Abstract
It has been suggested that platelet function in chronic immune thrombocytopenic purpura (ITP) may be abnormal. Thrombopoietin mimetics used for treatment can affect it, but the data remain limited. We investigated platelet function of 20 children diagnosed with severe ITP (aged 1–16 years, 12 females and eight males). Platelet functional activity in whole blood was characterized by flow cytometry before and after stimulation with SFLLRN plus collagen-related peptide. Levels of CD42b, PAC1, and CD62P, but not CD61 or annexin V, were significantly increased (P < 0.05) in resting platelets of patients before treatment compared with healthy donors. On average, PAC1 and CD62P in patients after activation were also significantly elevated, although some patients failed to activate integrins. Romiplostim (1–15 μg/kg/week s.c.) was prescribed to seven patients, with clinical improvement in six. Interestingly, one patient had clinical improvement without platelet count increase. Eltrombopag (25–75 mg/day p.o.) was given to four patients, with positive response in one. Others switched to romiplostim, with one stable positive response, one unstable positive response, and one non-responding. Platelet quality improved with romiplostim treatment, and their parameters approached the normal values. Our results suggest that platelets in children with severe ITP are pre-activated and abnormal, but improve with treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune thrombocytopenic purpura (ITP) is an autoimmune disease with complex pathogenesis that is caused by development of auto-antibodies against platelets and megakaryocytes and T cell-mediated lysis [1]. This dramatically promotes platelet clearance as well as disrupts their production leading to different degrees of thrombocytopenia and bleeding.
Importantly, not only platelet count, but also platelet function can be impaired in a fraction of the ITP patients according to some reports [2]. There is no reliable correlation between severity of hemorrhages and the platelet count [3]. The classic clinical assays like aggregometry cannot be used in thrombocytopenia, and the available data on platelet function in ITP are mostly from flow cytometry with divergence of the results and great differences in protocols. Some studies reported no differences in platelet activity between healthy adults and patients [2], and others reported that platelets in ITP are pre-activated [4].
An important recent option for treatment of ITP is new thrombopoietin mimetics, romiplostim and eltrombopag [5]. They were both approved for treatment of adult ITP, and were reported to affect not only platelet number (with some chance of long-term remission), but also platelet function. However, the data are scarce and conflicting as well. The research was almost exclusively done for eltrombopag. Some studies reported no significant effect of the drug on platelet function [6], while others observed some improvement in patients [4], possibly as a result of platelet count increase [4]. It is important to clarify this issue, because improvement of platelet function might mean that the drug can have beneficial effects even when it fails to raise the platelet count (which is essential information, because the expensive treatment could otherwise be discontinued). On the other hand, like with any pro-hemostatic drugs, improvement of platelet function beyond normal might mean thrombotic risks.
Childhood ITP affects 4–6 of 100 000 children per year, and is believed to be a relatively benign hematological disorder that resolves spontaneously in the majority of cases. However, at least 30% of patients have persistent disease, and 5–10% develop severe chronic disorder [7]. The problem of poor characterization of platelet function in the patients (with or without relation to treatment) is probably even more urgent for childhood ITP because there are less data. One study reported, using specially developed platelet reactivity and microaggregation assays, that platelet function is impaired in chronic childhood ITP depending on the bleeding phenotype [8]. Another report also found correlation between bleeding score and platelet function [9].
Romiplostim and eltrombopag are currently used off-label in childhood ITP. Several trials and case series [10–16] showed that they are effective [7]; the results of phase 3 trials are just becoming available now [17, 18]. The reports on their effect on platelet function are even more scarce; one study revealed changes in immature platelet fraction upon eltrombopag treatment [19].
Therefore, there is presently an unclear picture: (a) the data on platelet function in ITP and on the effect of eltrombopag are minimal and conflicting (particularly scarce for children); (b) there are no data at all for romiplostim, either for children or adults. To address this, we investigated a small group of 20 pediatric patients, to some of whom romiplostim or eltrombopag were prescribed. Flow cytometry revealed essential heterogeneity of platelet function in the untreated group, with both refractory and pre-activated platelets present in different patients, and found that treatment generally improved platelet function, sometimes independently of platelet count.
Materials and methods
Patients and donors
Children aged 1–16 years were included in this observational study. Blood obtained from adult healthy individuals served as positive controls. Investigations were performed in accordance with the Declaration of Helsinki under a protocol approved by the NSPC CHOI Ethical Committee, and written informed consent was obtained from all donors and patients, or their parents. Thrombocytopenia was defined as platelet count less than 100 × 103/µl, with persistent ITP lasting for 3–12 months and chronic ITP lasting for more than 12 months. Bleeding was evaluated using the SMO bleeding scale [20]. Blood samples from 10 healthy volunteers who claimed to abstain from platelet-affecting drugs were used as controls.
