Abstract
Post-transplant cytomegalovirus (CMV) disease can be almost completely avoided by current infection control procedures. However, CMV reactivation occurs in more than half of patients, and some patients can develop clinically resistant CMV infections. Whether resistance is due to the host’s immune status or a viral resistance mutation is challenging to confirm. Therefore, a prospective observational analysis of refractory CMV infection was conducted in 199 consecutive patients who received allogeneic hematopoietic stem cell transplantation at a single institution. Among them, 143 (72%) patients received anti-CMV drugs due to CMV reactivation, and only 17 (8.5%) exhibited refractory CMV infection. These patients had clinically refractory infection. However, viral genome analysis revealed that only one patient exhibited a mutation associated with the anti-CMV drug resistance. Clinical resistance was mainly correlated with host immune factors, and the incidence of resistance caused by gene mutations was low at the early stage after a transplantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cytomegalovirus (CMV) infection is a major complication of allogeneic hematopoietic stem cell transplantation (allo-SCT). Although there were advancements in the diagnosis and management of CMV disease, it is still considered a significant cause of morbidity and mortality after allo-SCT [1,2,3,4,5]. The incidence of CMV end-organ disease within 100 days after allo-SCT decreased by less than 10% with the early detection of CMV reactivation via CMV antigenemia or PCR assay and the use of preemptive antiviral therapy with ganciclovir (GCV), valganciclovir (VGCV), or foscarnet (FOS) [6,7,8,9,10]. However, up to 60% of patients develop CMV reactivation after allo-SCT. More recently, prophylaxis with letermovir (LTV) successfully decreased the risk of clinically significant CMV infection by about 40% until 24 weeks after allo-SCT [10]. Early CMV reactivation even without CMV disease is associated with a lower overall survival and higher non-relapse mortality [11, 12].
Refractory CMV infection rarely occurs in allo-HSCT recipients [1, 3, 4, 13,14,15]. However, due to the recent increase in the frequency of transplantation with profound immunosuppressive conditioning regimens including antithymocyte globulin or alemtuzumab [16,17,18], and the diversification of stem cell sources [19, 20], patients commonly develop refractory viral infections. Refractory CMV infection is mainly attributed to the host’s immunosuppressive status and is distinguished from drug resistance based on gene mutations. Previous reports showed that the risk factors for drug resistance included prolonged and repeated anti-CMV treatment. Antiviral drug resistance is suspected if the CMV viral load does not improve after 2 weeks of adequate antiviral therapy and if CMV end-organ disease occurs after more than 6 weeks of treatment [1, 3, 4, 13, 14].
Anti-CMV drug resistance is often caused by mutations in the CMV UL97 and UL54 genes. GCV or VGCV must be activated via phosphorylation with CMV phosphotransferase UL97 and inhibition of viral DNA polymerase UL54. By contrast, FOS and cidofovir (CDV) do not require phosphorylation. Thus, these agents directly inhibit viral DNA polymerase [21, 22]. These four anti-CMV drugs target the UL54 polymerase. Therefore, in CMV, several mutations in the UL54 gene are associated with drug resistance. In addition, the activation of GCV and VGCV requires UL97. Hence, some UL97 mutations cause resistance to drugs such as GCV and VGCV [14, 21]. By contrast, LTV inhibits CMV replication by binding to the components of the CMV terminase complex including UL51 and UL56. Thus, these mutations can induce drug resistance to LTV.
Several studies have shown drug-resistance mutations in the UL97 and UL54 genes particularly among patients with AIDS [23, 24] and solid-organ transplant (SOT) recipients [25, 26]. By contrast, there have been only several studies about resistance to antiviral drug against CMV in allo-SCT recipients [27,28,29,30,31,32]. Most of these analyses have included pediatric patients or were retrospective studies, and there have been only two reports analyzing antiviral resistance based on genetic testing prospectively in adult patients. Thus, the current study prospectively performed monitoring of the number of CMV pp65 antigen-positive (Ag+) cells and responses to anti-CMV drugs in 199 consecutive allo-SCT recipients in our institution. The DNA sequences of the UL54 and UL97 genes in patients with refractory CMV infection after transplantation were evaluated. Moreover, the actual incidence of virological resistance in this group of patients was validated.
