Abstract
Chemotherapy, all-trans retinoic acid (ATRA), and arsenic are effective options for acute promyelocytic leukemia (APL). We conducted a 20-year retrospective analysis of newly diagnosed (ND) APL patients treated with arsenic, ATRA and mitoxantrone. After achieving complete remission (CR), patients received 3–5 cycles of chemotherapy followed by AS4S4 maintenance for 3 years. Eighty-eight ND APL patients were treated with either oral AS4S4 (n = 42) or arsenic trioxide (ATO) (n = 46). The 8-year overall survival (OS) rate was 100% in the AS4S4 group and 90% in the ATO group. The disease-free survival (DFS) rates were 100% and 87.1% (p = 0.027), respectively. Patients in the ATO group had more side effects. A subsequent cohort of 33 ND APL patients received triple therapy with oral AS4S4, ATRA, and chemotherapy. The 13-year OS and DFS rates were 100% and 90.9%. Our long-term analyses show that APL patients with oral AS4S4 had better outcomes compared to ATO, with no need for hospitalization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute promyelocytic leukemia (APL) is a subtype of acute myeloid leukemia (AML) characterized by a balanced reciprocal translocation, t(15;17) (q22;q12-21), resulting in the fusion of promyelocytic leukemia (PML) gene with the retinoic acid receptor alpha (RARa) gene. Since the 1960s and 1970s, APL remains among the most aggressive and lethal AML subtypes with a high mortality rate [1, 2]. Currently, APL has become a curable form of AML with a complete remission (CR) rate of about 100%, best overall survival (OS) of more than 90%, and relapse-free survival (RFS) of more than 80% [3,4,5,6]. The approach to the treatment of APL has gone through several stages of progress. Anthracycline chemotherapy was first found to improve APL outcomes in the 1980s and 1990s [7, 8]. In the 1990s, a China-based study found that front-line all-trans retinoic acid (ATRA) therapy significantly improved the CR rate up to 90% and reduced mortality and incidence of disseminated intravascular coagulation (DIC) during the induction period, a significant milestone for APL therapy [9,10,11,12,13]. Subsequently, researchers in China found that adding arsenic (intravenous arsenic trioxide (ATO) or oral As4S4) to induction and maintenance therapy could significantly improve the CR rate and prolong RFS, resulting in another significant achievement in APL therapy. [14,15,16,17,18,19,20,21] Currently, the National Comprehensive Cancer Network (NCCN) and other guidelines suggest ATRA combined with ATO or chemotherapy as APL therapy and divide APL patients into low-risk and high-risk subgroups. Standard therapies vary depending on the patient’s risk characterization [22, 23] to overcome the inferior results of high-risk classified s patients.
In this study, we retrospectively analyzed 20 years of long-term follow-up data of newly diagnosed (ND) APL patients who received the triple therapy of arsenic (As4S4/ATO) combined with ATRA and anthracycline-based chemotherapy. All APL patients in the study achieved CR and long-term OS and RFS.
Patients and methods
We conducted a retrospective study of ND APL patients from 2001 to 2018 treated at the Beijing Daopei Hospital (2001–2008) and Hebei Yanda Lu Daopei Hospital (2008–2018). As defined by the 2021 NCCN guideline [22], patients who had abnormal promyelocytes in bone marrow with the abnormal karyotype t(15;17) or PML::RARA rearrangement were enrolled.
Ethics
The study protocol was approved by the Ethics Committee of Beijing Daopei Hospital and Hebei Yanda Lu Daopei Hospital according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients or their guardians.
Assessments
Cytogenetic analyses were performed on all patients with ND APL, and reverse transcription polymerase chain reaction (RT-PCR) for PML::RARA was performed on bone marrow (BM) aspirate samples. CR was defined, according to the 2021 NCCN guideline, by white blood cell (WBC), platelet counts and BM blasts [22]. The minimal residual disease (MRD) was assessed by quantitative monitoring of the PML::RARA fusion gene at a sensitivity threshold of 1 × 10–5.
