Abstract
Nivolumab is an anti-programmed cell death protein 1 monoclonal antibody that exhibits significant efficacy in treating melanoma and other malignancies. However, various nivolumab-induced immune-related adverse events (irAEs) have been reported, and differentiating irAEs from tumor progression is sometimes difficult. Here, we report a case of reactive lymphadenopathy occurring after treatment with nivolumab. A 56-year-old man with stage IIIC melanoma received adjuvant therapy with nivolumab after wide local excision. He developed systemic lymphadenopathy and autoimmune hemolytic anemia 1 month after receiving seven cycles of nivolumab. Pathological analysis of a cervical lymph node biopsy specimen revealed no metastatic lesion or any other malignancy, including lymphoma. Thus, the patient was diagnosed with nivolumab-induced reactive lymphadenopathy. Systemic corticosteroids were administered to reduce hemolysis, which led to the resolution of lymphadenopathy. When progressive lymphadenopathy is observed in a patient who received immune checkpoint inhibitor therapy, reactive lymphadenopathy should be carefully distinguished from progression to lymphoid metastasis, and biopsy should be performed if needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune checkpoint inhibitors (ICIs) enhance antitumor immunity by targeting molecules that downregulate the T-cell responses. The development of ICIs has resulted in a paradigm shift in treating various malignancies, providing patients with clinical benefits [1]. While the indications of ICIs are expanding, T cells activated by ICIs can cause immune-related adverse events (irAEs), which affect different target organs and have various clinical manifestations, including different timing of onset, duration of symptoms and severity [2]. With the widespread use of ICIs and increased incidence of irAE, it has become more difficult to determine whether the new-onset symptoms reflect disease progression or irAEs [3]. If an irAE is misdiagnosed as cancer progression, ICI may be discontinued, which may adversely affect the patient’s prognosis.
Nivolumab, a type of ICI, is an anti-programmed cell death-1 monoclonal antibody [4]. Although various irAEs have been reported with nivolumab use, to the best of our knowledge, no previous report has documented nivolumab-induced systemic lymphadenopathy.
Here, we report a case of irAE appearing as systemic lymphadenopathy after administration of seven cycles of nivolumab for stage III malignant melanoma.
Case Report
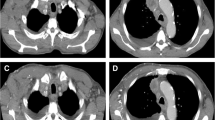
A 56-year-old man was admitted to our hospital with a black mass on the left side of his chest. Pathological analysis of the mass confirmed the diagnosis of malignant melanoma, and the tumor was classified as pT3bN1aM0 Stage IIIC according to AJCC 8th edition. He underwent left chest wall tumor resection and left axillary lymphadenectomy. Subsequently, he received nivolumab (240 mg/body every 2 weeks) as postoperative adjuvant therapy. After seven cycles of nivolumab, head computed tomography (CT) revealed a nodule in the right frontal lobe, suggesting brain metastasis of malignant melanoma. Chest and abdominal CT showed enlarged para-aortic and mesenteric lymph nodes compared to their appearance four months earlier, which was not considered to be lymphadenopathy because their maximum diameter was less than 10 mm. Nivolumab was discontinued, because the CT findings suggest relapse and brain metastasis of malignant melanoma. Instead, we started treatment with stereotactic radiation therapy for head lesions and introduced dabrafenib plus trametinib under BRAF V600K mutation. One month after discontinuation of nivolumab and initiation of dabrafenib and trametinib, he presented with generalized malaise, abdominal distention, polyarthralgia, and liver dysfunction. He showed no symptoms of fever or chills. He had no cutaneous lesions, uveitis, or granulomatous infiltration of scars associated with drug-induced sarcoidosis. Although both dabrafenib and trametinib were discontinued, his symptoms and liver dysfunction worsened. Laboratory investigations performed after discontinuation of dabrafenib and trametinib are shown in Table 1. Results of hematologic examination are as follows: white blood cell count 5,740 cells/mm3, platelet count 