Patients and methods
Patients
This prospective observational study was performed between January 2012 and December 2016. It was approved by the institutional review board of Kyushu University Hospital. All the consecutive adult patients who received allo-SCT at Kyushu University Hospital were included in the analysis. Patients underwent pp65 CMV antigenemia assay weekly from the time of neutrophil engraftment after allo-SCT [33, 34]. Then, they were followed up until 1 year after transplantation. Acyclovir was given for herpes simplex prophylaxis up to 35 days after transplantation, and thereafter, in some patients, low-dose acyclovir was given for prophylaxis against varicella-zoster virus. CMV infection was defined as a positive CMV antigenemia assay result, and CMV disease was diagnosed according to the published recommendations [3, 35]. Preemptive therapy for CMV was generally initiated when at least two CMV pp65 Ag+ cells per 50,000 white blood cells were detected. After viral therapy was started, the therapeutic efficacy of antiviral drugs was continually evaluated. Refractory CMV infection or clinical resistance indicates that the blood or plasma viral load, by PCR or the number of CMV Ag+ cells by antigenemia assay, increases or persists despite the appropriate antiviral therapy. In this study, the refractory CMV infection was defined as an increased number of CMV Ag+ cells after at least 2 weeks of appropriate anti-CMV therapy or persistent positive CMV antigenemia assay result after 6 weeks (including at least 2 weeks of appropriate antiviral therapy) [13]. On the other hand, drug resistance or virological resistance refers to the detection of genetic mutations that are confirmed to be drug resistant by phenotypic testing [13, 14]. There are two types of methods for diagnosing antiviral drug resistance: the classic phenotypic plaque reduction assay (PRA) and genotypic analysis [13, 14]. Although the PRA is the gold-standard method, it is time-consuming. Viral mutations have been intensively identified and most of them were linked to drug susceptibility or resistant phenotype, so genotypic assay is commonly used nowadays. Thus, genotypic assay was used to diagnose antiviral resistance in this study. Practically, the peripheral blood samples were collected from patients once the definition of refractory CMV infection was met, and the DNA sequences of the CMV UL54 and UL97 genes were analyzed to examine genetic mutations associated with drug resistance.
DNA sequence of the CMV, UL54, and UL97 genes
Viral DNA was extracted from the peripheral blood plasma using the NucleoSpin Virus Kit (Macherey‐Nagel, Düren, Germany). Four primer sets were designed to amplify the regions of mutation hotspot of drug resistance-covering codon 253-1021 in the UL54 gene (Supplementary Fig. 1, Supplementary Table 1). Moreover, the UL97 primer sets were designed to amplify codon 324-658 in the UL97 gene (Supplemental Figure, Supplemental Table). Mutation hotspots were included in this lesion, and all the regions were amplified via PCR using KOD FX Neo DNA polymerase (Toyobo, Osaka, Japan). Each PCR reaction contained a 50 μl mixture of the following: 25 μl PCR buffer for KOD FX Neo, 10 μl dNTPs, 13 μl template DNA, 0.5 μl of each primer (final: 0.25 μM), and 1 μl KOD FX Neo. Each DNA fragment was amplified using a PCR thermal cycler (Gene Atlas, the USA) with the following thermal cycling schedule: the first cycle consisted of 2 min at 94 °C, followed by 35 cycles of 10 s at 98 °C, 30 s at each temperature for annealing, 30 s at 68 °C, and a final cycle of 10 min at 68 °C. The reaction mixture was then cooled at 4 °C for 5 min. PCR products were purified with the Amicon Ultra Purification Kit (Merck, Millipore). Amplicons were sequenced in both forward and reverse directions with the same primers used for amplification using the ABI Prism BigDye v.1.1 terminator cycle sequencing kit (Life Technologies, Carlsbad, CA) per manufacturer’s directions on the Applied Biosystems 3130XL DNA analyzer. The sequence was analyzed using the Applied Biosystems SeqScape software. DNA sequences were analyzed with the Genetyx software (Genetyx, Tokyo, Japan) and were compared with that of the laboratory Towne and AD169 strain [14].