During the chemotherapy period, BM examination (including morphology and quantitative PML::RARA) and lumbar puncture to obtain cerebrospinal fluid (CSF, including morphology, protein, glucose, and flow cytometry) was conducted every month. After demonstrating that the PML::RARA gene was no longer detected, BM punctures were performed every three to six months for three years. If the CSF test was normal, lumbar puncture intrathecal injection could be conducted up to 6 times. Chromosomal testing methods, fusion genes, and mutation genes were described in the Supplementary Methods. Gene mutation tests were carried out since 2008.
Preparation of As 4 S 4
As4S4 was prepared from mined, natural realgar. Chia-Si Lu has interpreted the molecular structure of As4S4 for more than 50 years. Highly purified As4S4 was also mixed with an equal amount of ground Seman platycladi and put into capsules containing 250 mg of As4S4 [19, 20]. In the remission induction period, the oral dose and administration of As4S4 for newly diagnosed APL was 50–60 mg/kg of body weight per day divided into three daily doses.
Treatment process
Patients with genetical and chromosomal confirmed APL were treated with oral As4S4 at 50-60 mg/kg for 28 days or intravenous ATO 0.15 mg/kg for 28 days, combined with ATRA 25-45 mg/m2 for 28 days, and Mitoxantrone (NVT) 1.5-3 mg/m2 for 5–7 days in the first month. When the peripheral blood WBC was higher than 10 × 109/L during the first induction therapy, the ATRA was ceased until the WBC decreased to less than 10 × 109/L. After achieving CR, patients underwent 3–5 cycles of consolidation and intensive chemotherapy. The low-risk patients received three chemotherapy cycles, while high-risk (WBC > 10 × 109/L) patients underwent 4–5 chemotherapy cycles. The main chemotherapy regimen included (1) Idarubicin (IDA) 12 mg/m2 × 3 days, or Daunorubicin (DNR) 45-60 mg/m2 × 3 days, (2) Cytarabine (Ara-C) 75 mg/m2 × 7 days + Aclamycin (ACLA) 14 mg/m2 × 4 days, (3) NVT 6 mg/m2 × 3 days. For the patients with WBC > 10 × 109/L, and especially for patients with WBC > 50 × 109/L or extramedullary disease (EMD) in the first induction chemotherapy, add (4) Ara-C 1-2 g/m2 × 3 days, and/or (5) Thiotepa 100 mg/ m2 × 1 day.
During the chemotherapy period, 50–60 mg/kg of As4S4 or intravenous ATO 0.15 mg/kg × 15 days/30 days + ATRA 25-45 mg/m2 × 15 days/30 days (administered for 15 days followed by a 15-day off period every month) were continued for 6 months. If the neutropenia developed after chemotherapy, As4S4 or intravenous ATO would be stopped temporarily until the neutrophils recovered.
The APL patients then proceeded to a maintenance treatment: ATRA 25-45 mg/m2 × 15/30 days and suspended when the total course of therapy reached one year. Arsenic was given continuously (50–60 mg/kg of oral As4S4 × 15/30 days or intravenous ATO 0.15 mg/kg × 15/30 days), and the withdrawal time was delayed for 15 days every six months, until maintenance therapy every 15 days per 90 days. The total course of treatment continued for 3 years. (Fig. 1). Adverse events were assessed according to Common Terminology Criteria for Adverse Events (CTCAE) v5.0 [24]
The Treatment Scheme of the ATO/ AS4S4-Based Triple Agent Protocol. MICM Morphology, immunotherapy, cytogenetics, molecules, ATRA All trans retinoic acid, ATO arsenic trioxide, NVT Mitoxantrone; IDA Idarubicine, DNR daunorbicine, Ara-C Cytarabine, Acla Aclacinomycin, MD middle dose, IT Intrathecal chemotherapy
Statistic methods
Patients’ CR rate was analyzed by Chi-square test. The OS and RFS probabilities were estimated using the Kaplan–Meier method and were compared using the log-rank test. Calculations were performed with Prism software (version 5.0). P-values were determined by Chi-square Test.