Materials
Annexin V-Alexa647 and antibodies against P-selectin (CD62P-Alexa647), integrin αIIβ3 (CD61-PE), its activation (PAC1-FITC), glycoprotein I (CD42b-PE) were from Biolegend (San Diego, CA, USA). Collagen-related peptide (CRP) was kindly provided by Prof. R.W. Farndale (University of Cambridge, Cambridge, UK). All other reagents were from Sigma-Aldrich (St Louis, MO, USA).
Flow cytometry evaluation of platelet function
Blood was collected by venipuncture into 3-ml vacuum citrate tubes, which were then centrifuged for 3 min at 100 g to obtain platelet-rich plasma. The number of platelets was determined with an automated hematological analyzer; for donors, it was adjusted to 30 × 103 µl−1. Samples were diluted with buffer A (150 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 0.4 mM NaH2PO4, 20 mM HEPES, 5 mM glucose, 0.5% bovine serum albumin, pH 7.4 [21, 22]) when platelet concentration exceeded 200 × 103 µl−1. Platelets were either left intact or loaded with mepacrin (1 mM) for 30 min at 37 °C. Subsequently, they were either left unstimulated or stimulated with CRP at 20 µg/µl and SFLLRN at 12.5 µM for 10 min in the presence of 2.5 mM calcium chloride. Both resting and activated samples were incubated with antibodies against CD61, CD42b, CD62P, as well as PAC1 and annexin V for 10 min. Then, they were diluted 10-fold with buffer A containing 2.5 mM calcium, and analyzed using either BD Accuri C6 (BD Biosciences, San Jose, CA, USA) or Novocyte (Acea Bioscience, San Diego, CA, USA) flow cytometer.
Statistics
To test hypotheses, we used Student’s two-sample t test. The significance level was set as 95%.
Results
Baseline characteristics
A total of 20 patients, all outpatients at Federal Research and Clinical Center of Pediatric Hematology, Oncology and Immunology, were included in the study (Table 1). They were aged 1–16 years, with 12 girls and 8 boys. All had a previous history of unsuccessful treatment (2–6 lines of therapy). Duration of the disease was 3–132 months from the diagnosis; severe chronic form was diagnosed in 14 of the patients; others had persistent ITP. All patients had thrombocytopenic purpura, and 15 out of 20 had significant hemorrhage (nasal, oral, uterine, intestinal, CNS).
Platelet function in patients with ITP before treatment
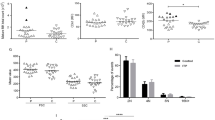
All patients had their blood collected before treatment, but only 10 of them produced reliable platelet measurements, probably because of pre-activation and aggregation as described below (patient numbers 1, 2, 3, 5, 8, 9, 11, 13, 15, 16). Figure 1 shows that patients have significantly higher CD42b (by a factor of 1.5–2.1, P < 0.05), but not CD61 (Fig. 1a). Integrins were generally pre-activated (Fig. 1b), and the number of active integrins remained higher even after activation. The same was observed for P-selectin (Fig. 1c), but not procoagulant activity, where effects were not significant (Fig. 1d). Dense granule function in the patients was normal (Fig. 1e).
Baseline parameters of platelet functional activity in ITP patients. Platelets of 10 patients and 10 healthy donors were characterized by flow cytometry before and after stimulation with CRP plus TRAP as described under “Materials and methods”. a Levels of CD42b and CD61 prior to stimulation. b PAC1, c P-selectin, d the percentage of procoagulant platelets before and after stimulation. The symbols show mean values, horizontal lines are medians, boxes show 25–75 percentiles, error bars show 5–95% intervals, and X symbols show maximal and minimal values. Asterisks indicate statistical significance at P < 0.05
Pre-activation of platelets in ITP compared with normal ones is illustrated for integrins in online supplement Fig. S1A. Two of the patients exhibited refractory behavior in combination with pre-activation. Representative data are shown in Fig. S1B; for comparison, a typical healthy donor is shown in Fig. S1C. As a result of spontaneous activation, resting platelets from an number of ITP patients aggregated and were poorly observable (Fig. S1D), and this was corrected after 5 months of treatment (Fig. S1E).
