Abstract
Inflammatory cytokines play a role in hematopoiesis and development of myelodysplastic syndromes (MDS). Although increased serum levels of inflammatory cytokines are associated with poor survival in MDS patients, clinical management does not include assessment of inflammation. We investigated the significance of inflammation in MDS using serum C-reactive protein (CRP) levels, an indicator of the degree of systemic inflammation that can be used in routine practice. We hypothesized that serum CRP levels can be used to further classify low-risk MDS. We conducted a retrospective analysis of 90 patients with low-risk MDS, defined by the international prognostic scoring system (IPSS). We examined the prognostic relevance of CRP and known prognostic factors at diagnosis. Increased serum CRP (≥ 0.58 mg/dL) was associated with poor survival (hazard ratio [HR]: 17.63, 95% confidence interval [CI] 5.83–53.28, P < 0.001) both overall and among the 73 patients with low-risk MDS as defined by the revised IPSS (HR: 28.05, 95% CI 6.15–128.04, P < 0.001). Increased CRP might predict poor prognosis and serum CRP levels can indicate clonal hematopoiesis and non-hematological comorbidity in patients with low-risk MDS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myelodysplastic syndromes (MDS) are defined as a disease of clonal hematopoiesis, characterized by genetic alteration of hematopoietic stem cells [1]. MDS encompasses diseases associated with poor prognosis that quickly develop into leukemia, as well as those that are associated with a relatively good prognosis and long overall survival (OS). Increased percentage of blasts in the bone marrow, decreased neutrophil and hemoglobin levels, reduced platelet count, and specific chromosomal abnormalities are factors associated with poor prognosis in patients with MDS [2, 3]. As not all MDS patients classified as “low-risk” have good clinical outcomes, further stratification of low-risk MDS is required to improve patient outcomes and the associated quality of life.
A recent experimental study has shown the importance of inflammatory signals associated with hematopoiesis in the pathology of MDS [4]. Specifically, interleukin (IL)-6 was shown to cause inhibition of erythropoiesis by inducing potent upregulation of reactive oxygen species and caspase-mediated cell death in the inflammatory microenvironment of the bone marrow [5]. Clinically, numerous studies have reported on the significance of serum cytokine profiles in MDS pathology [6, 7]. Among them, elevated levels of serum IL-6 have been associated with poor prognosis in patients with MDS [8].
Despite this evidence, assessing the degree of inflammation is not part of the clinical management of patients with MDS. In this study, we investigated the significance of inflammation in MDS using serum C-reactive protein (CRP) levels, which are used as an indicator of systemic inflammation in routine practice. We hypothesized that serum CRP levels can be used to further classify low-risk MDS.
Materials and methods
Patients and clinical variables
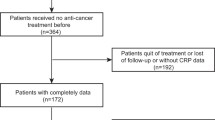
A total of 91 patients newly diagnosed with low-risk MDS at our hospital between January 2008 and December 2018 were eligible for this study. One patient, who lacked data on CRP levels, was excluded. The final sample included 90 patients. The candidate prognostic factors of interest in this study were CRP levels, as well as serum levels of neutrophils and hemoglobin, platelet count, the percentage of blasts in the bone marrow, and the chromosome subtype-based score obtained at the time of diagnosis, which are known prognostic factors based on the International prognostic scoring system (IPSS) [2] and the revised IPSS (IPSS-R) [3]. In addition, among the prognostic factors previously reported, age [9] and serum lactate dehydrogenase (LDH) levels [10, 11], which were available in this retrospective study, were investigated.
The diagnosis of MDS was based on the World Health Organization 2017 classification of myeloid neoplasms and acute leukemia [12]. MDS classified as low and intermediate-1 based on the IPSS was defined as low-risk MDS [2]. As a subgroup, we defined an IPSS-R score of 3.5 or less as low-risk MDS [13].
All patients underwent blood and bone marrow evaluations at diagnosis. Complete blood count was measured using a fully automated XN-1000 hematology analyzer system (Sysmex, Chuo-ku, Japan). CRP and biochemical parameters were measured using a JCA-BM6010 (JEOL Ltd., Tokyo, Japan). The proportion of blasts in the bone marrow was evaluated by two laboratory technicians, based on Giemsa specimens. Chromosomes of bone marrow cells were analyzed using the G-banding method.