Results
The long-term survival of newly diagnosed APL patients, enrolled between 2001 and 2008
Eighty-eight patients with ND APL were enrolled from June 2001 to December 2008 and followed until December 2012. The first treatment was oral As4S4 (n = 42) or intravenous ATO (n = 46). Detailed patient characteristics are provided in Table 1. All patients received combined chemotherapy regimens of ATRA and NVT. Both groups achieved hematological CR within one month, and no treatment-related deaths occurred. All patients achieved PML::RARA molecular complete remission (MCR) confirmed by RT-PCR at a median time of 2 months (range: 1–7 months). In the As4S4 group, both the 8-year OS and RFS rate was 100%, with a median follow-up time of 66 months (range: 15–106 months). In the ATO group, the 8-year OS was 90.0%, and the RFS was 87.1%, with a median follow-up time of 42 months (range: 14–104 months). There was a significant difference in the RFS between the two groups (p = 0.027) (Fig. 2). However, no significant difference in OS was observed between the groups (p = 0.177). Five patients (10.7%) in the ATO group experienced relapse, including four patients with central nervous system leukemia (CNSL) and one patient with myeloid sarcoma in the external auditory meatus. They subsequently received oral As4S4 alone with local radiotherapy and have been alive for more than 62 months with sustained HCR and MCR without relapse. All four patients with CNSL received additional chemotherapy with IDA or MD Ara-C and intrathecal chemotherapy. Two of the CNSL patients received oral As4S4 concurrently and have been alive for more than 59 and 94 months, respectively, with consistent MCR and HCR without CNS relapse. The remaining two patients subsequently had hematological relapse. In the As4S4 group, 23 patients were followed up for 18 years until November 2021. Except for one patient who died of cervical cancer at the 7th year of follow-up, and another patient with myasthenia gravis at the 10th year of follow-up, the remaining 21 patients are alive and healthy, with an 18-year OS and RFS of 95.6%.
OS and RFS of 88 newly diagnosed APL patients in the oral AS4S4 and ATO groups from 2001 to 2008. The 8-year OS and RFS rates were 100% in the AS4S4 group, and in the ATO group, the 8-year OS rate was 90.0%, and the RFS rate was 87.1%. There was a significant difference of RFS between the two groups (p = 0.027), while the OS were not significantly different (p = 0.177)
Adverse effects of ATO and the As 4 S 4 triple therapy among newly diagnosed APL patients enrolled between 2001 and 2008
There were no arsenic-related deaths in the cohort. Among the 88 patients, patients in the ATO group experienced more adverse effects than those in the As4S4 group (32/46 vs. 12/42, p < 0.05) during induction period, but without significant differences in the individual impact factors (Table 2). There were five patients with cardiac toxicity in the ATO group, including 2 with Grade 1–2 heart failure, 2 with mild tachycardia, and 1 with mild premature ventricular beat. Two patients had incidents of mild asymptomatic QTc prolongation in the As4S4 group. The incidence of Grade 1–2 abnormal liver function was also higher in the ATO group than in the As4S4 group but the difference was not statistically significant (Table 2). During the treatment, no patient discontinued therapy due to abnormal organ function. At the end of the chemotherapy cycles and during the maintenance therapy with ATO or As4S4 group, the main side effect was a slight decrease of WBC and neutrophils (neutrophils > 1 × 109/L), with no incidence of severe neutropenia. In the As4S4 group, 23 patients were followed up for 18 years until November 2021. Four of the patients have given birth to healthy children following their recovery.
The long-term survival of 33 ND APL patients, enrolled between 2008 and 2018
Because the RFS and safety was better in the As4S4 group than in the ATO group from the data enrolled from 2001 to 2008, since 2008, we mainly utilized the triple therapy of oral arsenic As4S4 combined with ATRA and chemotherapy for APL patients. From September 2008 to May 2018, 33 ND APL patients (15 children and 18 adults) were enrolled and followed until November 2021. Among them, 11 (33%) patients had additional chromosomal abnormalities other than t(15;17). During the first course of treatment, 20 patients (61%) had a highest WBC count of greater than 10 × 109/L. There were four patients (12%) with CNSL. Among patients with the PML::RARA fusion gene type, there were 23 cases with the long type, 4 cases with the short (S) type, and 2 cases with the variant (V) type. Among the 20 patients with gene mutation data, 3 were FLT3-ITD positive, and other gene mutations occasionally detected included NRAS, RUNX1, WT1, CSF3R, and ASXL1.
