Abstract
Disseminated intravascular coagulation (DIC) is a common and life-threatening complication in sepsis. Sepsis-associated DIC is recognized as the systemic activation in coagulation with suppressed fibrinolysis that leads to organ dysfunction in combination with systemic intravascular inflammation. In this process, thrombin contributes a key role in connecting both coagulation and inflammation. Endothelial injury, a result of sepsis, causes DIC due to the effect of multiple activated factors that include neutrophils, platelets, and damage-associated molecular patterns. Recent advances in the understanding of pathophysiology have made it possible to diagnose sepsis-associated DIC at earlier timing with better accuracy. However, progress in the treatment is still limited, and new therapeutics for sepsis-associated DIC are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
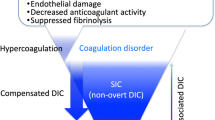
The concept of disseminated intravascular coagulation (DIC) has been considered as a clinically manifested hemostatic disorder that arises from various underlying diseases [1], and over time, it was realized the unique feature of DIC is the coexistence of the manifestations of both thrombosis and bleeding [2] (Fig. 1). The fundamental pathological condition of DIC is characterized by extensive thrombus formation in the microvasculature due to coagulopathy despite differences in underlying causes. As a result, DIC can be categorized by specific diagnostic criteria [3]. One of the important events in defining DIC was the establishment of the definition and diagnostic criteria by the International Society on Thrombosis and Haemostasis (ISTH) in 2001 [4]. However, almost 20 years later, the concept and diagnosis of DIC continue to be better defined. In this review, we introduce the recent advances in the pathophysiology, diagnosis, and treatment strategy of sepsis-associated DIC.
Intravital microscopic view of the microcirculation in a rat model of sepsis. The rat model of sepsis was made by lipopolysaccharide (LPS) injection. 10 min after LPS injection, bleeding was recognized (black arrows). At 30 min, the bleeding area was expanded. 60 min after LPS injection, thrombus was formed in the microvessel (white arrows). 120 min after LPS injection, the blood flow was stopped in the distal side of the vessel (white arrowheads). The above changes are seen in the venule and minimal change was observed in the arteriole
Advance in the understanding of the pathophysiology of sepsis-associated DIC
Systemic activation in coagulation and fibrinolysis suppression
The fundamental concept of DIC was defined as “an acquired syndrome characterized by the intravascular activation of coagulation with loss of localization arising from different causes. It can originate from and cause damage to the microvasculature, which, if sufficiently severe, can produce organ dysfunction” [4]. This definition reminds us that “systemic activation in coagulation” is the critical aspect of DIC, however, since it was not possible to construct diagnostic criteria that depict “systemic activation in coagulation” with the conventional coagulation biomarkers, the ISTH published overt DIC diagnostic criteria to better define a consumptive coagulopathy. As a result, coagulopathy and its advanced stage of DIC are often considered as the hemostatic disorder [5]. Recently, the importance of early coagulopathy detection before patients decompensate is important for sepsis management [6]. Currently, the accepted concept is that systemic activation of coagulation and the suppressed fibrinolytic pathways are the major facilitators of multiorgan dysfunction and death [7]. The mechanisms of the activation in coagulation and inhibition of fibrinolysis are multifactorial [8], but the principal initiator of the coagulation cascades is considered to be tissue factor that is expressed on macrophages [9] and other cells, including neutrophils and endothelial cells [10]. Furthermore, the extracellular vesicles released from various cells also express the tissue factor in sepsis-associated DIC [11]. Among the coagulation factors, thrombin is considered to be the central factor in the pathogenesis of sepsis-associated DIC. Thrombin has a multitude of effects beyond clot formation that include endothelium activation by binding to protease activated receptor 1 (PAR-1) expressed on the endothelial surface and platelets to amplify inflammation [12].
The suppression of fibrinolysis, often referred to as fibrinolytic shutdown, plays a major role in accelerating the prothrombotic condition. Virtually, the thrombotic-type DIC is characterized by the suppressed fibrinolysis [13], and plasminogen activator inhibitor-1 (PAI-1) produced by endothelial cells contributes to the fibrinolysis suppression [14]. Perhaps, this fibrinolysis suppression has the purpose of restricting the pathogen spread and tissue healing but leads the unfavorable microcirculatory disturbance. Multiple studies have reported the increased level of PAI-1 is a useful marker of poor outcome in sepsis [15]. Upon these findings, balancing coagulation and fibrinolysis is expected to provide an opportunity for future therapeutic agent development. However, the appropriate control of fibrinolysis is not easy, and the fibrinolytic approach may not be a good choice. Since the fibrinolytic suppression is a result of activated coagulation, we think anticoagulant therapy may be the better choice. The natural anticoagulants such as antithrombin, protein C, and thrombomodulin are the first candidates that should be examined. The ideally designed studies that determine to whom, when to start, for how long, and how much, etc. are necessary for the success in the development of new therapy.
