Abstract
We present the case of a 63-year-old male with pure white cell aplasia (PWCA), a rare complication of thymoma, who was successfully treated with cyclosporine A (CyA) and thymectomy. The patient presented with high fever and agranulocytosis. Complete blood count revealed a white blood cell count of 0.9 × 109/L (3% neutrophils), a hemoglobin level of 15.8 g/dL, and a platelet count of 308 × 109/L. Bone marrow (BM) aspiration revealed a hypocellular marrow lacking granulocytes. Computed tomography showed a large anterior mediastinal mass, and the patient was diagnosed with PWCA associated with thymoma. Thirteen days after the initiation of CyA treatment, myeloid cells appeared in the BM, and the neutrophil count in peripheral blood started to increase on day 18. Thymectomy was performed 3 months later. Although CyA treatment was discontinued after thymectomy, complete remission has been maintained for over 4 years. In vitro colony-forming unit granulocyte–macrophage (CFU-GM) assay using the patient’s serum showed severe suppression of CFU-GM colonies in the presence of the patient’s serum, suggesting the presence of CFU-GM inhibitor in the patient’s serum. The efficacy of the immunosuppressive therapy and the CFU-GM assay suggests the potential involvement of an immunological mechanism in patients with thymoma-associated PWCA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pure white cell aplasia (PWCA) is a rare hematological disease [1] with an unknown incidence rate. As far as we know, there are only 20 cases of PWCA that have been reported in 10 literature. PWCA is characterized by agranulocytosis in the peripheral blood and is associated with a profound suppression of entire granulopoiesis in bone marrow (BM), whereas erythropoiesis and megakaryocytopoiesis remain intact [2]. The detailed pathogenesis of PWCA is unclear; however, an association with the inhibitory activity of immunoglobulins on granulopoiesis has been reported [2,3,4]. The association of PWCA with thymoma has also been described [5]. No treatment protocols for PWCA have yet been established. Here, we describe the successful treatment of a patient with PWCA accompanied with thymoma using cyclosporine A (CyA) followed by thymectomy.
Case report
A 63-year-old male experienced temporal agranulocytosis after a viral infection in May 2 years ago of admission to the hospital. His medical history was unremarkable. Three days prior to admission, the patient had developed fever and had been initiated on an oral antibiotic (400 mg of garenoxacin, once daily) by his primary care physician. However, the fever did not improve, and he was admitted to our hospital.
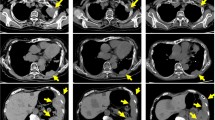
Laboratory blood test results revealed neutropenia (Table 1). The patient’s renal function and coagulation tests were within normal limits. Additionally, his immunoglobulin levels were low (Table 1), and he was negative for anti-nuclear and anti-neutrophil antibodies. Flow cytometric analysis did not detect T/B-cell monoclonal expansion, and no monoclonal rearrangements of the T-cell receptors were evident in peripheral blood. Further tests for hepatitis B and C viruses, Lyme disease, Epstein–Barr virus, cytomegalovirus, Mycoplasma, Chlamydia, Ehrlichia, tuberculosis, and human immunodeficiency virus in serum and bacterial cultures from blood and urine were negative. BM aspiration revealed a normocellular marrow without granulocytes (Table 1). Flow cytometry of the BM aspirate and peripheral cells did not show any evidence for T- or B-cell monoclonality. Lymphocyte subsets were also normal in the peripheral blood and BM. Karyotypes of BM cells appeared normal. Chest X-ray and computed tomography revealed evidence of thymoma (Fig. 1). Based on laboratory and imaging results, the patient was diagnosed with PWCA associated with thymoma, and treatment with granulocyte colony-stimulating factor (G-CSF; filgrastim, 5 µg/kg/day) was initiated on the day of admission to the hospital. However, there was no improvement in the neutrophil counts after 4 days of G-CSF, and BM aspiration did not show any improvement in myelopoiesis after 10 days of G-CSF administration. Therefore, CyA (150 mg/day for three consecutive days) was added 10 days after initiation of G-CSF administration. BM reexamination 13 days after the start of CyA treatment confirmed that normal granulopoiesis had resumed. An increase in neutrophil counts to normal levels occurred following treatment, accompanied by normalization of body temperature and C-reactive protein levels. Laboratory data including BM examination were almost normal 3 months later (Table 1), at which time thymectomy was performed, and CyA treatment was suspended. At the time, laboratory values including BM examination were almost normal (Table 1). Histopathology of the thymectomy specimen revealed benign spindle-cell thymoma. The patient has been in complete remission for over 4 years (Fig. 2).
An in vitro colony-forming unit (CFU) granulocyte–macrophage (CFU-GM) assay was performed in triplicate using normal human mononuclear cells in a methylcellulose-based semisolid culture medium (MethoCult H4434; STEMCELL Technologies, Vancouver, BC, Canada), containing various concentrations [0% (control normal serum only), 5%, 10%, and 20%] of patient serum for 14 days. As shown in Fig. 3, severe suppression of CFU-GM colonies was observed in the presence of 20% patient serum, suggesting the presence of a CFU-GM inhibitor in the patient’s serum.