139,000 cells/mm3, hemoglobin 6.6 g/dl, and reticulocytes 911,800 cells/mm3. Results of biochemical tests are as follows: aspartate aminotransferase, 79 U/L; alanine aminotransferase, 111 U/L; gamma-glutamyl transpeptidase, 119 U/L; alkaline phosphatase, 1359 U/L; total bilirubin, 2.2 mg/dL, direct bilirubin, 1.4 mg/dL, lactate dehydrogenase, 492 U/L, and haptoglobin < 3 mg/dL. Immunological examination revealed a positive direct antiglobulin test (DAT), cold agglutinin titer of 1:128, and soluble interleukin-2 receptor level of 5586 U/mL. Cytomegalovirus (CMV) IgG antibody titer was positive, CMV IgM antibody titer was negative, suggesting the previous infection. Epstein-Barr virus (EBV) IgG antibody to viral capsid antigen (VCA-IgG) was positive, VCA-IgM was negative, EBV nuclear antigen antibody was positive, suggesting the previous infection. The human immunodeficiency virus was negative on screening testing. The presence of anemia, reticulocytosis, low haptoglobin, and positive DAT was suggestive of autoimmune hemolytic anemia (AIHA). Furthermore, evaluated direct bilirubin and serum alkaline phosphatase suggested complications of hepatobiliary disorders. Abdominal ultrasonography showed that the spleen was enlarged to the longest diameter of 14.4 cm. Positron emission tomography-CT scans revealed systemic lymphadenopathy in the cervical (12 mm, SUVmax 7.4), axillary (12 mm, SUVmax 7.7), hepatic (27 mm, SUVmax 17.4), para-aortic (14 mm, SUVmax 13.6), mesenteric (21 mm, SUVmax 12.4), external iliac (28 mm, SUVmax 14.8), and inguinal (16 mm, SUVmax 4.5) regions with abnormal fluorodeoxyglucose uptake in each region (Fig. 1A–C). Since we suspected lymphoma and metastasis of malignant melanoma, we performed a biopsy from the right cervical lymph node (Fig. 1D). The lymph node showed a preserved architecture with reactive follicular hyperplasia and no granulomatous lesions. Immunohistochemical staining showed that the cells were negative for Melan-A and HMB45, suggesting that there was no melanoma invasion. The germinal centers were CD20 ( +), CD10 ( +), BCL-6 ( +), and BCL-2 (–), suggesting reactive lymphoid hyperplasia and ruling out lymphoma. Thus, we established the diagnosis of benign reactive lymphoid hyperplasia with no evidence of malignant melanoma or lymphoma. We also performed a bone marrow biopsy to find the cause of AIHA. Bone marrow smear revealed cellular hyperplasia and erythroid predominance with no abnormal cells. Flow cytometric and karyotype analyses revealed no abnormalities, and there was no Ig and TCR gene rearrangement. Thus, we concluded that AIHA was caused by nivolumab and was not associated with lymphoproliferative disease Fig 2.
Radiological findings. PET-CT and CT revealed systemic lymphadenopathy when systemic symptoms appeared (A, B, and C). We performed a biopsy from the right cervical lymph node (D). Lymphadenopathy disappeared completely after treatment with corticosteroids (E). CT Computed tomography, PET Positron emission tomography
Pathological finding. Cervical lymph node biopsy. A Hematoxylin and eosin staining; the lymph node shows preserved architecture. B, C, D, E, and F depict immunohistochemistry for CD3, CD20, CD10, BCL-6, and BCL-2, respectively; B and T-cells appear to have a normal distribution, and the germinal center is positive for CD20, CD10, and BCL-6, and negative for BCL-2. Bar, 500 μm
He received corticosteroids (prednisone, 1 mg/kg per day) for AIHA treatment. Remarkable improvement in symptoms, including lymphadenopathy, was observed immediately after steroid therapy initiation. The arthralgia, liver damage and hemolysis improved, and the anemia did not progress again after the first transfusion. Chest and abdominal CT performed 1 week after administration of corticosteroids showed a reduction in the size of the systemic lymph nodes (Fig. 1E). Corticosteroids were promptly tapered and discontinued.
After the symptoms subsided and corticosteroid therapy was discontinued, he was re-administered dabrafenib and trametinib to treat the malignant melanoma relapse. One year after re-administration of dabrafenib and trametinib, CT findings revealed that the lymph nodes continued to shrink.
Discussion
We experienced a case of nivolumab-induced systemic lymphadenopathy, which responded well to corticosteroid therapy.