End points
The primary end point was the frequency of virological resistance, which was defined by genetic analysis. The secondary end point included the frequency of refractory CMV infection after allo-SCT and the risk factors for refractory CMV infection.
Statistical analysis
Using the Chi-square test, a univariate analysis was performed to examine categorical variables including age, sex, primary diseases, disease status at allo-SCT, conditioning regimen, graft source, donor type, prior HSCT, CMV serostatus, maximum numbers of CMV pp65 Ag+ cells, acute GVHD, and usage of corticosteroids. CMV serostatus of cord blood was treated as CMV seronegative. A P value of < 0.05 was considered statistically significant. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University), a graphic user interface for R (version 3.2.4; The R Foundation for Statistical Computing, Vienna, Austria) [36].
Results
Incidence of refractory CMV infection
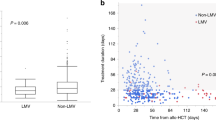
Initially, 199 patients who received allo-SCT were enrolled in our study. Early mortality, defined as death occurring before neutrophil engraftment, was observed in eight patients. Thirty-six patients did not develop CMV antigenemia during the clinical course and until 1 year after transplantation. Moreover, 155 patients had positive CMV antigenemia test results at least once at a median of 34 (range 9–97) days after transplantation. The median maximum number of CMV Ag+ cells per 50,000 leukocytes was 5 (range 1–332) during the follow-up period. In total, 12 experienced spontaneous remission of CMV reactivation without antiviral therapy, and 143 received preemptive therapy with antiviral agents including GCV, VGCV, and FOS. Further, 17 patients [8.5% of all patients (n = 199) and 11.9% of all patients who received preemptive treatment (n = 143)], met the criteria on refractory CMV infection, as described above (Fig. 1).
Clinical findings of patients with refractory CMV infection
Table 1 depicts the clinical features of 143 patients who received anti-CMV drugs. There was no correlation between refractory CMV infection and pre-transplantation characteristics, including age, sex, disease status at transplantation, and intensity of conditioning. Moreover, the incidence of refractory CMV infection was not affected by stem cell sources, donor type, prior history of transplantation, or CMV serostatus, which are the determinants of CMV reactivation [1, 37]. However, because of the limited sample size and the lack of multivariate analysis, it is difficult to conclude that these factors did not influence refractory CMV infection in this study.
By contrast, patients with resistance to anti-CMV drugs and those without differed in terms of post-transplant factors. The development of grade II to IV acute GVHD did not affect responses to anti-CMV drugs. However, the administration of corticosteroids as prophylaxis or treatment for acute GVHD was associated with an increased incidence of refractory CMV infection (P = 0.0189). As expected, the maximum number of CMV Ag+ cells before the first genetic tests in patients with refractory CMV infection was high (median 58 per 50,000 leukocytes; range 18–423) compared to the other patients, where the maximum number of CMV Ag+ cells was less than 20 per 50,000 leukocytes in 88% of patients.
Low incidence of actual antiviral drug resistance even in recipients with refractory CMV infection
Figure 2 and Table 2 depict the characteristic of patients with refractory CMV infection. As described above, the recipients had a high number of CMV Ag+ cells (median 58, range 18–227 Ag+ cells), and 12 (71%) of 17 recipients received corticosteroids. In recipients UPN1, UPN4, UPN5, UPN7, UPN10, UPN13, UPN14, UPN15, and UPN16, the number of CMV Ag+ cells increased after more than 2 weeks of anti-CMV therapy. However, eventually, CMV reactivation improved in these recipients without the need to change antiviral agents. In recipients UPN2, UPN3, UPN6, UPN8, UPN9, and UPN12, treatment with antivirals was modified because the number of CMV Ag+ cells increased after more than 2 weeks or persistent viremia for more than 6 weeks after the initial treatment. However, CMV reactivation improved in these recipients after therapy modification. Recipient UPN17 received FOS and GCV for 20 weeks or more. However, CMV retinitis developed, and the number of CMV Ag+ cells increased even after treatment with FOS. The details of recipient UPN17 are described below.
