation, CONS/MT consolidation and/or maintenance therapy (JPEG 113 KB)
12185_2023_3682_MOESM3_ESM.jpeg
Supplementary file3 Fig. S3. The cumulative incidence of discontinuation due to adverse events. The 4month, 1, 2 and 4years-cumulative incidence rates of discontinuation of post-transplantation therapy due to adverse events were 13.3%, 15.7%, 28.9% and 33.3%, respectively (JPEG 62 KB)
About this article
Cite this article
Suzuki, K., Gunji, T., Kawashima, M. et al. Contribution of post-transplantation therapy to sustained MRD negativity in multiple myeloma: a retrospective analysis. Int J Hematol 119, 39–49 (2024). https://doi.org/10.1007/s12185-023-03682-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-023-03682-z