Chromosome subtypes were classified based on the MDS cytogenetic scoring system [14]: very good [− Y, del(11q)], good [normal, del(5q), del(12p), del(20q), double, including del(5q)], intermediate [del(7q), + 8, + 19, i(17q), any other single or double independent clones], poor [− 7. inv(3)/t(3q)/del(3q), double including − 7/del(7q), and complex 3 abnormalities], and very poor (complex > 3 abnormalities). Chromosome subtype-based prognostic scores were derived from the IPSS-R [3] as follows: very good (0 points), good (1 point), intermediate (2 points), poor (3 points), and very poor (4 points). This study was approved by the Institutional Review Board of Showa University (approval number 3057). Informed consent was obtained in the form of opt-out.
Statistical analysis
We explored the prognostic value of CRP levels on the OS of patients with low-risk MDS. The cutoff value of CRP levels relevant to prognosis was determined based on receiver-operating characteristic curve analysis. The sensitivity, specificity, and area under the curve (AUC) values were estimated. Cutoff scores were calculated based on the Youden index, which corresponds to the highest sum of specificity and sensitivity values. OS was analyzed using the Kaplan–Meier method, and the log-rank test was used for comparison according to CRP level, neutrophil count, hemoglobin levels, platelet count, the percentage of blasts in the bone marrow, the chromosome subtype-based score, age, and LDH level. Multivariate analysis was performed using the Cox proportional hazard model. Fisher’s exact test was performed to assess the difference in comorbidities between the higher and lower CRP groups. Comparisons of clinical variables between the higher and lower CRP groups were analyzed by the Mann–Whitney U test. Logistic regression was performed to determine the association between CRP and progression to high-risk MDS or acute myeloid leukemia (AML).
As this was an exploratory study aimed to assess the prognostic relevance of CRP levels in patients with low-risk MDS, the sample size was not pre-specified. All analyses were performed with two-sided tests at a significance level of P < 0.05. JMP® 15 software was employed for all statistical analyses (SAS Institute, Cary, NC).
Results
Patients’ characteristics
Patients’ characteristics are presented in Table 1. This study included 90 patients. The median age of the patients was 76 years (23–96 years). The median follow-up duration was 882 days (42–3819 days). There were 73 cases that could be defined as low-risk MDS by the IPSS-R. There were no cases classified as high-risk by the IPSS and low-risk by the IPSS-R. In this subgroup, the median age of the patients was 75 years (23–92 years), and the median follow-up duration was 1022 days (47–3819 days).
Prognostic significance of CRP levels and other factors
The median CRP level at the time of diagnosis was 0.17 mg/dL (0.04–20.26 mg/dL). Even in cases with increased CRP levels, none of the patients presented with severe infection at the time of diagnosis.
The prognostically relevant level of CRP was 0.58 mg/dL, with sensitivity and specificity values of 78.3% and 85.1%, respectively (Fig. 1). The AUC value was 0.80. A CRP level ≥ 0.58 mg/dL was associated with reduced OS (HR: 17.63, 95% CI 5.83–53.28, P < 0.001) (Fig. 2 and Table 2).
Receiver-operating characteristic curve of C-reactive protein (CRP) levels in the prognostic assessment of patients with low-risk myelodysplastic syndromes based on the international prognostic scoring system. The prognostically relevant level of CRP was 0.58 mg/dL, with sensitivity and specificity values of 78.3% and 85.1%, respectively. The area under the curve (AUC) value was 0.80
In patients with low-risk MDS based on the IPSS, none of the following factors had prognostic significance for survival: age (P = 0.11), neutrophil (P = 0.27) or hemoglobin (P = 0.09) levels, platelet count (P = 0.89), or chromosome subtype-based score (P = 0.70). An increased LDH level (> reference range) (HR: 2.84, 95% CI 1.16–6.94, P = 0.02) and a percentage of blasts in the bone marrow of > 2% (HR: 2.90, 95% CI 1.27–6.60, P = 0.008) was associated with reduced survival.