An analysis of viral DNA revealed that 17 patients with refractory CMV infection had five common amino acid substitutions (V355A, N685S, A688V, A885T, and N898D) in the UL54 gene and one common substitution (D605E) in the UL97 gene. In addition to this set of substitutions, A336V (n = 8), D711E (n = 1), V781I (n = 1), and L897S (n = 1) were detected in the UL54 gene. However, there were no other substitutions in the UL97 gene.
Most substitutions in the UL54 gene and D605E in the UL97 gene were considered drug-sensitive [14]. Therefore, these mutations were not associated with refractory infection in these patients. Few reports showed an association between D711E in the UL54 gene and drug resistance [28]. Nevertheless, this substitution was also found in the sample of patients who did not exhibit persistent antigenemia. Therefore, it was not considered a drug-resistant substitution. V781I in the UL54 gene was found in the sample of recipient UPN17 and was considered drug-resistant substitution [14]. Thus, the incidence rate of refractory CMV infection with drug-resistant substitution was only 5.9% in patients with refractory CMV infection and 0.50% in all patients.
Prolonged and insufficient antiviral treatment might cause drug resistance
Figure 3A shows the clinical course of recipient UPN17 who exhibited actual antiviral drug resistance. The patient was a 45-year-old man with diffuse large B cell lymphoma. He received allo-SCT from an HLA one-locus mismatched unrelated donor for relapsed and refractory disease after autologous SCT. The conditioning regimen comprised fludarabine, melphalan, and antithymocyte globulin. Prophylaxis for acute GVHD comprised short-term methotrexate and tacrolimus. The patient developed grade II acute GVHD at day 24 after transplantation, which subsequently resolved without the administration of corticosteroids. The patient exhibited CMV colitis at day 32 after transplantation. Hence, he received full-dose GCV for 4 weeks. Then, the symptoms improved, and CMV Ag+ cells disappeared. After 2 weeks, CMV Ag+ cells were detected again. Since the patient experienced prolonged neutropenia, FOS was used as the second-line treatment. However, drugs at optimal doses could not be administered because of renal dysfunction, and then, CMV Ag+ cells repeatedly turned negative and positive with treatment with antiviral drugs at suboptimal doses. The patient developed extensive chronic GVHD around day 120 after transplantation and low-dose prednisolone were added. After 15 weeks of antiviral treatment, the number of CMV Ag+ cells increased despite being under FOS treatment, and viral DNA analysis was performed on day 255 after transplantation. The cumulative duration of antiviral treatment including both of GCV and FOS until first genetic test was 155 days, which is extremely long compared to that in patients (UPN1-16) who had refractory CMV infection but without genetic mutations (median 27 days; range 18–68 days). Wild-type and mutant sequences coexisted at codon 781 (V781V/I) of the UL54 gene (Fig. 3B). V781I was associated with FOS resistance and reduced susceptibility to GCV although the results of the susceptibility to GCV vary among reports [13, 14, 38, 39]. Since the number of CMV Ag+ cells gradually decreased and neutropenia persisted, FOS treatment was continued. However, the patient developed CMV retinitis, and the number of CMV Ag+ cells increased. Therefore, a viral DNA analysis was performed again on day 320 after transplantation. The mutant V781I became dominant, and this mutation was found to be associated with FOS resistant. Thus, treatment with anti-CMV drug was changed to GCV, and the number of CMV Ag+ cells gradually decreased with G-CSF support. Unfortunately, the patient died of sepsis caused by Candida krusei at day 835 after transplantation.