Clinical implication of the case
Treatment with ICIs has improved the prognosis of various malignancies. IrAEs are drug reactions unique to ICIs and caused by excessive activation of the autoimmune responses. They can affect any organ system, including the skin, gastrointestinal tract, lungs, thyroid, adrenal, pituitary glands, musculoskeletal, renal, nervous, hematologic, cardiovascular, and ocular systems [5].
ICI-induced lymphadenopathy has been reported in several cases. Among them, we found 12 previously reported cases of nivolumab-induced lymphadenopathy [6,7,8,9,10]. The median age of the patients was 53.5 years (range 32–71 years). All patients had a single enlarged lymph node localized to the mediastinal and/or hilar region in 11 patients and the cervical region in one patient. Eleven of these 12 patients developed 1 of the symptoms of drug-induced sarcoidosis-like reactions (DISR), but there were no findings suggestive of DISR in this case. Among reports of irAEs due to other ICIs, two cases were reported as ICI-related systemic lymphadenopathy, occurring in a 41-year-old man and a 37-year-old woman with melanoma who were treated with ipilimumab and pembrolizumab, respectively [11].
Our patient showed systemic lymphadenopathy after adjuvant therapy with nivolumab. We initially suspected metastasis of melanoma or the occurrence of a concurrent malignancy, including lymphoma. However, pathological findings revealed that the lymph node was not malignant rather, it showed reactive lymphoid hyperplasia. The patient also developed other symptoms such as arthritis, liver dysfunction, splenomegaly, and AIHA, which were already reported as irAEs. We concluded that this systemic lymphadenopathy was a nivolumab-induced irAE, based on the fact that this occurred simultaneously as the other irAE-like symptoms described above and the patient clinical course improved quickly with corticosteroids and did not relapse. To our knowledge, this is the first case of nivolumab-induced systemic lymphadenopathy. It should be kept in mind that besides ipilimumab or pembrolizumab, nivolumab may also cause ICI-related systemic lymphadenopathy. It is also essential to elucidate the cause of lymphadenopathy, because the appropriate treatment can vary completely depending on the diagnosis. Regarding PET-CT results, the SUV max of the enlarged lymph nodes ranged from 7.4 to 17.4 in our case and 8–16.2 in previous cases, which was high enough to suspect malignancies and suggested that PET-CT alone was not sufficient for differential diagnosis [11,12,13,14]. Thus, a thorough investigation, including biopsy, should be performed when patients present with lymphadenopathy during or after treatment with ICIs instead of assuming that cancer progression is causing lymphadenopathy.
Possible mechanism
Lymphadenopathy refers to abnormal consistency or enlargement of lymph nodes (usually greater than 1 cm in size) [15]. The primary function of lymph nodes is to filter for foreign antigens. There are two mechanisms of lymphadenopathy in general: hyperplasia and infiltration. Hyperplasia occurs in response to immunologic or infectious stimuli, and infiltration occurs when various cells, including cancer cells, invade the body [16]. Although the precise mechanisms of irAEs have not been fully elucidated, they are thought to occur due to pre-existing tolerated self-reactive T-cells that are deregulated in the periphery and/or immune cells that have cross-reactivity between the target of an individual patient’s antitumor immune response and normal tissues [17, 18]. In our case, lymphadenopathy might have occurred due to lymphoid hyperplasia in response to immunologic stimuli induced by nivolumab.
Conclusion
We reported the first case of systematic lymphadenopathy caused by nivolumab. Hematologists need to be aware of this rare event for the differential diagnosis of systematic lymphadenopathy. Our case also emphasizes the need to confirm the underlying etiology of lymphadenopathy that develops during treatment with ICIs by performing lymph node biopsy rather than image finding alone. Assuming that the patient has disease progression based on radiologic images alone may result in unnecessary changes to the treatment plan.
Availability of data and materials
All data analyzed during this study are included in this manuscript.
References
von Itzstein MS, Khan S, Gerber DE. Investigational biomarkers for checkpoint inhibitor immune-related adverse event prediction and diagnosis. Clin Chem. 2020;66:779–93. https://doi.org/10.1093/clinchem/hvaa081.
Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28:2377–85. https://doi.org/10.1093/annonc/mdx286.
Wu CE, Yang CK, Peng MT, Huang PW, Chang CF, Yeh KY, et al. The association between immune-related adverse events and survival outcomes in Asian patients with advanced melanoma receiving anti-PD-1 antibodies. BMC Cancer. 2020;20:1018. https://doi.org/10.1186/s12885-020-07508-7.