Increased CRP levels (HR: 18.22, 95% CI 5.64–58.84, P < 0.001), increased LDH level (HR: 3.46, 95% CI 1.38–8.65, P = 0.01), and increased percentage of blasts in the bone marrow (HR: 2.52, 95% CI 1.06–5.95, P = 0.04) were associated with reduced OS in the multivariate analysis.
We performed additional analysis limited to the low-risk MDS, based on the IPSS-R. The prognostically relevant level of CRP was also 0.58 mg/dL (Supplementary Fig. 1). A CRP level ≥ 0.58 mg/dL was associated with reduced OS (HR: 28.05, 95% CI 6.15–128.04, P < 0.001) (Supplementary Fig. 2 and Supplementary Table 1). An increased LDH level (> reference range) was associated with reduced survival (HR: 3.29, 95% CI 1.04–10.40, P = 0.04). The other factors had no prognostic significance for survival. Increased CRP (HR: 34.17, 95% CI 7.10–164.55, P < 0.001) and LDH (HR: 4.15, 95% CI 1.26–13.67, P = 0.02) levels showed significant associations with reduced OS in the multivariate analysis.
Comorbidities and clinical variables
Patient comorbidities at the time of diagnosis of low-risk MDS obtained from medical records are presented in Supplementary Table 2. The diseases listed below did not differ between the higher CRP group and the lower CRP group.
Patient comorbidities associated with atherosclerotic disease obtained from medical records were as follows: hypertension (28, 14 patients), diabetes mellitus (13, 5 patients), dyslipidemia (8, 2 patients), chronic kidney disease (8, 4 patients), heart failure (7, 5 patients), angina pectoris or myocardial infarction (3, 2 patients), and cerebral infarction (2, 3 patients) (CRP levels < 0.58 mg/dL, ≥ 0.58 mg/dL, respectively). Patient comorbidities associated with other tumors were as follows: esophageal cancer (2, 2 patients) and colorectal cancer (1, 3 patients) (CRP levels < 0.58 mg/dL, ≥ 0.58 mg/dL, respectively). The other comorbidities associated with CRP level were as follows: rheumatoid arthritis (2, 1 patients), Behçet’s disease (2, 1 patients), and systemic lupus erythematosus (1, 1 patients) (CRP levels < 0.58 mg/dL, ≥ 0.58 mg/dL, respectively).
The clinical variables of patients at the time of diagnosis are presented in Supplementary Table 3. Hemoglobin and hematocrit levels were significantly lower in the higher CRP group (P = 0.02, P = 0.02, respectively). There were no significant differences in the other clinical variables (white blood cell count, neutrophil count, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, reticulocyte count, red cell distribution width, platelet count, and LDH, aspartate aminotransferase, alanine aminotransferase, and creatinine levels) between the groups.
Clinical courses
Patient treatments and main causes of death are presented in Table 1. Azacitidine was administered to 19 patients. The 17 patients that converted from low-risk MDS to high-risk MDS were treated with azacitidine. Only 2 patients with low-risk MDS (CRP level < 0.58 mg/dL) were treated with azacitidine at the discretion of the attending physician. Seven of the 19 patients who were administered azacitidine progressed to AML and 10 died. Lenalidomide was administered to 4 patients. Immunosuppressive therapy was administered to 7 patients. Erythropoietin was administered to 17 patients. Combined chemotherapy was administered to 9 patients that converted from low-risk MDS into AML. All the 5 who were administered chemotherapy alone died, 2 died after allogeneic stem cell transplant, and 2 survived long-term after allogeneic stem cell transplant. Allogeneic stem cell transplant was performed in 5 patients, including 1 patient with high-risk MDS and 4 patients with AML. Three of the 5 patients had long-term survival and 2 died.