Derangement of endothelial function
The vascular endothelial cell is the primary target of injury in sepsis-associated DIC from polymorphonuclear neutrophils that are activated, releasing neutrophil extracellular traps (NETs), reactive oxygen species, and other proinflammatory mediators [16]. Intravital microscopic observation of sepsis models demonstrate the injurious effects of neutrophil adhesion, platelet aggregation, and morphological changes of endothelial cells [17] (Fig. 2). The immunothrombus formed by leukocyte-platelet aggregates and fibrin deposition in the vascular lumen is the hallmark of sepsis-associated DIC [18]. NETs are composed of DNA, histones, and other cytotoxic substances that immobilize and eradicate invading pathogens [19]. NETs are also known to convert the anticoagulant property of the vascular endothelium to procoagulant. Stiel et al. [20] reported the increased capability of NETs formation of the neutrophils obtained from sepsis-associated DIC. In addition to the effects of NETs, damage-associated molecular patterns including cell-free DNA, histones, and high-mobility group box 1 (HMGB 1) are released into the bloodstream from the damaged or necrotic cells that can activate procoagulant effects [21] (Fig. 3). For example, HMGB 1 stimulates coagulation cascades by upregulating tissue factor expression and promoting the externalization of phosphatidylserine to the outer surface of the cell membranes [22].
Intravital microscopic view of the microcirculation in normal and sepsis model of rat. Smooth blood flow was observed in arteriole (bottom) and venule (top). In normal rat, the space between endothelial surface and red cell column represents the thickness of the glycocalyx (between the white arrows). In a sepsis model of rat, leukocytes adhere to the endothelium of the venule (white arrowhead), and platelets aggregated in the vascular lumen (white arrows). The endothelium is thickened and the surface became rough. The leukocytes transmigrated to the tissue (black arrowhead)
Pathogenesis of sepsis-associated coagulopathy. Macrophages recognize pathogen invasion by pathogen recognition receptors (PRRs) and express tissue factor (TF) on its surface and initiates the coagulation cascades. Macrophage also releases proinflammatory cytokines and microvesicles in sepsis. Activated neutrophil releases neutrophil extracellular traps (NETs) and cytotoxic mediators such as lysosome and oxygen radicals that damage the endothelial cells. The endothelial cell loses its anticoagulant properties by losing glycocalyx and reduces nitric oxide (NO), prostaglandin I2 (PG I2), and tissue factor pathway inhibitor (TAFI) release. Damaged endothelial produce plasminogen activator inhibitor 1 and releases VWF and express adhesion molecules. The complement system is activated and procoagulant C3a-5a is increased, and membrane attack complex (MAC) damages the endothelial cell. The damaged cells release damage-associated molecular patterns such as cell-free DNA, histones, and high-mobility group box 1 (HMGB1) and accelerates the thrombus formation
The vascular endothelium is a multifunctional antithrombotic barrier between the blood stream and tissues. The endothelial cell produces and releases antithrombotic substances such as nitric oxide (NO), prostaglandin I2, and tissue factor pathway inhibitor (TFPI) [23]. The endothelial cell also maintains antithrombotic properties by expressing glycocalyx, thrombomodulin, and protein C receptors. The glycocalyx is composed of proteoglycan and glycosaminoglycans that cover the endothelial surface to provide antithrombotic activity and Stile facilitate blood flow. One of its major components is heparan sulfate, that functions as the binding site for antithrombin to maximize its anticoagulant activity [24]. Thrombin, a key mediator in coagulation, provides a contrasting and critical prothrombotic effect by inducing Weibel–Palade body exocytosis that releases stored von Willebrand factor (VWF) endothelial cells [25]. Thrombin also can damage endothelial cells by cleaving complements and forming membrane attack complexes (MACs) [26].
Recently, the increased levels of angiotensin II and the decreased activity of angiotensin-converting enzyme 2 (ACE2) have been recognized in sepsis [27] (Fig. 4). Consequently, the beneficial effects of angiotensin1–7, such as anti-inflammation and antithrombosis are reduced [28]. Other than above, the endothelial responses via disruption of angiopoietin/Tie2 system in sepsis-associated DIC have attracted attention [29]. Angiopoietin-1 and -2 are the ligands for the vascular endothelial receptor tyrosine kinase Tie2, and signaling by angiopoietin-1 suppresses endothelial cell apoptosis, maintains the vascular endothelial permeability, and reduces leucocyte adhesion. Systemically, angiopoietin-2 signaling converts antiinflammatory and antithrombotic properties to a prothrombotic effect. During sepsis, angiopoietin-2 stored in Weibel–Palade body is released and preferentially binds to Tie2 over angiopoietin-1 [30].
Pathogenesis of sepsis-associated coagulopathy. Thrombin is the key mediator that activates endothelial cells and platelets. Thrombin binds to protease-activated receptor 1 (PAR1) and induces proinflammatory reaction, prothrombotic change, and activates platelet aggregation. Damaged endothelial cell release angiopoietin 2 (Ang2) that counteracts Ang1′s anti-inflammatory actions through its receptor Tie2. angiotensin 2 receptor (ACE2). Ang2 also increases vascular permeability that leads to the loss of anticoagulant proteins. The activity of the angiotensin converting enzyme 2 (ACE2) on the endothelial surface decreases in sepsis and the level of angiotensin II (Ang II) increases, which leads to vasoconstriction and hyperinflammation
Anticoagulation dysregulation in sepsis
Semeraro et al. [31] described important factors in massive fibrin deposition recognized in sepsis-associated DIC: (a) the expression of tissue factor and subsequent activation in coagulation, (b) the suppression of fibrinolysis due to the production of PAI-1, and (c) the impairment of physiological anticoagulant pathways, orchestrated mainly by endothelial cell dysfunction. The physiological anticoagulants such as protein C and antithrombin are significantly decreased during sepsis, and the levels are known to correlate with mortality [32]. The monitoring of anticoagulants in DIC is important, because not only levels reflect both activation in coagulation and vascular damage, but also important information to decide when to initiate potential anticoagulation repletion.