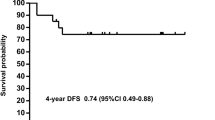
All 33 patients achieved MCR. The 10-year OS rate was 100%, and the 10-year RFS rate was 90.9% (Fig. 3). The median follow-up time was 98 months (range: 45–154 months). Of the 33 patients, three patients relapsed (1 hematological relapse, 1 MRD relapse, and 1 CNSL relapse), all of the three patients were in the high-risk group with the WBC > 10 × 109/L, and had long type PML::RARA fusion gene, without FLT3-ITD. There were no significant differences in relapse rate revealed in an analysis of patient characteristics, including age, gender, presence of CNSL, WBC count, and others (Table 3). Two of them discontinued the As4S4 or had a dose reduction during the As4S4 oral maintenance period. The triple therapy and lumbar puncture injection of chemotherapy drugs proceeded again after relapse. All 3 patients achieved CR again, and the long-term BM and CSF obtained were MRD negative till now, and their updated OS was 138 months, 82 months, and 79 months, respectively.
Discussion
With the discovery and wide application of ATRA and ATO, APL has changed from the leukemia with the highest mortality to one with the best therapeutic outcomes [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18]. Since the 1990s, Dr. Daopei Lu from Beijing discovered that another oral arsenic agent, realgar (whose main component is As4S4), also produces a high CR rate of 87.5% among APL patients, as well as RFS rates at 1 and 3 years of approximately 86.1% and 76.6%, respectively, and published by 2002 [19,20,21]. Later results of other Chinese cohort studies based on the oral arsenic agent resulted in excellent outcomes including a one-year OS rate of 100% [25]. Based on substantial medical evidence, including from the Southwest Oncology Group, and the Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) pilot study, the NCCN acute leukemia guideline and Chinese guidelines for diagnosis and treatment of APL state that the long-term survival rate of APL has been significantly improved following the utilization of ATRA combined with arsenic or chemotherapy [22, 26]. Still, the 3-year and 5-year OS rate of APL patients remained about 90–100%, and the RFS rate is about 80–90%, which is not enough to enable all APL patients to survive long-term without leukemia [3, 22, 27, 28]. Moreover, there was still no clear conclusion on how to use the three types of drugs, including the best sequence and combinations to maximize patient outcomes.
With many years of experience in researching APL therapies, Dr. Daopei Lu demonstrated that a three-drug combination regimen consisting of arsenic, ATRA and chemotherapies (mainly with anthracyclines) starting from induction chemotherapy and followed by maintenance treatment with arsenic for up to 3 years could make 100% of APL patients achieve MCR, with long-term OS and RFS rates of 100% and 94%, respectively [20, 29, 30]. Our retrospective follow-up analysis of approximately 20 years demonstrated that the triple therapy was safe and highly effective for APL patients.
The current APL risk stratification is based on the GIMEMA and PETHEMA clinical trials completed in 2000 [23]. Patients segregated into low-risk (WBC counts ≤ 10 × 109/L) and high-risk (WBC counts > 10 × 109/L) subgroups have distinctive RFS curves (p < 0.0001) [22, 23]. Generally, during the induction therapy with ATRA, WBC can increase significantly, up to as high as 100 × 109/L. In such high WBC scenarios, serious side effects of DIC probably occur. Chemotherapies such as NVT and arsenic are recommended to be used simultaneously to effectively clear the APL cells and reduce the severity of DIC. Another benefit of such triple therapy is that it reduces the migration of excess APL cells to EMD, thereby preventing long-term relapse, such as the CNSL. After passing this potentially dangerous period of the induction phase, chemotherapy should be performed for 3 cycles with mainly anthracycline chemotherapy. Especially for those patients with a high WBC count, the chemotherapy should be increased from 3 to 4–5 cycles. Our data showed that the 10-year OS was 100%. Notably, increasing the number of chemotherapy cycles are beneficial for those high-risk patients such as the patients with extremely high WBC, and S or V type PML::RARA gene [31, 32].