Therapy and clinical response
Out of 20 patients, thrombopoietin mimetics were prescribed to 11 (Table 2). They were given as a monotherapy. In the patients, who did not respond by platelet increase and/or had bleedings, rescue therapy was provided as a supplement to thrombopoietin mimetics as listed in Table 2. Romiplostim (1–15 μg/kg/week s.c.) was prescribed to 7 patients, with clinical improvement in 6 of them. Interestingly, one patient had clinical improvement without platelet count increase. Eltrombopag (25–75 mg/day p.o.) was given to 4 patients, with positive response in 1. Other 3 patients were switched to romiplostim, with 1 positive response, 1 unstable positive response, and 1 non-responding. As a summary clinical outcome, thrombopoietin mimetics significantly improved hemostasis in 9 patients out of 11 to whom they had been prescribed; only 2 patients still had hemorrhages, none of them severe. We did not observe relation of the response to age: age of the responders was uniformly ranged from 20 months to 16 years, with both non-responders aged 5 years.
Platelet function during treatment
Five patients were followed during their therapy with romiplostim; the data of flow cytometry characterization of their function are shown in supplementary Fig. S2.
For patient 10, who did not respond either with platelet count or bleeding reduction, the number of platelets in almost all time points was too small for reliable measurements (likely because of pre-activation and aggregation). The only exception is point 3, where extremely high pre-activation (PAC1 data in Fig. S2C, P-selectin data in Fig S2E, annexin V-binding in Fig. S2G) is obvious.
Patient 4 is the one who did not respond to eltrombopag but responded to romiplostim successfully. At point 0, platelet function measurement was not possible; at later stages, platelets are pre-activated (PAC1 data in Fig. S2C; note that point 2 seems better than point 1), but detectable. Like with patient 10, response of her platelets to stimulation is quite high.
Patient 16 is an example of unstable response to treatment. Interestingly, his platelets are also of poor quality, with significant pre-activation where analysis was possible. The data in Fig. S3 indicate that, at point 2, his platelets did not bind antibodies against integrins (for comparison, data for patient 4 are shown side by side, for integrin and for glycoproteins Ib.
One of the most interesting dynamic responses to romiplostim was that of patient 2, who demonstrates stable clinical improvement without platelet count increase. The laboratory parameters of platelet function show steady improvement, though platelets are pre-activated in almost all cases.
Finally, patient 6 is the only one among these five with steady and stable response to therapy (another is patient 4, but she was on eltrombopag first). All parameters of her platelets (including integrins, alpha-granules, procoagulant activity, both for pre-activation and post-stimulation) are steadily improved over the course of therapy. The only exception is dense granule function (Fig. S2B) that was originally normal and remained so.
Discussion
This small-scale observational study investigated the baseline state of platelet function in children with chronic ITP and their response to therapy with thrombopoietin mimetics (mostly romiplostim) using flow cytometry. The following conclusions were reached:
-
1)
Platelet function in this patient group before treatment is significantly abnormal. Their level of background platelet activation (judging by integrin activation and P-selectin externalization) is significantly increased. Platelet functions after stimulation were mostly normal or higher than normal, though in some patients pre-activation was combined with refractoriness.
-
2)
Initial clinical response to romiplostim and eltrombopag agrees well with previous reports [10, 11, 13, 15, 16]. Most intriguingly, one patient responded to romiplostim clinically without platelet count increase.
-
3)
Platelet functions significantly change during romiplostim therapy in each patient. Background activation seems to decrease, while responses to activation may go up and down, but not below the normal level. This could possibly explain the clinical response in the romiplostim patient who did not have a platelet count increase.
The observation of platelet pre-activation is novel for childhood ITP to the best of our knowledge, but the result agrees well with the previous report on increased platelet background activation in adult ITP [4]. Both these studies disagree with report [2] that found no differences. Additional research is required to ascertain the significance of the observed pre-activation, but the fact that some patients had 30–60% level of integrin activation in resting platelets, quite overlapping with the range for normal stimulated platelets, does suggest some possible clinical relevance. The group size is not sufficient to look for correlation of bleeding phenotype with platelet function (like in [8, 9]), but some patients did show impaired responses to stimulation.
The clinical response of children to romiplostim is relatively well characterized, and the present study agrees well both initial studies and the recent phase 3 trial [17]. It is, however, interesting that a patient with no stable increase in platelet count but a clear clinical improvement was observed even in the small selection of the present study. This additionally stimulates speculation on the possible changes in platelet function.
In contrast to eltrombopag, there were no data on the effect of romiplostim on platelet function in either adults or children with ITP. The data of the present study suggest that the platelet functions do change significantly, but the sample size does not allow statistical analysis of the groups. Decrease of background activation and normalization of the platelet response can be traced for individual patients, which might be important to avoid thrombosis risks (otherwise, increase of platelet count without decreasing ITP-dependent pre-activation could be dangerous). Platelet function improvement is observed for all patients with positive clinical response (it is particularly interesting for the patient with no platelet count increase). However, additional study of a larger group is required to quantitatively characterize this.