Comparison to COVID-19-associated coagulopathy
The new severe acute respiratory syndrome coronavirus (SARS‐CoV‐2) infection is known to be frequently complicated with thrombotic events. Multiple factors such as intravascular inflammation, activated coagulation, platelet aggregation, and vascular endothelial damage are involved in the development of thrombosis [33]. Nicolai et al. [34] described the histopathology of the lung and described that activated neutrophil and platelets play the key role in the formation of immunothrombus, as seen in sepsis. With regard to hypercoagulability, viscoelastic testing demonstrates a procoagulant profile represented by an increased clot strength, and the increase was attributed to platelet activation and increased fibrinogen levels [35]. These observations suggest an important role of activated platelets in the thrombogenicity in COVID-19. Although thrombocytopenia is the most frequent laboratory disorder in infection-associated DIC, it is much less common in COVID‐19, at least in its early phase. We believe this is due to microclot formation that occurs in the lung microcirculation due to the direct SARS-CoV-2 infection of the pulmonary endothelial cells. As a result, the systemic consumption of the platelets does not occur until the infection is at a more advanced and systemic phase [36]. Viscoelastic testing also suggests that systemic fibrinolysis is considerably suppressed, as noted in sepsis-associated DIC [37]. The postmortem findings suggest that the histologic findings of COVID-19 of the lung are in accord with that of DIC [38], and increased NETs forming ability, and increased platelet aggregation were also reported [34]. In the later stages of COVID-19, systemic activation of coagulation becomes evident and finally reaches to the same condition defined as thrombotic type DIC [39]. Regarding the effect of anticoagulant therapy, the effect of heparin or low-molecular-weight heparin (LMWH) was reported in severe COVID-19 suggesting that the heparins may be effective not only for deep vein thrombosis (DVT) prevention but also for microthrombosis prevention in the lung [40].
Comparison to malignancy-associated coagulopathy
Cancer is also complicated by DIC. Similar to sepsis, DIC in cancer is thought to be driven by the induction of cytokine and expression of procoagulant factors on cancer cells, such as tissue factor or factor Xa cancer procoagulant [41]. Interestingly, recent studies reported the involvement of the derangement of vascular endothelium in the pathogenesis of cancer-induced DIC [42]. Since the cancer-associated DIC progresses slowly compared to that in sepsis, and coagulation factors and platelets are usually compensated. Therefore, cancer-associated DIC is usually classified in the balanced type. At this stage, the symptom is subclinical, but thrombotic events are sometimes seen in the advanced stages. The exception is the hematological malignancy-associated DIC that can show abrupt life-threatening hemorrhage associated with sudden thrombocytopenia and consumptive coagulation factor deficiency. It is noteworthy that the exacerbation of DIC also occurs after the successful chemotherapy in this situation.
As the disease progresses, bleeding can occur and this process may further lead to exhaustion of platelets and coagulation factors. The bleeding can be the first clinical symptom indicating the presence of DIC. In some cases, the clinical presentation of cancer-associated DIC is reminiscent of thrombotic microangiopathies rather than sepsis-associated DIC. The therapeutic cornerstone of DIC in malignancy is the treatment of underlying disorder but supportive treatment, specifically aimed at the hemostatic system may be required. At the same time, it should be kept in mind that antifibrinolytic agents are sometimes helpful to manage excessive hyperfibrinolysis and bleeding [43].
Advance in the diagnosis of sepsis-associated DIC
Coagulation and fibrinolysis markers
The key mediator that propagates systemic coagulation and inflammation in sepsis is thrombin [12]. Thrombin stimulates the release of proinflammatory cytokines and chemokines from the immune cells as well as from the endothelial cells [44]. Therefore, it is important to monitor thrombin generation for the correct evaluation of the severity of sepsis-associated DIC. In the randomized controlled trial (RCT) that evaluated recombinant thrombomodulin, the placebo group demonstrated an increased mortality along with increased baseline levels of thrombin–antithrombin complex (TAT) and prothrombin fragment1.2 [45]. While such a relation was not observed between D-dimer levels and mortality, D-dimers elevated in response to the fibrin generation but the level stayed the same and did not escalate with increased severity [9]. This finding is because the fibrinolytic system was suppressed in severe cases by increased PAI-1 production, and the D-dimer level is not a reflection of fibrin formation [46]. Rather, the combination of the biomarkers for thrombin generation and fibrinolytic suppression such as TAT and PAI-1 increase the predictive value for DIC and mortality [47].