Detailed clinical course of recipients exhibiting refractory CMV infection and genetic resistance to anti-CMV agents. Clinical course, treatment for CMV and GVHD, viral load of CMV, and CMV end-organ disease in recipient UPN17. DNA sequences of CMV-UL54 in recipient UPN17. (Left) Sequences on day 255. Mix of wild-type (GTT = Val) and mutant (ATT = Ile) sequences. (Right) Sequences on day 320. Drug-resistant mutant virus became dominant
Discussion
We performed a prospective observational analysis of refractory CMV infection in 199 consecutive allo-SCT patients at a single institution. Among them, 143 (72%) received anti-CMV drugs due to CMV reactivation. However, only 17 (8.5%) patients exhibited refractory CMV infection. However, an analysis of viral genome showed that only one patient exhibited mutation associated with anti-CMV drug resistance. Thus, the refractory CMV infection is mainly associated with host immune factor. Of note, 5 (29%) of 17 patients with refractory CMV infection experienced progression to CMV end-organ disease. Furthermore, the mortality in this population was significantly higher than that in the whole cohort. Although most patients did not exhibit viral mutations associated with drug resistance, they are at high risk for CMV end-organ disease. The patients who had refractory CMV infection should be cautiously monitored to validate the absence of late-onset CMV end-organ disease.
The frequency of virological resistance with genetic mutations in allo-SCT recipients ranged from 1.7 to 7.7% [27,28,29,30,31,32]. A prospective study of a French group revealed that clinical resistance was suspected in 4 (6.8%) of 59 allo-SCT recipients, and only one (1.7%) had virological resistance [32]. This result is well matched with ours. Another prospective study from Israeli group included 410 allo-SCT patients over 5 years. In this study, clinical resistance was observed in 20 patients (4.9%) and of those, drug-resistant mutations were detected in 10 patients (2.4%). However, different from our results, clinical and virological resistances were exclusively identified in haploidentical donor-SCT patients. Our cohort included 36 haploidentical donor transplants, but the incidence of refractory CMV infection (11%) was not significantly different from those in patients transplanted with other stem cell sources (14%), and no virological resistance was identified in haploidentical donor-SCT patients. The reason for this discrepancy is unknown, but they showed that prolonged antiviral treatment and higher preceding viral load were risk factors for virological resistance, and this is consistent with our results although there was only 1 patient with virological resistance in our study. The other studies have performed a retrospective analysis of a small number of cases, and some have included pediatric patients, and the frequency of virological resistance was also low in these studies. By contrast, the incidence of genetically proven resistant virus was quite high in patients with AIDS [23, 24] or SOT recipients [25, 40] compared with allo-SCT patients. One possible reason is that the number of T cells may progressively decrease in patients with AIDS. Moreover, SOT recipients receive long-term treatment with calcineurin inhibitors.
The current study aimed to assess clinical factors affecting drug resistance. However, a statistical analysis was challenging to perform because only one patient exhibited genetic resistance. Previous reports have shown that the risk factors for drug resistance included prolonged antiviral therapy particularly with anti-CMV drugs at suboptimal doses, and profound immunosuppression was not sufficient to inhibit viral replication [3, 13, 24, 25, 32]. Indeed, recipient UPN17 received prolonged treatment (> 20 cumulative weeks). However, the dose of antiviral agents was not optimal. Thus, this patient was at a high risk for drug resistance.
This study showed that virological resistance was rare even in patients with refractory CMV infection. There is no consensus on when genotype assay should be performed. We defined refractory CMV infection as increased number of CMV Ag+ cells after at least of 2 weeks of appropriate anti-CMV therapy, or persistent viremia even after 6 weeks (including at least 2 weeks of appropriate antiviral therapy). Since persistent CMV infection was not observed outside this population, the definition can be used as the initial criterion for suspected drug resistance. Furthermore, if the antiviral drug administration period is prolonged due to repeated flare-ups of viremia, or if CMV end-organ disease develops during treatment with antiviral drugs, genetic analysis should be performed, and a repeat examination must be conducted after a single negative test result. In our case, the virus with a drug-resistant mutation was initially minor. However, it became dominant over time, thereby causing clinically refractory disease. When the patient exhibited clinically refractory infection and drug-resistant mutations are detected, treatment should be promptly switched to another non-cross resistant drug, thereby preventing the continuous administration of ineffective and unnecessary drugs. Moreover, the development of CMV end-organ disease can be prevented. However, multidrug-resistance mutations are still challenging to manage with alternative treatments. CMV treatment strategies after transplantation have shifted from the conventional preemptive treatment to preventive therapy with the induction of LTV. Therefore, the number of patients who require preemptive therapy will decrease in the future. However, even with LTV, clinically significant CMV infection was detected in about 20% of patients 24 weeks after transplantation. Therefore, CMV monitoring after transplantation is essential [10]. In the future, it is necessary to perform an analysis of LTV-resistant mutations.