Eighteen patients progressed to high-risk MDS (observed in 9 and 9 patients with CRP levels < 0.58 mg/dL and ≥ 0.58 mg/dL, respectively). The conversion from low-risk to high-risk MDS was confirmed by decreased blood counts, elevated blast count, and change in cytogenesis. For all 3 (2, 2 patients); for decreased blood count and elevated blast count (2, 2 patients); for decreased blood count (including based only on IPSS-R) (3, 2 patients); and for elevated blast count (2, 3 patients) (with CRP levels < 0.58 mg/dL, ≥ 0.58 mg/dL, respectively). Ten patients progressed to AML (observed in 4 and 6 patients with CRP levels < 0.58 mg/dL, ≥ 0.58 mg/dL, respectively). In the < 0.58 mg/dL CRP group, 2 patients with MDS with single lineage dysplasia, 1 patient with MDS with multilineage dysplasia, and 1 patient with MDS with ring sideroblasts and multilineage dysplasia at initial diagnosis progressed to AML. In the ≥ 0.58 mg/dL CRP group, 1 patient with MDS with single lineage dysplasia, 1 patient with MDS with multilineage dysplasia, and 4 patients with MDS with excess blasts-1 at initial diagnosis progressed to AML. Higher CRP level (≥ 0.58 mg/dL) was not associated with progression to high-risk MDS (HR: 2.79, 95% CI 0.96–8.07, P = 0.06), but was associated with progression to AML (HR: 3.95, 95% CI 1.02–15.36, P = 0.04).
During the observation period, 23 patients died (including 5 and 18 patients with CRP levels < 0.58 mg/dL, ≥ 0.58 mg/dL, respectively). The main cause of death related to MDS was classified into organ dysfunction by expanding blasts, infection, and bleeding. In 16 patients, the main cause of death was related to MDS (observed in 3 and 13 patients with CRP levels < 0.58 mg/dL, ≥ 0.58 mg/dL, respectively). In 7 patients, the main cause of death was unrelated to MDS (observed in 2 and 5 patients with CRP levels < 0.58 mg/dL, ≥ 0.58 mg/dL, respectively): heart failure (n = 3; observed in 1 and 2 patients with CRP levels < 0.58 mg/dL, ≥ 0.58 mg/dL, respectively), graft-versus-host disease after allogeneic stem cell transplant (n = 1), colon cancer (n = 1), asphyxia (n = 1), and thyroid crisis (n = 1). The patient that died of graft-versus-host disease had no progression of MDS.
Discussion
The present findings suggest that CRP levels might be prognostically useful in assessing patients with low-risk MDS, alongside factors defined by the IPSS or IPSS-R. In the present study, in addition to known prognostic factors, such as increased LDH level and percentage of blasts in the bone marrow, patients with low-risk MDS with serum CRP levels ≥ 0.58 mg/dL at diagnosis presented with an OS shorter than that in their counterparts.
Our study reveals that a high CRP level is characteristic of low-risk MDS, suggesting that the assessment of inflammation can benefit the clinical management of patients with MDS. The hemoglobin and hematocrit levels were significantly lower in the higher CRP group. Higher CRP level tended to be associated with progression to AML. This suggests that high CRP levels might be associated with anemia caused by chronic inflammation and decreased availability of iron and reflect clonal hematopoiesis of the pre-phase of pancytopenia in peripheral blood or an increase of blasts in bone marrow. Of note, there are many factors that lead to high CRP levels, which are involved in infection, inflammation, tissue damage, and even in development of malignant neoplasms. In this study, the comorbidities at the time of diagnosis of low-risk MDS obtained from medical records did not differ between the higher and lower CRP groups. None of the patients presented with severe infection at the time of diagnosis. The high CRP level characteristic of low-risk MDS might suggest that one of the reasons why patients with elevated CRP levels have poor prognosis is the progression of clonal hematopoiesis or AML. In clinical management, patients with higher CRP levels should be carefully monitored as a group with a possibility of poor prognosis in low-risk MDS, while confirming other potential causes of high CRP.
The association between inflammation and hematopoiesis has been previously reported. Previous studies have revealed that an aberrant plasma cytokine profile is associated with both the pathological process and prognosis of MDS [8, 15, 16], which is in agreement with our study. Accumulating evidence indicates a close link between inflammation and hematopoiesis in the hematopoietic microenvironment. A study revealed aberrant expression of cytokines such as TNF and interferon by bone marrow cells in MDS patients, suggesting that the disruption of hematopoiesis in MDS might be related to the production of these cytokines [17]. Another study with an experimental mouse model has shown the anti-inflammatory function of Tet2 [18], and its associated mutation is frequently found in clonal hematopoiesis in MDS [19]. The inflammatory microenvironment of the bone marrow in MDS patients may result in the deterioration of clonal hematopoiesis, leading to ineffective hematopoiesis. The inflammatory microenvironment of the bone marrow might play a role in the process of clonal hematopoiesis. The assumption that serum CRP level is associated with an inflammatory microenvironment of the bone marrow may explain the shorter survival of patients with increased CRP levels.