Patrinely JR Jr, Johnson R, Lawless AR, Bhave P, Sawyers A, Dimitrova M, et al. Chronic immune-related adverse events following adjuvant anti–PD-1 therapy for high-risk resected melanoma. JAMA Oncol. 2021;7:744–8. https://doi.org/10.1001/jamaoncol.2021.0051.
Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–68. https://doi.org/10.1200/JCO.2017.77.6385.
Chorti E, Kanaki T, Zimmer L, Hadaschik E, Ugurel S, Gratsias E, et al. Drug-induced sarcoidosis-like reaction in adjuvant immunotherapy: increased rate and mimicker of metastasis. Eur J Cancer. 2020;131:18–26. https://doi.org/10.1016/j.ejca.2020.02.024.
Taira K, Kimura A, Nakata A, Nadatani Y, Fukunaga S, Otani K, et al. (2021) A case of nivolumab-induced cervical lymphadenopathy in a patient with gastric cancer. J Gastrointest Oncol 12:880–4. https://doi.org/10.21037/jgo-20-315
Danlos FX, Pagès C, Baroudjian B, Vercellino L, Battistella M, Mimoun M, et al. Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest. 2016;149:e133–6. https://doi.org/10.1016/j.chest.2015.10.082.
Montaudié H, Pradelli J, Passeron T, Lacour JP, Leroy S. Pulmonary sarcoid-like granulomatosis induced by nivolumab. Br J Dermatol. 2017;176:1060–3. https://doi.org/10.1111/bjd.14808.
Kim C, Gao J, Shannon VR, Siefker-Radtke A. Systemic sarcoidosis first manifesting in a tattoo in the setting of immune checkpoint inhibition. BMJ Case Rep. 2016. https://doi.org/10.1136/bcr-2016-216217.
Firwana B, Ravilla R, Raval M, Hutchins L, Mahmoud F. Sarcoidosis-like syndrome and lymphadenopathy due to checkpoint inhibitors. J Oncol Pharm Pract. 2017;23:620–4. https://doi.org/10.1177/1078155216667635.
González-Cruz C, Bodet D, Muñoz-Couselo E, García-Patos V. Mediastinal FDG-positive lymph nodes simulating melanoma progression: drug-induced sarcoidosis like/lymphadenopathy related to ipilimumab. BMJ Case Rep. 2021;14: e237310. https://doi.org/10.1136/bcr-2020-237310.
Cha J, Kim S, Wang J, Yun M, Cho A. Evaluation of 18F-FDG PET/CT parameters for detection of lymph node metastasis in cutaneous melanoma. Nucl Med Mol Imaging. 2018;52:39–45. https://doi.org/10.1007/s13139-017-0495-4.
Barrington SF, Mikhaeel NG. When should FDG-PET be used in the modern management of lymphoma? Br J Haematol. 2014;164:315–28. https://doi.org/10.1111/bjh.12601.
Gaddey HL, Riegel AM. Unexplained lymphadenopathy: evaluation and differential diagnosis. Am Fam Physician. 2016;94:896–903.
Verma R, Khera S. Cervical lymphadenopathy: a review. Int J Health Sci Res. 2020;10:292–8.
Yoest JM. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: a short review. ImmunoTargets Ther. 2017;6:73–82. https://doi.org/10.2147/ITT.S126227.
Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–55. https://doi.org/10.1056/NEJMoa1609214.
Acknowledgements
The authors thank the medical and nursing staff at the Osaka University Hospital.
Funding
The authors received no funding. The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
All authors have read the final version of this manuscript and agree to the content of the manuscript and its submission. TK and YS wrote the manuscript. KF, JF, AH, AT, and HK contributed to the diagnosis and treatment. MK performed the pathological examination.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial and non-financial interests.
Ethics approval and consent to participate
Publication of this case report does not require approval from the institutional review board of our hospital. Written informed consent was obtained from the patient.
Consent for publication
Written informed consent for publication was obtained from the patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kubo, T., Hino, A., Fukushima, K. et al. Nivolumab-induced systemic lymphadenopathy occurring during treatment of malignant melanoma: a case report. Int J Hematol 116, 302–306 (2022). https://doi.org/10.1007/s12185-022-03312-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-022-03312-0