In addition, based on the conclusion of the GIMEMA and PETHEMA clinical trials, patients in many studies were treated with identical induction (AIDA schedule) regimens. After completion of consolidation therapy, patients with PML::RARA negative disease were given oral mercaptopurine (6-MP), intramuscular methotrexate (MTX), and oral ATRA [23]. A large amount of published clinical data show that efficacy outcomes with ATRA plus arsenic as maintenance therapy are significantly better compared to outcomes using ATRA, 6-MP and MTX. The long-term RFS increased from 70 to 80% to 80–90% with the ATRA plus arsenic as maintenance therapy [11, 18, 27, 28]. According to the NCCN guideline and Chinese guidelines for diagnosis and treatment of APL, for the low-risk APL subgroup, the guideline-recommended regimen is retinoic acid ± arsenic or retinoic acid ± chemotherapy as induction therapy, followed by retinoic acid and arsenic for 0.5–2 years as maintenance chemotherapy. For the high-risk APL subgroup, the recommended regimen is a combination of two or three drugs and subsequent maintenance therapy also for 0.5–2 years [22, 26]. Currently, the 3-year or 5-year long-term RFS is about 80–100%, but longer term follow-up is still pending [22, 25, 33]. Our clinical data shows that extending the maintenance therapy with ATO/ As4S4 up to about 3 years can result in long-term RFS without increasing adverse effects. In our study, all 33 ND APL patients achieved a nearly 100% OS for more than 10 years. Although three of the patients have relapsed midway, two of them admitted that they stopped receiving maintenance oral As4S4 therapy for a period of time. These patients also achieved MRD (−) CR again after restarting the As4S4 and have remained relapse-free till now.
Because APL cells have pseudopodia and are highly migratory, they are prone to produce extramedullary lesions, especially CNSL [34, 35]. Our study confirmed that intensive chemotherapy, especially drugs that can cross the blood brain barrier (BBB), including arsenic [36], as well as IDA, NVT, Thiotepa and enhanced lumbar puncture chemotherapy, are helpful for APL patients to achieve CR and longer term remission [8, 37]. Extending the maintenance oral As4S4 therapy to 3 years can also increase the probability of achieving long-term RFS for the high-risk APL patients.
Since 2008, we have added gene mutation screening in the first BM tests. There is ample evidence that some gene mutations, such as FLT3-ITD, are poor prognostic factors for patients with AML [22, 38]. Meanwhile, some studies reported that gene mutations such as FLT3-ITD do not affect the prognosis and treatment of APL [39]. In our analysis, we found that gene mutations, particularly FLT3-ITD, did not affect the overall prognosis of APL patients.
The most commonly used arsenic therapy type is intravenous ATO. The side effects of ATO and the risk of sudden death have been reported in the early literature on the use of ATO [40,41,42]. In recent years, with the control of the dosage and purity of ATO, no serious toxicity has been reported. Our analysis from 2001 to 2008 also showed that ATO was associated with side effects, but no deaths occurred. Meanwhile, oral As4S4 is more convenient for patients and has fewer side effects than intravenous ATO (although there is no statistically significant difference). Most patients did not develop cardiac or hepatic toxicities or neutropenia [29, 30], [43]. Following induction and consolidation chemotherapy, patients could return to normal life without hospitalization. The more than 20-year long-term follow-up data showed the As4S4 treatment was safe without increasing the risk of secondary tumors [20, 29, 30, 33]. Notably, several female patients have given birth to healthy children following the treatment.