A recent study has demonstrated an association between clonal hematopoiesis and inflammation. The subjects with clonal hematopoiesis of indeterminate potential (CHIP) had higher levels of high-sensitivity CRP than those without CHIP [20]. The most common mutations associated with clonal hematopoiesis are in DNMT3A, TET2, and ASXL1 [21], and these somatic mutations are early events in MDS and AML [22]. At the stage of CHIP before MDS, inflammation can be detected by high-sensitivity CRP. A previously study showed that driver mutation concerning CHIP was a poor prognostic factor in MDS. Larger clones in MDS patients with type-2 mutations including TET2 and ASXL1 were associated with a significantly shorter overall survival [23]. Mutations in DNMT3A [24] and ASXL1 [25] are predictors of poor overall survival in patients with MDS. Our study is consistent with this association between clonal hematopoiesis and inflammation.
However, clonal hematopoiesis cannot fully explain why patients with elevated CRP levels in low-risk MDS have a poor prognosis. In our analysis, causes of death included those other than the progression of MDS. A recent study revealed that the presence of these somatic mutations, such as in DNMT3A, TET2, and ASXL1, are associated with increased risks of hematological malignancy, all-cause mortality, incident coronary heart disease, and ischemic stroke in patients with CHIP [21, 26]. It also revealed that clonal hematopoiesis accelerates heart failure [27]. Another study revealed that patients with MDS who may have clonal hematopoiesis are more likely to die of cardiovascular disease (CVD) than the general population, and low-risk MDS patients are at particularly high risk of death by CVD [28]. Moreover, the complications of CVD, arteriosclerosis, or heart failure might be detrimental to the treatment of MDS or infection. In this study, high CRP levels indicate poor prognosis, due to the progression of MDS or non-hematological comorbidity. Therefore, this study presents evidence that serum CRP levels may be useful in the clinical management of MDS by managing both MDS and non-hematological comorbidities.
The present study has the following limitations. First, we did not investigate the genetic mutations. Second, this study did not fully account for all factors associated with inflammation. Since this was a retrospective study, the comorbidities at diagnosis were limited to the information obtained from medical records. Third, we could not evaluate the correlation between post-treatment CRP levels and therapeutic effect because there are many factors affecting CRP level, such as infections or concomitant medications. Furthermore, this was an exploratory study with a relatively small sample size. Future studies should involve larger samples to validate the present findings and confirm their clinical relevance. The question of how to preventively intervene for non-hematological diseases in patients with MDS remains.
In conclusion, the present study findings suggest that increased CRP levels are associated with poor prognosis in patients with low-risk MDS. Furthermore, the serum CRP levels may be classified for patients who are carefully managed for clonal hematopoiesis and non-hematological comorbidities in patients with low-risk MDS.
References
Kennedy JA, Ebert BL. Clinical implications of genetic mutations in myelodysplastic syndrome. J Clin Oncol. 2017;35:968–74.
Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–88.
Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–65.
Sallman DA, List A. The central role of inflammatory signaling in the pathogenesis of myelodysplastic syndromes. Blood. 2019;133:1039–48.
Mei Y, Zhao B, Basiorka AA, Yang J, Cao L, Zhang J, et al. Age-related inflammatory bone marrow microenvironment induces ineffective erythropoiesis mimicking del(5q) MDS. Leukemia. 2018;32:1023–33.
Kornblau SM, McCue D, Singh N, Chen W, Estrov Z, Coombes KR. Recurrent expression signatures of cytokines and chemokines are present and are independently prognostic in acute myelogenous leukemia and myelodysplasia. Blood. 2010;116:4251–61.
Feng X, Scheinberg P, Wu CO, Samsel L, Nunez O, Prince C, et al. Cytokine signature profiles in acquired aplastic anemia and myelodysplastic syndromes. Haematologica. 2011;96:602–6.