Vascular biomarkers
Since DIC is based on the vascular injury, constructing the diagnostic evaluation using vascular biomarkers may be a rational approach. Endothelium injury has been most frequently evaluated with soluble thrombomodulin released from the endothelial surface into circulation and PAI-1 produced by the endothelial cells [48]. Other biomarkers include circulating components of the glycocalyx, such as syndecan-1 and hyaluronic acid that covers endothelial surface, as the damage markers. Inkinen et al. [49] reported syndecan-1, angiopoietin-2, and soluble thrombomodulin were independently associated with an increased risk of mortality in sepsis. Additional potential biomarkers for assessing endothelial injury include vascular endothelial growth factor receptors 2 (VEGFR2) and the urokinase plasminogen activator receptor (uPAR), both are the receptors expressed on vascular endothelial cells, are released into the circulation during sepsis. Lafon et al. [50] reported soluble VEGFR2 and soluble uPAR as the severity markers in sepsis. Additional research is underway to examine better endothelial damage markers in sepsis and DIC.
Importance of detecting the early-stage of DIC
DIC has been recognized as a critical event in patients with sepsis, and therefore, common and readily available biomarkers are needed for the diagnosis. The ISTH overt-DIC criteria were composed of platelet count, prothrombin time (PT), fibrin degradation products, and fibrinogen, however, since overt-DIC criteria were developed to identify the consumptive coagulopathy, the delay for diagnosis is a major drawback [51]. To overcome this issue, sepsis-induced coagulopathy (SIC) has been proposed by ISTH. SIC is composed of three items: (a) the presence of organ dysfunction, (b) decreased platelet count, and (c) increased PT-INR, and the validation study has shown that SIC precedes overt-DIC and almost all of overt DIC patients are diagnosed as SIC [52]. Jackson Chornenki et al. [53] also reported a high predictive value of the combination of platelet count and PT-INR for DIC (area under the curve > 0.8). In addition to the need for accurate prediction of DIC, and an important question is whether the timing of SIC diagnosis is appropriate for initiating anticoagulant therapy. One observational study from Japan reported that SIC could determine the timing of anticoagulation properly [54], and recent RCT for recombinant thrombomodulin was performed using similar criteria as SIC [55]. In contrast, Ding et al. [56] reported there was no advantage in diagnosing SIC in terms of mortality prediction compared with overt DIC. Helms et al. [57] also reported similar results. However, we believe these studies did not appropriately examine the concept of SIC. The SIC was designed to detect the risk of DIC at an earlier timing with fewer, more readily available, and less-expense biomarkers, and the prediction of outcomes is not the first priority. ISTH recommends a two-step approach to diagnose sepsis-associated DIC that includes screening first by SIC, then diagnosing by overt DIC criteria [58]. As for the severity and mortality evaluation of sepsis, the superiority of the SOFA score over the DIC score has already been reported [59].
Other diagnostic criteria
To diagnose acute DIC at an earlier timing, the Japanese Society for Acute Medicine (JAAM) released the DIC criteria in 2007, and JAAM criteria has been used for the diagnosis of sepsis-associated DIC in most Japanese institutions [60]. The JAAM criteria are easy to use, the score is reported to correlate with mortality, and does not include fibrinogen levels. However, JAAM-DIC criteria have rarely been used outside Japan.
The DIC diagnostic criteria have been designed in various ways. Japanese Society on Thrombosis and Hemostasis (JSTH) proposed new diagnostic criteria based on the different concepts from SIC [61, 62]. The feature of the JSTH criteria was trying to diagnose various types of DIC by one scoring system. Any hematopoietic disorder type of DIC, infectious type DIC, and basic type of DIC based can be diagnosed using partially overlapping subclassified criteria. The other feature of JSTH criteria is the inclusion of molecular markers. As for molecular markers, antithrombin activity, TAT, soluble fibrin, and prothrombin fragment 1.2 were employed to increase the sensitivity [63]. TAT and soluble fibrin were also adopted for the exclusion of other conditions that mimic DIC. In the cases of infectious types of DIC, diagnosis is made based on the scores of the platelet count, FDP, PT ratio, antithrombin activity, and other molecular markers (TAT, soluble fibrin, or prothrombin fragment1.2). Though molecular markers may increase the sensitivity and specificity, theses markers are costly and unavailable in the local laboratories. Overall, JSTH DIC diagnostic criteria are capable of detecting DIC more specifically and better suitable for research purposes.
Advance in the treatment of sepsis-associated DIC
Recombinant thrombomodulin
The effect of recombinant thrombomodulin for sepsis-associated coagulopathy was recently examined in a phase III study on 800 cases. In this study, the 28-day-all-cause mortality rate was not statistically significantly different between the treatment group and the placebo group (26.8 vs 29.4%, respectively; P = 0.32) [64]. However, the post hoc analysis demonstrated the greater absolute risk reductions in subgroups with higher baseline prothrombin fragment1.2 or TAT. The higher risk reductions were also recognized in subgroups with baseline coagulation biomarker levels above the median of the entire study population at the reduction range from 4.2 to 5.5% [42]. The effect of recombinant thrombomodulin is still inconclusive, and the study will be continued.