In conclusion, our 20-year retrospective study demonstrated that the triple therapy including ATRA combined with arsenic and anthracycline-based chemotherapy had excellent efficacy and safety for newly diagnosed APL patients. If the arsenic regimen was maintained for about 3 years, the long-term RFS of more than 10 years was nearly 100%. Moreover, oral As4S4 was safe and convenient for patients, and did not require hospitalization. However, because this was a relatively small study, it is necessary to accumulate more cases to provide convincing data on the efficacy outcomes of the triple therapy in APL patients.
Data Availability
All authors declare no competing financial interests.
References
Stone RM, Mayer RJ. The unique aspects of acute promyelocytic leukemia. J Clin Oncol. 1990;8:1913–21.
Warrell RP Jr, de The H, Wang ZY, Degos L. Acute promyelocytic leukemia. N Engl J Med. 1993;329:177–89.
Adès L, Guerci A, Raffoux E, Sanz M, Chevallier P, Lapusan S, et al. European APL Group. Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL Group experience. Blood. 2010;115(9):1690–6. https://doi.org/10.1182/blood-2009-07-233387.
Burnett AK, Russell NH, Hills RK, Bowen D, Kell J, Knapper S, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16(13):1295–305. https://doi.org/10.1016/S1470-2045(15)00193-X.
Abaza Y, Kantarjian H, Garcia-Manero G, Estey E, Borthakur G, Jabbour E, et al. Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood. 2017;129(10):1275–83. https://doi.org/10.1182/blood-2016-09-736686.
Platzbecker U, Avvisati G, Cicconi L, Thiede C, Paoloni F, Vignetti M, et al. Improved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapy in non-high-risk acute promyelocytic leukemia: final results of the randomized Italian-German APL0406 trial. J Clin Oncol. 2017;35(6):605–12. https://doi.org/10.1200/JCO.2016.67.1982.
Head D, Kopecky KJ, Weick J, Files JC, Ryan D, Foucar K, et al. Effect of aggressive daunomycin therapy on survival in acute promyelocytic leukemia. Blood. 1995;86(5):1717–28.
Avvisati G, Petti MC, Lo-Coco F, Vegna ML, Amadori S, Baccarani M, GIMEMA (Gruppo Italiano Malattie Ematologische dell’Adulto) Italian Cooperative Group, et al. Induction therapy with idarubicin alone significantly influences event-free survival duration in patients with newly diagnosed hypergranular acute promyelocytic leukemia: final results of the GIMEMA randomized study LAP 0389 with 7 years of minimal follow-up. Blood. 2002;100(9):3141–6. https://doi.org/10.1182/blood-2002-02-0352.
Sun GL, Huang YG, Chang XF. Clinical study of treatment with all-trans-retinoic acid in 544 cases of acute promyelocytic leukemia. Chin J Hematol. 1992;13:135–7.
Fenaux P, LeDeley MC, Castaigne S, Archimbaud E, Chomienne C, Link H, et al. Effect of all transretinoic acid in newly diagnosed acute promyelocytic leukemia. Results of a multicenter randomized trial. European APL 91 Group. Blood. 1993;82(11):3241–9.
Sanz MA, Vellenga E, Rayón C, Díaz-Mediavilla J, Rivas C, Amutio E, et al. All-trans retinoic acid and anthracycline monochemotherapy for the treatment of elderly patients with acute promyelocytic leukemia. Blood. 2004;104(12):3490–3. https://doi.org/10.1182/blood-2004-04-1642.
Fenaux P, Chastang C, Chevret S, Sanz M, Dombret H, Archimbaud E, et al. A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood. 1999;94(4):1192–200.
Burnett AK, Grimwade D, Solomon E, Wheatley K, Goldstone AH. Presenting white blood cell count and kinetics of molecular remission predict prognosis in acute promyelocytic leukemia treated with all-trans retinoic acid: result of the randomized MRC trial. Blood. 1999;93:4131–43.
Zhang P, Wang SY, Hu LH, Shi FD, Qiu FQ, Hong LJ, et al. Treatment of 72 cases of acute promyelocytic leukemia with intravenous arsenic trioxide. Chin J Hematol. 1996;17:58–62.
Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339(19):1341–8. https://doi.org/10.1056/NEJM199811053391901.
Niu C, Yan H, Yu T, Sun HP, Liu JX, Li XS, et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94(10):3315–24.
Hu J, Shen ZX, Sun GL, Chen SJ, Wang ZY, Chen Z. Long-term survival and prognostic study in acute promyelocytic leukemia treated with alltrans-retinoic acid, chemotherapy, and ATO: an experience of 120 patients at a single institution. Int J Hematol. 1999;70:248–60.
Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, Gruppo Italiano Malattie Ematologiche dell’Adulto; German-Austrian. Acute Myeloid Leukemia Study Group; Study Alliance Leukemia, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–21. https://doi.org/10.1056/NEJMoa1300874.
Compiling Committee of Pharmacopoeia of People’s Republic of China. Pharmacopoeia of People’s Republic of China, vol. 1. 95th ed. Guangzhou: Guangdong Press of Science and Technique; 1995. p. 298–9.
Lu DP, Qiu JY, Jiang B, Wang Q, Liu KY, Liu YR, et al. Tetra-arsenic tetra-sulfide for the treatment of acute promyelocytic leukemia: a pilot report. Blood. 2002;99(9):3136–43. https://doi.org/10.1182/blood.v99.9.3136.
Lu DP, Jiang B, Qiu JY. Acute promyelocytic leukemia treated by diarsenic trisulfide—the first case report. J Beijing Med Univ. 2000;32:256–7.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Acute Myeloid LeukemiaVersion 1.2021. https://www.nccn.org/guidelines/guidelines-process
Sanz MA, Lo Coco F, Martín G, Avvisati G, Rayón C, Barbui T, et al. Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96(4):1247–53.
Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017. https://www.meddra.org/. Accessed 27 Nov 2017.
Zhu HH, Huang XJ. Oral arsenic and retinoic acid for non-high-risk acute promyelocytic leukemia. N Engl J Med. 2014;371(23):2239–41. https://doi.org/10.1056/NEJMc1412035.
Chinese Society of Hematology, Chinese Medical Doctor Association; Chinese Medical Association, Chinese Medical Doctor Association. Chinese guidelines for diagnosis and treatment of acute promyelocytic leukemia (2018). Zhonghua Xue Ye Xue Za Zhi. 2018;39(3):179–83. https://doi.org/10.3760/cma.j.issn.0253-2727.2018.03.002. (Chinese).
Sanz MA, Montesinos P, Vellenga E, Rayón C, de la Serna J, Parody R, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans retinoic acid and anthracycline monochemotherapy: long-term outcome of the LPA 99 multicenter study by the PETHEMA Group. Blood. 2008;112(8):3130–4. https://doi.org/10.1182/blood-2008-05-159632.
Sanz MA, Montesinos P, Rayón C, Holowiecka A, de la Serna J, Milone G, PETHEMA and HOVON Groups, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome. Blood. 2010;115(25):5137–46. https://doi.org/10.1182/blood-2010-01-266007.
Lu DP, Wang Q. Current study of APL treatment in China. Int J Hematol. 2002;76(Suppl 1):316–8. https://doi.org/10.1007/BF03165273.
Tong Wu, Zhao J, Jiang B, Shu-Lan Wu, Yang P-D, Shan F-X, et al. Tetra-arsenic tetra-sulfide containing triple-agent regimen as the first line therapy for acute promyelocytic leukemia: expeditiously consecutive complete remission and improved disease-free survival. Blood. 2007;110(11):591. https://doi.org/10.1182/blood.V110.11.591.591.
Gallagher RE, Willman CL, Slack JL, Andersen JW, Li YP, Viswanatha D, et al. Association of PML-RAR alpha fusion mRNA type with pretreatment hematologic characteristics but not treatment outcome in acute promyelocytic leukemia: an intergroup molecular study. Blood. 1997;90(4):1656–63.