Pardanani A, Finke C, Lasho TL, Al-Kali A, Begna KH, Hanson CA, et al. IPSS-independent prognostic value of plasma CXCL10, IL-7 and IL-6 levels in myelodysplastic syndromes. Leukemia. 2012;26(4):693–9.
Kantarjian H, O’Brien S, Ravandi F, Cortes J, Shan J, Bennett JM, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original international prognostic scoring system. Cancer. 2008;113:1351–61.
Wimazal F, Sperr WR, Kundi M, Meidlinger P, Fonatsch C, Jordan JH, et al. Prognostic value of lactate dehydrogenase activity in myelodysplastic syndromes. Leuk Res. 2001;25:287–94.
Germing U, Hildebrandt B, Pfeilstöcker M, Nösslinger T, Valent P, Fonatsch C, et al. Refinement of the international prognostic scoring system (IPSS) by including LDH as an additional prognostic variable to improve risk assessment in patients with primary myelodysplastic syndromes (MDS). Leukemia. 2005;19:2223–31.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Pfeilstöcker M, Tuechler H, Sanz G, Schanz J, Garcia-Manero G, Solé F, et al. Time-dependent changes in mortality and transformation risk in MDS. Blood. 2016;128:902–10.
Schanz J, Tüchler H, Solé F, Mallo M, Luño E, Cervera J, et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol. 2012;30:820–9.
Shi X, Zheng Y, Xu L, Cao C, Dong B, Chen X. The inflammatory cytokine profile of myelodysplastic syndromes: a meta-analysis. Medicine (Baltimore). 2019;98:e15844.
Galili N, Marionneaux S, Lascher S, Mazumder A, Vesole D, Mumtaz M, et al. C-reactive protein (CRP) associated with higher risk patients with myelodysplastic syndromes (MDS). J Clin Oncol. 2008;26:18009.
Kitagawa M, Saito I, Kuwata T, Yoshida S, Yamaguchi S, Takahashi M, et al. Overexpression of tumor necrosis factor (TNF)-alpha and interferon (IFN)-gamma by bone marrow cells from patients with myelodysplastic syndromes. Leukemia. 1997;11:2049–54.
Zhang Q, Zhao K, Shen Q, Han Y, Gu Y, Li X, et al. Tet 2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature. 2015;525:389–93.
Haferlach T, Nagata Y, Grossmann V, Okuno Y, Bacher U, Nagae G, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 2014;28:241–7.
Busque L, Sun M, Buscarlet M, Ayachi S, Feroz Zada Y, Provost S, et al. High-sensitivity C-reactive protein is associated with clonal hematopoiesis of indeterminate potential. Blood Adv. 2020;4:2430–8.
Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–98.
Shlush I, Sasan Z, Mitchell A, Chen WC, Brandwein JM, Gupta V, et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506:328–33.
Akishima H, Yoshizato T, Yoshida K, Sekeres MA, Radivoyevitch T, Suzuki H, et al. Dynamics of clonal evolution in myelodysplastic syndromes. Nat Genet. 2017;49:204–12.
Walter MJ, Ding L, Shen D, Shao J, Grillot M, McLellan M, et al. Recurrent DNMT3A mutations in patients with myelodysplastic syndromes. Leukemia. 2011;25:1153–8.
Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364:2496–506.
Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–21.
Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, et al. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1β/NLRP3 inflammasome. J Am Coll Cardiol. 2018;71:875–86.
Brunner AM, Blonquist TM, Hobbs GS, Amrein PC, Neuberg DS, Steensma DP, et al. Risk and timing of cardiovascular death among patients with myelodysplastic syndromes. Blood Adv. 2017;1:2032–40.
Acknowledgements
All the authors reviewed the paper and agreed with the final version. We thank all the physicians, nurses, and staff who contributed to the patient care. We thank Eisuke Inoue (Showa University Research Administration Center) for their generous support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Baba, Y., Saito, B., Shimada, S. et al. Increased serum C-reactive protein is an adverse prognostic factor in low-risk myelodysplastic syndromes. Int J Hematol 114, 441–448 (2021). https://doi.org/10.1007/s12185-021-03187-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-021-03187-7