Antithrombin
The largest RCT examined the effect of antithrombin was performed in 2001 [65]. In this study, antithrombin not only failed to show a beneficial effect on mortality but also increased the bleeding risk. However, we should remind this study targeted the severe sepsis and not the sepsis-associated DIC, therefore, Umemura et al. [66] performed a meta-analysis using the reported RCTs and showed a reduction of mortality (risk ratio 0.63; 95% CI: 0.45–0.90) in the subgroup of septic patients with DIC. Also, a recent summary of systematic reviews found some evidences of its efficacy with low certainty [67], and the Japanese Clinical Practice Guidelines for Management of Sepsis and Septic shock weakly recommended the use of antithrombin for sepsis-associated DIC with reduced antithrombin activities [68].
Heparins
Heparins are frequently used in septic patients admitted to ICU for venous thromboembolism prevention. However, its efficacy on DIC and patients’ outcome is still uncertain. Recent studies performed in China reported the beneficial effects on morbidity and mortality but increased bleeding risk [69]. The overall benefit should be examined in future studies.
Summary
DIC has been thought of as consumptive coagulopathy since its diagnostic criteria define the decompensated coagulopathy that occurs. However, its definition clearly describes that DIC is a systemic activation of coagulation along with endothelial dysfunction in the pathophysiology. To bridge the gap between the definition and the diagnostic criteria, the establishment of individual criteria depending on the underlying diseases is intended to improve diagnosis and management. In the case of sepsis-associated DIC, new strategies, including the release of a two-step diagnosis using SIC and the introduction of new biomarkers were intended. Along with ongoing advances in research, the approach to determine the specific implications of DIC should continue to be examined in both laboratory and clinical research.
References
Feinstein DI. Diagnosis and management of disseminated intravascular coagulation: the role of heparin therapy. Blood. 1982;60(2):284–7.
Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145(1):24–33.
Tanaka K, Imamura T. Incidence and clinicopathological significance of DIC in autopsy cases. Bibl Haematol. 1983;49:79–93.
Taylor FB Jr, Toh CH, Hoots WK, Wada H, Levi M. Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–30.
Hunt BJ. Bleeding and coagulopathies in critical care. N Engl J Med. 2014;370(9):847–59.
Liu XL, Wang XZ, Liu XX, Hao D, Jaladat Y, Lu F, Sun T, Lv CJ. Low-dose heparin as treatment for early disseminated intravascular coagulation during sepsis: a prospective clinical study. Exp Ther Med. 2014;7(3):604–8.
Gando S, Fujishima S, Saitoh D, Shiraishi A, Yamakawa K, Kushimoto S, Ogura H, Abe T, Mayumi T, Sasaki J, Kotani J, Takeyama N, Tsuruta R, Takuma K, Yamashita N, Shiraishi SI, Ikeda H, Shiino Y, Tarui T, Nakada TA, Hifumi T, Otomo Y, Okamoto K, Sakamoto Y, Hagiwara A, Masuno T, Ueyama M, Fujimi S, Umemura Y. The significance of disseminated intravascular coagulation on multiple organ dysfunction during the early stage of acute respiratory distress syndrome. Thromb Res. 2020;191:15–21.
Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost. 2018;16(2):231–41.
Ahamed J, Niessen F, Kurokawa T, Lee YK, Bhattacharjee G, Morrissey JH, Ruf W. Regulation of macrophage procoagulant responses by the tissue factor cytoplasmic domain in endotoxemia. Blood. 2007;109(12):5251–9.
Iba T, Miki T, Hashiguchi N, Tabe Y, Nagaoka I. Is the neutrophil a “prima donna” in the procoagulant process during sepsis? Crit Care. 2014;18(4):230.
Matsumoto H, Yamakawa K, Ogura H, Koh T, Matsumoto N, Shimazu T. Enhanced expression of cell-specific surface antigens on endothelial microparticles in sepsis-induced disseminated intravascular coagulation. Shock. 2015;43(5):443–9.
Conway EM. Thrombin: coagulation’s master regulator of innate immunity. J Thromb Haemost. 2019;17(11):1785–9.
Asakura H. Classifying types of disseminated intravascular coagulation: clinical and animal models. J Intensive Care. 2014;2(1):20.
Madoiwa S. Recent advances in disseminated intravascular coagulation: endothelial cells and fibrinolysis in sepsis-induced DIC. J Intensive Care. 2015;3:8.
Hoshino K, Kitamura T, Nakamura Y, Irie Y, Matsumoto N, Kawano Y, Ishikura H. Usefulness of plasminogen activator inhibitor-1 as a predictive marker of mortality in sepsis. J Intensive Care. 2017;5:42.
Delabranche X, Helms J, Meziani F. Immunohaemostasis: a new view on haemostasis during sepsis. Ann Intensive Care. 2017;7(1):117.
Iba T, Kidokoro A, Fukunaga M, Nagakari K, Shirahama A, Ida Y. Activated protein C improves the visceral microcirculation by attenuating the leukocyte-endothelial interaction in a rat lipopolysaccharide model. Crit Care Med. 2005;33(2):368–72.
Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13(1):34–45.