The current study had several limitations. First, the standard quantitative CMV PCR assay was not performed because only the CMV antigenemia test is widely used for CMV monitoring and is reimbursed by the National Health Insurance in Japan. The CMV antigenemia assay has a slightly lower sensitivity for detecting CMV reactivation than the quantitative PCR assay [3, 4, 41]. Although the PCR assay may detect more persistent infections, the incidence of refractory CMV infection was not likely underestimated with the antigenemia assay because such cases involve a high viral load. Second, mutations were detected using the conventional Sanger sequencing method, not by next-generation sequencing-based target sequencing. Our method had a detection limit of about 10% for mutant strains. Thus, minor drug-resistant clones are challenging to identify with this method particularly in the early stage of emergence. However, since viruses with drug-resistant mutations become dominant in patients with refractory CMV infection, our method may have sufficient sensitivity for detecting drug-resistant mutations.
In conclusion, drug-resistant CMV mutation is rare even in clinically refractory allo-SCT recipients. Refractory CMV infection is mainly associated with host immunity. In most cases, continuous treatment with the same antiviral drugs could resolve persistent viremia. However, patients with refractory CMV infection have a higher mortality rate than others even if drug-resistant mutations are not detected. Although the frequency of gene mutations is low, it might be appropriate to consider genetic analysis when patients exhibit refractory infection. Especially, in cases with persistent CMV infection, repeated genetic analysis might be helpful.
References
Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Infect Dis Clin North Am. 2010;24(2):319–37.
Ljungman P, de la Camara R, Cordonnier C, Einsele H, Engelhard D, Reusser P, et al. Management of CMV, HHV-6, HHV-7 and Kaposi-sarcoma herpesvirus (HHV-8) infections in patients with hematological malignancies and after SCT. Bone Marrow Transplant. 2008;42(4):227–40.
Ljungman P, de la Camara R, Robin C, Crocchiolo R, Einsele H, Hill JA, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European conference on infections in leukaemia (ECIL 7). Lancet Infect Dis. 2019;19(8):e260–72.
Boeckh M, Ljungman P. How I treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113(23):5711–9.
Boeckh M, Murphy WJ, Peggs KS. Recent advances in cytomegalovirus: an update on pharmacologic and cellular therapies. Biol Blood Marrow Transplant. 2015;21(1):24–9.
Green ML, Leisenring W, Xie H, Mast TC, Cui Y, Sandmaier BM, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol. 2016;3(3):e119–27.
Marty FM, Ljungman P, Papanicolaou GA, Winston DJ, Chemaly RF, Strasfeld L, et al. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis. 2011;11(4):284–92.
Marty FM, Winston DJ, Rowley SD, Vance E, Papanicolaou GA, Mullane KM, et al. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med. 2013;369(13):1227–36.
Marty FM, Winston DJ, Chemaly RF, Mullane KM, Shore TB, Papanicolaou GA, et al. A randomized, double-blind, placebo-controlled phase 3 trial of oral brincidofovir for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25(2):369–81.
Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377(25):2433–44.
Takenaka K, Nishida T, Asano-Mori Y, Oshima K, Ohashi K, Mori T, et al. Cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation is associated with a reduced risk of relapse in patients with acute myeloid leukemia who survived to day 100 after transplantation: the japan society for hematopoietic cell transplantation transplantation-related complication working group. Biol Blood Marrow Transplant. 2015;21(11):2008–16.