Slack JL, Willman CL, Andersen JW, Li YP, Viswanatha DS, Bloomfield CD, et al. Molecular analysis and clinical outcome of adult APL patients with the type V PML-RARalpha isoform: results from intergroup protocol 0129. Blood. 2000;95(2):398–403.
Zhu HH, Hu J, Lo-Coco F, Jin J. The simpler, the better: oral arsenic for acute promyelocytic leukemia. Blood. 2019;134(7):597–605. https://doi.org/10.1182/blood.2019000760.
Liso V, Specchia G, Pogliani EM, Palumbo G, Mininni D, Rossi V, et al. Extramedullary involvement in patients with acute promyelocytic leukemia: a report of seven cases. Cancer. 1998;83(8):1522–8.
Specchia G, Lo Coco F, Vignetti M, Avvisati G, Fazi P, Albano F, et al. Extramedullary involvement at relapse in acute promyelocytic leukemia patients treated or not with all-trans retinoic acid: a report by the Gruppo Italiano Malattie Ematologiche dell’Adulto. J Clin Oncol. 2001;19(20):4023–8. https://doi.org/10.1200/JCO.2001.19.20.4023.
Guo M, Zhao Q, Fan S, Wu Z, Lin L, Chen H, et al. Characteristics of arsenic species in cerebrospinal fluid (CSF) of acute promyelocytic leukaemia (APL) patients treated with arsenic trioxide plus mannitol. Br J Clin Pharmacol. 2021;87(10):4020–6. https://doi.org/10.1111/bcp.14804.
Mistry AR, Felix CA, Whitmarsh RJ, Mason A, Reiter A, Cassinat B, et al. DNA topoisomerase II in therapy-related acute promyelocytic leukemia. N Engl J Med. 2005;352(15):1529–38. https://doi.org/10.1056/NEJMoa042715.
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209–21. https://doi.org/10.1056/NEJMoa1516192.
Poiré X, Moser BK, Gallagher RE, Laumann K, Bloomfield CD, Powell BL, et al. Arsenic trioxide in front-line therapy of acute promyelocytic leukemia (C9710): prognostic significance of FLT3 mutations and complex karyotype. Leuk Lymphoma. 2014;55(7):1523–32. https://doi.org/10.3109/10428194.2013.842985.
Huang SY, Chang CS, Tang JL, Tien HF, Kuo TL, Huang SF, et al. Acute and chronic arsenic poisoning associated with treatment of acute promyelocytic leukaemia. Br J Haematol. 1998;103(4):1092–5. https://doi.org/10.1046/j.1365-2141.1998.01079.x.
Ohnishi K, Yoshida H, Shigeno K, Nakamura S, Fujisawa S, Naito K, et al. Prolongation of the QT interval and ventricular tachycardia in patients treated with arsenic trioxide for acute promyelocytic leukemia. Ann Intern Med. 2000;133(11):881–5. https://doi.org/10.7326/0003-4819-133-11-200012050-00012.
Westervelt P, Brown RA, Adkins DR, Khoury H, Curtin P, Hurd D, et al. Sudden death among patients with acute promyelocytic leukemia treated with arsenic trioxide. Blood. 2001;98(2):266–71. https://doi.org/10.1182/blood.v98.2.266.
Shen JC, Liu KY, Jiang B, Lu XJ, Lu DP. [Effect of the tetra-arsenic tetra-sulfide (As4S4) on the corrected QT interval in the treatment of acute promyelocytic leukemia]. Zhonghua Xue Ye Xue Za Zhi. 2004 Jun;25(6):359-61.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12185_2022_3507_MOESM1_ESM.docx
Supplementary file1.Table S1. Comparison of baseline characteristics of the APL patients treated with As4S4 (2001-2008), ATO(2001-2008) and As4S4 (2008-2018). Cytogenetics. Fusion genes. (DOCX 18 KB)
About this article
Cite this article
Zhang, X., Wu, S., Yang, J. et al. Long-term retrospective study of retinoic acid combined with arsenic and chemotherapy for acute promyelocytic leukemia. Int J Hematol 117, 530–537 (2023). https://doi.org/10.1007/s12185-022-03507-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-022-03507-5