Pfeiler S, Massberg S, Engelmann B. Biological basis and pathological relevance of microvascular thrombosis. Thromb Res. 2014;133(Suppl 1):S35–7.
Stiel L, Mayeur-Rousse C, Helms J, Meziani F, Mauvieux L. First visualization of circulating neutrophil extracellular traps using cell fluorescence during human septic shock-induced disseminated intravascular coagulation. Thromb Res. 2019;183:153–8.
Liaw PC, Ito T, Iba T, Thachil J, Zeerleder S. DAMP and DIC: the role of extracellular DNA and DNA-binding proteins in the pathogenesis of DIC. Blood Rev. 2016;30(4):257–61.
Yang X, Cheng X, Tang Y, Qiu X, Wang Z, Fu G, Wu J, Kang H, Wang J, Wang H, Chen F, Xiao X, Billiar TR, Lu B. The role of type 1 interferons in coagulation induced by gram-negative bacteria. Blood. 2020;135(14):1087–100.
Levi M, vander Poll T. Coagulation in patients with severe sepsis. Semin Thromb Hemost. 2015;41(1):9–15.
Iba T, Levy JH. Derangement of the endothelial glycocalyx in sepsis. J Thromb Haemost. 2019;17(2):283–94.
Senis YA, Richardson M, Tinlin S, Maurice DH, Giles AR. Changes in the pattern of distribution of von Willebrand factor in rat aortic endothelial cells following thrombin generation in vivo. Br J Haematol. 1996;93(1):195–203.
Krisinger MJ, Goebeler V, Lu Z, Meixner SC, Myles T, Pryzdial EL, Conway EM. Thrombin generates previously unidentified C5 products that support the terminal complement activation pathway. Blood. 2012;120(8):1717–25.
Bitker L, Burrell LM. Classic and nonclassic renin-angiotensin systems in the critically ill. Crit Care Clin. 2019;35(2):213–27.
Pai WY, Lo WY, Hsu T, Peng CT, Wang HJ. Angiotensin-(1–7) inhibits thrombin-induced endothelial phenotypic changes and reactive oxygen species production via NADPH Oxidase 5 downregulation. Front Physiol. 2017;8:994.
Higgins SJ, De Ceunynck K, Kellum JA, Chen X, Gu X, Chaudhry SA, Schulman S, Libermann TA, Lu S, Shapiro NI, Christiani DC, Flaumenhaft R, Parikh SM. Tie2 protects the vasculature against thrombus formation in systemic inflammation. J Clin Invest. 2018;128(4):1471–84.
Moss A. The angiopoietin: Tie 2 interaction: a potential target for future therapies in human vascular disease. Cytokine Growth Factor Rev. 2013;24(6):579–92.
Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis, thrombosis and organ dysfunction. Thromb Res. 2012;129(3):290–5.
Choi Q, Hong KH, Kim JE, Kim HK. Changes in plasma levels of natural anticoagulants in disseminated intravascular coagulation: high prognostic value of antithrombin and protein C in patients with underlying sepsis or severe infection. Ann Lab Med. 2014;34(2):85–91.
Iba T, Levy JH, Levi M, Connors JM, Thachil J. Coagulopathy of Coronavirus Disease 2019. Crit Care Med. 2020. https://doi.org/10.1097/CCM.0000000000004458 (PMID: 32467443; PMCID: PMC7255402).
Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, Muenchhoff M, Hellmuth JC, Ledderose S, Schulz H, Scherer C, Rudelius M, Zoller M, Höchter D, Keppler O, Teupser D, Zwißler B, Bergwelt-Baildon M, Kääb S, Massberg S, Pekayvaz K, Stark K. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020. https://doi.org/10.1161/CIRCULATIONAHA.120.048488.
Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M, Falco M, Albano G, Menicanti L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18(7):1747–51.
Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–32.
Wright FL, Vogler TO, Moore EE, Moore HB, Wohlauer MV, Urban S, Nydam TL, Moore PK, McIntyre RC Jr. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg. 2020;231(2):193-203.e1.
Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, FerrazdaSilva LF, de PierreOliveira E, NascimentoSaldiva PH, Mauad T, MarciaNegri E. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost. 2020. https://doi.org/10.1111/jth.14844.
Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–7.
Paranjpe I, Fuster V, Lala A, Russak AJ, Glicksberg BS, Levin MA, Charney AW, Narula J, Fayad ZA, Bagiella E, Zhao S, Nadkarni GN. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients With COVID-19. J Am Coll Cardiol. 2020;76(1):122–4.
Asakura H, Kamikubo Y, Goto A, Shiratori Y, Yamazaki M, Jokaji H, Saito M, Uotani C, Kumabashiri I, Morishita E, et al. Role of tissue factor in disseminated intravascular coagulation. Thromb Res. 1995;80(3):217–24.
Levi M. Management of cancer-associated disseminated intravascular coagulation. Thromb Res. 2016;140(Suppl 1):S66-70.
Koseki M, Asada N, Uryu H, Takeuchi M, Asakura H, Matsue K. Successful combined use of tranexamic acid and unfractionated heparin for life-threatening bleeding associated with intravascular coagulation in a patient with chronic myelogenous leukemia in blast crisis. Int J Hematol. 2007;86(5):403–6.