Teira P, Battiwalla M, Ramanathan M, Barrett AJ, Ahn KW, Chen M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127(20):2427–38.
El Chaer F, Shah DP, Chemaly RF. How I treat resistant cytomegalovirus infection in hematopoietic cell transplantation recipients. Blood. 2016;128(23):2624–36.
Lurain NS, Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev. 2010;23(4):689–712.
Drew WL. Cytomegalovirus resistance testing: pitfalls and problems for the clinician. Clin Infect Dis. 2010;50(5):733–6.
Chang YJ, Wang Y, Mo XD, Zhang XH, Xu LP, Yan CH, et al. Optimal dose of rabbit thymoglobulin in conditioning regimens for unmanipulated, haploidentical, hematopoietic stem cell transplantation: long-term outcomes of a prospective randomized trial. Cancer. 2017;123(15):2881–92.
Mardani M, Abolghasemi S, Shabani S, Tavakoli F, Saeedi A, Parkhideh S, et al. The association of conditioning regimen with cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation. Iran J Microbiol. 2020;12(6):636–43.
Delgado J, Pillai S, Benjamin R, Caballero D, Martino R, Nathwani A, et al. The effect of in vivo T cell depletion with alemtuzumab on reduced-intensity allogeneic hematopoietic cell transplantation for chronic lymphocytic leukemia. Biol Blood Marrow Transplant. 2008;14(11):1288–97.
Goldsmith SR, Slade M, DiPersio JF, Westervelt P, Lawrence SJ, Uy GL, et al. Cytomegalovirus viremia, disease, and impact on relapse in T cell replete peripheral blood haploidentical hematopoietic cell transplantation with post-transplant cyclophosphamide. Haematologica. 2016;101(11):e465–8.
Walker CM, van Burik JA, De For TE, Weisdorf DJ. Cytomegalovirus infection after allogeneic transplantation: comparison of cord blood with peripheral blood and marrow graft sources. Biol Blood Marrow Transplant. 2007;13(9):1106–15.
Boivin G, Goyette N, Gilbert C, Covington E. Analysis of cytomegalovirus DNA polymerase (UL54) mutations in solid organ transplant patients receiving valganciclovir or ganciclovir prophylaxis. J Med Virol. 2005;77(3):425–9.
Cihlar T, Fuller MD, Mulato AS, Cherrington JM. A point mutation in the human cytomegalovirus DNA polymerase gene selected in vitro by cidofovir confers a slow replication phenotype in cell culture. Virology. 1998;248(2):382–93.
Jabs DA, Enger C, Dunn JP, Forman M. Cytomegalovirus retinitis and viral resistance: ganciclovir resistance. CMV retinitis and viral resistance study group. J Infect Dis. 1998;177(3):770–3.
Boivin G, Gilbert C, Gaudreau A, Greenfield I, Sudlow R, Roberts NA. Rate of emergence of cytomegalovirus (CMV) mutations in leukocytes of patients with acquired immunodeficiency syndrome who are receiving valganciclovir as induction and maintenance therapy for CMV retinitis. J Infect Dis. 2001;184(12):1598–602.
Limaye AP, Corey L, Koelle DM, Davis CL, Boeckh M. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants. Lancet. 2000;356(9230):645–9.
Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300(4):413–22.
van der Beek MT, Marijt EW, Vossen AC, van der Blij-de Brouwer CS, Wolterbeek R, Halkes CJ, et al. Failure of pre-emptive treatment of cytomegalovirus infections and antiviral resistance in stem cell transplant recipients. Antivir Ther. 2012;17(1):45–51.
Choi SH, Hwang JY, Park KS, Kim Y, Lee SH, Yoo KH, et al. The impact of drug-resistant cytomegalovirus in pediatric allogeneic hematopoietic cell transplant recipients: a prospective monitoring of UL97 and UL54 gene mutations. Transpl Infect Dis. 2014;16(6):919–29.