Discussion
Several mechanisms have been suggested to underlie the pathogenesis of PWCA, such as autoimmunity, drug treatment, and viral infections [1, 2, 6, 7]. In this patient, severe neutropenia and complete lack of granulocytic series in BM were observed 3 days after the initial dose of antibiotics (garenoxacine). We believed that the occurrence of drug-induced agranulocytosis was less likely in 3 days. Specific CFU-GM suppression factors have also been speculated in the pathogenesis of PWCA. In some cases, immunoglobulin G specific for a transiently expressed antigen on immature myeloid cells was proposed to act as a suppressant leading to neutrophil destruction [3, 4]. Our results from the in vitro CFU-GM assay also suggested the presence of suppressive factor(s) in our patient’s serum. The reported efficacy of treatment approaches for PWCA, such as high-dose gamma globulins, plasmapheresis, and antibody therapy, to suppress B-cell activity [8, 9] suggests the involvement of humoral immune responses in the pathogenesis of PWCA. However, the transient effect of treatment and relapse of the disease during the course of such treatments were also reported. Thus, treatment against the humoral immune response might not be sufficient for sustained remission of PWCA.
PWCA is accompanied with thymoma in some cases, and the efficacy of thymectomy in PWCA was reported in several reports [8, 10,11,12,13], suggesting the involvement of thymoma as a pathogenic mechanism in PWCA. As the thymus selects functional, self-tolerant T-cells in a major histocompatibility complex/human leukocyte antigen-dependent manner, thymectomy in PWCA patients might compromise the reactivity and specificity of the selected T-cell repertoire [14]. Thus, deregulation of T-cell immune function might also be involved in the pathogenesis of PWCA in patients with thymoma.
The efficacy of CyA, which has immunosuppressive effects on T-cells, was also reported in the treatment of PWCA and Pure red cell aplasia [10, 15, 16]. CyA and thymectomy, which both might impact the deregulated T-cell function, were effective in our patient with thymoma and PWCA, who might have had suppressive factors circulating in his serum. Thus, these treatment options might also indirectly impact humoral immune responses, probably through a direct effect on the cellular immune responses.
In conclusion, the current case suggested that both the cellular and the humoral immune responses might be involved in the pathogenesis of PWCA in patients with thymoma. Thymectomy followed by CyA might be a useful treatment option that can induce prolonged complete remission in these patients.
References
Levitt LJ. Chlorpropamide-induced pure white cell aplasia. Blood. 1987;69:394–400.
Tamura H, Okamoto M, Yamashita T, Sato C, Watanabe A, Kondo A, et al. Pure white cell aplasia: report of the first case associated with primary biliary cirrhosis. Int J Hematol. 2007;85:97–100.
Levitt LJ, Ries CA, Greenberg PL. Pure white-cell aplasia. Antibody-mediated autoimmune inhibition of granulopoiesis. N Engl J Med. 1983;308:1141–6.
Iida S, Noda T, Banno S, Nitta M, Takada K, Yamamoto M. Pure white cell aplasia (PWCA) with an inhibitor against colony-forming unit of granulocyte-macrophage (CFU-GM). Rinsho Ketsueki. 1990;31:1726–30.
Ackland SP, Bur ME, Adler SS, Robertson M, Baron JM. White blood cell aplasia associated with thymoma. Am J Clin Pathol. 1988;89:260–3.
Mamus SW, Burton JD, Groat JD, Schulte DA, Lobell M, Zanjani ED. Ibuprofen-associated pure white-cell aplasia. N Engl J Med. 1986;314:624–5.
Herzog-Tzarfati K, Shiloah E, Koren-Michowitz M, Minha S, Rapoport MJ. Successful treatment of prolonged agranulocytosis caused by acute parvovirus B19 infection with intravenous immunoglobulins. Eur J Intern Med. 2006;17:439–40.
Yip D, Rasko JE, Lee C, Kronenberg H, O’Neill B. Thymoma and agranulocytosis: two case reports and literature review. Br J Haematol. 1996;95:52–6.
Risitano AM, Selleri C, Serio B, Torelli GF, Kulagin A, Maury S, et al. Alemtuzumab is safe and effective as immunosuppressive treatment for aplastic anaemia and single-lineage marrow failure: a pilot study and a survey from the EBMT WPSAA. Br J Haematol. 2010;148:791–6.
Alvares CL, Svasti-Salee D, Rowley M, Gordon-Smith EC, Marsh JCW. Remission induced by Campath-1H for thymoma-associated agranulocytosis. Ann Hematol. 2004;83:398–400.
Postiglione K, Ferris R, Jaffe JP, Stroncek D. Immune mediated agranulocytosis and anemia associated with thymoma. Am J Hematol. 1995;49:336–40.
Degos L, Faille A, Housset M, Boumsell L, Rabian C, Parames T. Syndrome of neutrophil agranulocytosis, hypogammaglobulinemia, and thymoma. Blood. 1982;60:968–72.
Kobayashi M, Hasegawa T, Iwabuchi S, Fukushima M, Koie H, Kannari K. The effect of thymectomy on myasthenia gravis, thrombocytopenia, and granulocytopenia associated with thymoma: report of a case. Surg Today. 1995;25:1061–5.
Baldwin TA, Hogquist KA, Jameson SC. The fourth way? Harnessing aggressive tendencies in the thymus. J Immunol. 2004;173:6515–20.
Fumeaux Z, Beris P, Borisch B, Sarasin FP, Roosnek E, Dayer J-M, et al. Complete remission of pure white cell aplasia associated with thymoma, autoimmune thyroiditis and type 1 diabetes. Eur J Haematol. 2003;70:186–9.
Wu X, Wang S, Lu X, Shen W, Qiao C, Wu Y, et al. Response to cyclosporine A and corticosteroids in adult patients with acquired pure red cell aplasia: serial experience at a single center. Int J Hematol. 2018;108:123–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Kobayashi, Y., Ando, K., Hata, T. et al. Complete remission of pure white cell aplasia associated with thymoma after thymectomy and cyclosporine administration. Int J Hematol 109, 346–350 (2019). https://doi.org/10.1007/s12185-018-02573-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-018-02573-y