Posma JJ, Posthuma JJ, Spronk HM. Coagulation and non-coagulation effects of thrombin. J Thromb Haemost. 2016;14(10):1908–16.
Levi M, Vincent JL, Tanaka K, Radford AH, Kayanoki T, Fineberg DA, Hoppensteadt D, Fareed J. Effect of a recombinant human soluble thrombomodulin on baseline coagulation biomarker levels and mortality outcome in patients with sepsis-associated coagulopathy. Crit Care Med. 2020;48(8):1140–7.
Semeraro F, Ammollo CT, Caironi P, Masson S, Latini R, Panigada M, Semeraro N, Gattinoni L, Colucci M. Low D-dimer levels in sepsis: good or bad? Thromb Res. 2019;174:13–5.
Koyama K, Madoiwa S, Nunomiya S, Koinuma T, Wada M, Sakata A, Ohmori T, Mimuro J, Sakata Y. Combination of thrombin-antithrombin complex, plasminogen activator inhibitor-1, and protein C activity for early identification of severe coagulopathy in initial phase of sepsis: a prospective observational study. Crit Care. 2014;18(1):R13.
Müller RB, Ostrowski SR, Haase N, Wetterslev J, Perner A, Johansson PI. Markers of endothelial damage and coagulation impairment in patients with severe sepsis resuscitated with hydroxyethyl starch 130/0.42 vs Ringer acetate. J Crit Care. 2016;32:16–20.
Inkinen N, Pettilä V, Lakkisto P, Kuitunen A, Jukarainen S, Bendel S, Inkinen O, Ala-Kokko T, Vaara ST, FINNAKI Study Group. Association of endothelial and glycocalyx injury biomarkers with fluid administration, development of acute kidney injury, and 90-day mortality: data from the FINNAKI observational study. Ann Intensive Care. 2019;9(1):103.
Lafon T, Cazalis MA, Vallejo C, Tazarourte K, Blein S, Pachot A, Laterre PF, Laribi S, François B, TRIAGE study group. Prognostic performance of endothelial biomarkers to early predict clinical deterioration of patients with suspected bacterial infection and sepsis admitted to the emergency department. Ann Intensive Care. 2020;10(1):113.
Iba T, Umemura Y, Watanabe E, Wada T, Hayashida K, Kushimoto S, Japanese Surviving Sepsis Campaign Guideline Working Group for disseminated intravascular cogulation. Diagnosis of sepsis-induced disseminated intravascular coagulation and coagulopathy. Acute Med Surg. 2019;6(3):223–32.
Iba T, Arakawa M, Di Nisio M, Gando S, Anan H, Sato K, Ueki Y, Levy JH, Thachil J. Newly proposed sepsis-induced coagulopathy precedes International Society on Thrombosis and Haemostasis overt-disseminated intravascular coagulation and predicts high mortality. J Intensive Care Med. 2020;35(7):643–9.
Jackson Chornenki NL, Dwivedi DJ, Kwong AC, Zamir N, Fox-Robichaud AE, Liaw PC, Canadian Critical Care Translational Biology Group. Identification of hemostatic markers that define the pre-DIC state: a multi-center observational study. J Thromb Haemost. 2020. https://doi.org/10.1111/jth.14973.
Yamakawa K, Yoshimura J, Ito T, Hayakawa M, Hamasaki T, Fujimi S. External validation of the two newly proposed criteria for assessing coagulopathy in sepsis. Thromb Haemost. 2019;119(2):203–12.
Vincent JL, Francois B, Zabolotskikh I, Daga MK, Lascarrou JB, Kirov MY, Pettilä V, Wittebole X, Meziani F, Mercier E, Lobo SM, Barie PS, Crowther M, Esmon CT, Fareed J, Gando S, Gorelick KJ, Levi M, Mira JP, Opal SM, Parrillo J, Russell JA, Saito H, Tsuruta K, Sakai T, Fineberg D, SCARLET Trial Group. Effect of a recombinant human soluble thrombomodulin on mortality in patients with sepsis-associated coagulopathy. JAMA. 2019;321(20):1993–2002.
Ding R, Wang Z, Lin Y, Liu B, Zhang Z, Ma X. Comparison of a new criteria for sepsis-induced coagulopathy and International Society on Thrombosis and Haemostasis disseminated intravascular coagulation score in critically ill patients with sepsis 30: a retrospective study. Blood Coagul Fibrinolysis. 2018;29(6):551–8.
Helms J, Severac F, Merdji H, Clere-Jehl R, François B, Mercier E, Quenot JP, Meziani F, CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). Performances of disseminated intravascular coagulation scoring systems in septic shock patients. Ann Intensive Care. 2020;10(1):92.
Iba T, Levy JH, Yamakawa K, Thachil J, Warkentin TE, Levi M, Scientific and Standardization Committee on DIC of the International Society on Thrombosis and Haemostasis. Proposal of a two-step process for the diagnosis of sepsis-induced disseminated intravascular coagulation. J Thromb Haemost. 2019;17(8):1265–8.