Allice T, Busca A, Locatelli F, Falda M, Pittaluga F, Ghisetti V. Valganciclovir as pre-emptive therapy for cytomegalovirus infection post-allogenic stem cell transplantation: implications for the emergence of drug-resistant cytomegalovirus. J Antimicrob Chemother. 2009;63(3):600–8.
Kim YJ, Boeckh M, Cook L, Stempel H, Jerome KR, Boucek R Jr, et al. Cytomegalovirus infection and ganciclovir resistance caused by UL97 mutations in pediatric transplant recipients. Transpl Infect Dis. 2012;14(6):611–7.
Shmueli E, Or R, Shapira MY, Resnick IB, Caplan O, Bdolah-Abram T, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis. 2014;209(4):557–61.
Hantz S, Garnier-Geoffroy F, Mazeron MC, Garrigue I, Merville P, Mengelle C, et al. Drug-resistant cytomegalovirus in transplant recipients: a French cohort study. J Antimicrob Chemother. 2010;65(12):2628–40.
Gondo H, Minematsu T, Harada M, Akashi K, Hayashi S, Taniguchi S, et al. Cytomegalovirus (CMV) antigenaemia for rapid diagnosis and monitoring of CMV-associated disease after bone marrow transplantation. Br J Haematol. 1994;86(1):130–7.
Takenaka K, Gondo H, Tanimoto K, Nagafuji K, Fujisaki T, Mizuno S, et al. Increased incidence of cytomegalovirus (CMV) infection and CMV-associated disease after allogeneic bone marrow transplantation from unrelated donors. The Fukuoka bone marrow transplantation group. Bone Marrow Transplant. 1997;19(3):241–8.
Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34(8):1094–7.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8.
Boeckh M, Nichols WG, Papanicolaou G, Rubin R, Wingard JR, Zaia J. Cytomegalovirus in hematopoietic stem cell transplant recipients: current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9(9):543–58.
Cihlar T, Fuller MD, Cherrington JM. Characterization of drug resistance-associated mutations in the human cytomegalovirus DNA polymerase gene by using recombinant mutant viruses generated from overlapping DNA fragments. J Virol. 1998;72(7):5927–36.
Mousavi-Jazi M, Schloss L, Drew WL, Linde A, Miner RC, Harmenberg J, et al. Variations in the cytomegalovirus DNA polymerase and phosphotransferase genes in relation to foscarnet and ganciclovir sensitivity. J Clin Virol. 2001;23(1–2):1–15.
Limaye AP, Raghu G, Koelle DM, Ferrenberg J, Huang ML, Boeckh M. High incidence of ganciclovir-resistant cytomegalovirus infection among lung transplant recipients receiving preemptive therapy. J Infect Dis. 2002;185(1):20–7.
de la Camara R. CMV in hematopoietic stem cell transplantation. Mediterr J Hematol Infect Dis. 2016;8(1):e2016031.
Acknowledgements
This work was supported by JSPS KAKENHI (Grant No: 18H02840).
Author information
Authors and Affiliations
Contributions
FJ and KT designed the study, performed the statistical analyses, wrote the manuscript, and collected the patient data. YM, GY, TY, TN, AY, MH, JY, SD, TS, JO, ST, KK, KK, TM and KA collected the patient data. All the authors analyzed and reviewed the data and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial interests to disclose. The authors declare no competing financial interests in relation to this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12185_2021_3218_MOESM1_ESM.pdf
Supplementary Figure 1 (PDF 111 KB) Maps of the UL54 and UL97 genes and amplified lesions. Red bars represent confirmed mutations associated with drug resistance (14). Four primer pairs were designed for the UL54 gene and two pairs for the UL97 gene. The arrows indicate the position and direction of the primers.
About this article
Cite this article
Jinnouchi, F., Mori, Y., Yoshimoto, G. et al. Incidence of refractory cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation. Int J Hematol 115, 96–106 (2022). https://doi.org/10.1007/s12185-021-03218-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-021-03218-3