Iba T, Arakawa M, Mochizuki K, Nishida O, Wada H, Levy JH. Usefulness of measuring changes in SOFA score for the prediction of 28-day mortality in patients with sepsis-associated disseminated intravascular coagulation. Clin Appl Thromb Hemost. 2019;25:1076029618824044.
Gando S, Iba T, Eguchi Y, Ohtomo Y, Okamoto K, Koseki K, Mayumi T, Murata A, Ikeda T, Ishikura H, Ueyama M, Ogura H, Kushimoto S, Saitoh D, Endo S, Shimazaki S, Japanese Association for Acute Medicine Disseminated Intravascular Coagulation (JAAM DIC) Study Group. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med. 2006;34(3):625–31.
Asakura H, Takahashi H, Uchiyama T, Eguchi Y, Okamoto K, Kawasugi K, Madoiwa S, Wada H. Proposal for new diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb J. 2016;14:42.
Wada H, Takahashi H, Uchiyama T, Eguchi Y, Okamoto K, Kawasugi K, Madoiwa S, Asakura H. The approval of revised diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb J. 2017;15:17.
Aota T, Wada H, Fujimoto N, Yamashita Y, Matsumoto T, Ohishi K, Suzuki K, Imai H, Usui M, Isaji S, Uchiyama T, Seki Y, Katayama N. Evaluation of the diagnostic criteria for the basic type of DIC established by the Japanese Society of Thrombosis and Hemostasis. Clin Appl Thromb Hemost. 2017;23:838–43.
Vincent JL, Francois B, Zabolotskikh I, Daga MK, Lascarrou JB, Kirov MY, Pettilä V, Wittebole X, Meziani F, Mercier E, Lobo SM, Barie PS, Crowther M, Esmon CT, Fareed J, Gando S, Gorelick KJ, Levi M, Mira JP, Opal SM, Parrillo J, Russell JA, Saito H, Tsuruta K, Sakai T, Fineberg D, SCARLET Trial Group. Effect of a recombinant human soluble thrombomodulin on mortality in patients with sepsis-associated coagulopathy: the SCARLET randomized clinical trial. JAMA. 2019;321(20):1993–2002.
Warren BL, Eid A, Singer P, Pillay SS, Carl P, Novak I, Chalupa P, Atherstone A, Pénzes I, Kübler A, Knaub S, Keinecke HO, Heinrichs H, Schindel F, Juers M, Bone RC, Opal SM, KyberSept Trial Study Group. Caring for the critically ill patient High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286(15):1869–78.
Umemura Y, Yamakava K, Ogura H. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: a meta-analysis of randomized controlled trials. J Thromb Haemost. 2015;14:518–30.
Wiedermann CJ. Antithrombin concentrate use in disseminated intravascular coagulation of sepsis: meta-analyses revisited. J Thromb Haemost. 2018;16(3):455–7.
Nishida O, Ogura H, Egi M, Fujishima S, Hayashi Y, Iba T, Imaizumi H, Inoue S, Kakihana Y, Kotani J, Kushimoto S, Masuda Y, Matsuda N, Matsushima A, Nakada TA, Nakagawa S, Nunomiya S, Sadahiro T, Shime N, Yatabe T, Hara Y, Hayashida K, Kondo Y, Sumi Y, Yasuda H, Aoyama K, Azuhata T, Doi K, Doi M, Fujimura N, Fuke R, Fukuda T, Goto K, Hasegawa R, Hashimoto S, Hatakeyama J, Hayakawa M, Hifumi T, Higashibeppu N, Hirai K, Hirose T, Ide K, Kaizuka Y, Kan’o T, Kawasaki T, Kuroda H, Matsuda A, Matsumoto S, Nagae M, Onodera M, Ohnuma T, Oshima K, Saito N, Sakamoto S, Sakuraya M, Sasano M, Sato N, Sawamura A, Shimizu K, Shirai K, Takei T, Takeuchi M, Takimoto K, Taniguchi T, Tatsumi H, Tsuruta R, Yama N, Yamakawa K, Yamashita C, Yamashita K, Yoshida T, Tanaka H, Oda S. The Japanese clinical practice guidelines for management of sepsis and septic shock 2016 (J-SSCG 2016). J Intensive Care. 2018;6:7.
Fan Y, Jiang M, Gong D, Zou C. Efficacy and safety of low-molecular-weight heparin in patients with sepsis: a meta-analysis of randomized controlled trials. Sci Rep. 2016;6:25984.
Funding
This work was supported in part by a Grant-in-Aid for Special Research in Subsidies for ordinary expenses of private schools from The Promotion and Mutual Aid Corporation for Private Schools of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Iba T has received a research grant from Japan Blood Products Organization and JIMRO. Connors JM receives personal fees from Bristol-Meyer Squibb, Abbott, Portola, and research funding to the institution from CSL Behring. Levy JH serves on the Steering or Advisory Committees for Instrumentation Laboratories, Merck, Octapharma, and Leading Biosciences. The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Iba, T., Connors, J.M., Nagaoka, I. et al. Recent advances in the research and management of sepsis-associated DIC. Int J Hematol 113, 24–33 (2021). https://doi.org/10.1007/s12185-020-03053-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-020-03053-y