References
Nomura S. Advances in diagnosis and treatments for immune thrombocytopenia. Clin Med Insights Blood Disord. 2016;9:15–22.
Connor DE, Ma DD, Joseph JE. Flow cytometry demonstrates differences in platelet reactivity and microparticle formation in subjects with thrombocytopenia or thrombocytosis due to primary haematological disorders. Thromb Res. 2013;132:572–7.
Cuker A, Cines DB, Neunert CE. Controversies in the treatment of immune thrombocytopenia. Curr Opin Hematol. 2016;23:479–85.
Psaila B, Bussel JB, Linden MD, Babula B, Li Y, Barnard MR, et al. In vivo effects of eltrombopag on platelet function in immune thrombocytopenia: no evidence of platelet activation. Blood. 2012;119:4066–72.
Schifferli A, Kuhne T. Thrombopoietin receptor agonists: a new immune modulatory strategy in immune thrombocytopenia? Semin Hematol. 2016;53(Suppl 1):S31–4.
Haselboeck J, Kaider A, Pabinger I, Panzer S. Function of eltrombopag-induced platelets compared to platelets from control patients with immune thrombocytopenia. Thromb Haemost. 2013;109:676–83.
Journeycake JM. Childhood immune thrombocytopenia: role of rituximab, recombinant thrombopoietin, and other new therapeutics. Hematology Am Soc Hematol Educ Program. 2012;2012:444–9.
van Bladel ER, Laarhoven AG, van der Heijden LB, Heitink-Polle KM, Porcelijn L, van der Schoot CE, et al. Functional platelet defects in children with severe chronic ITP as tested with 2 novel assays applicable for low platelet counts. Blood. 2014;123:1556–63.
Frelinger AL 3rd, Grace RF, Gerrits AJ, Berny-Lang MA, Brown T, Carmichael SL, et al. Platelet function tests, independent of platelet count, are associated with bleeding severity in ITP. Blood. 2015;126:873–9.
Bussel JB, Buchanan GR, Nugent DJ, Gnarra DJ, Bomgaars LR, Blanchette VS, et al. A randomized, double-blind study of romiplostim to determine its safety and efficacy in children with immune thrombocytopenia. Blood. 2011;118:28–36.
Bussel JB, de Miguel PG, Despotovic JM, Grainger JD, Sevilla J, Blanchette VS, et al. Eltrombopag for the treatment of children with persistent and chronic immune thrombocytopenia (PETIT): a randomised, multicentre, placebo-controlled study. Lancet Haematol. 2015;2:e315–25.
Pasquet M, Aladjidi N, Guiton C, Courcoux MF, Munzer M, Auvrignon A, et al. Romiplostim in children with chronic immune thrombocytopenia (ITP): the French experience. Br J Haematol. 2014;164:266–71.
Bussel JB, Hsieh L, Buchanan GR, Stine K, Kalpatthi R, Gnarra DJ et al. Long-term use of the thrombopoietin-mimetic romiplostim in children with severe chronic immune thrombocytopenia (ITP). Pediatr Blood Cancer 2014;62:208–213.
Mokhtar GM, Tantawy AA, El Sherif NH. Romiplostim therapy in children with unresponsive chronic immune thrombocytopenia. Platelets. 2012;23:264–73.
Escudero Vilaplana V, Aragones JH, Fernandez-Llamazares CM, Bieler CB, Rodriguez SM, Saez MS. Use of romiplostim for primary immune thrombocytopenia in children. Pediatr Hematol Oncol. 2012;29:197–205.
Elalfy MS, Abdelmaksoud AA, Eltonbary KY. Romiplostim in children with chronic refractory ITP: randomized placebo controlled study. Ann Hematol. 2011;90:1341–4.
Tarantino MD, Bussel JB, Blanchette VS, Despotovic J, Bennett C, Raj A, et al. Romiplostim in children with immune thrombocytopenia: a phase 3, randomised, double-blind, placebo-controlled study. Lancet. 2016;388:45–54.
Grainger JD, Locatelli F, Chotsampancharoen T, Donyush E, Pongtanakul B, Komvilaisak P, et al. Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;386:1649–58.
Gerrits AJ, Leven EA, Frelinger AL 3rd, Brigstocke SL, Berny-Lang MA, Mitchell WB, et al. Effects of eltrombopag on platelet count and platelet activation in Wiskott-Aldrich syndrome/X-linked thrombocytopenia. Blood. 2015;126:1367–78.
Rodeghiero F, Michel M, Gernsheimer T, Ruggeri M, Blanchette V, Bussel JB, et al. Standardization of bleeding assessment in immune thrombocytopenia: report from the International Working Group. Blood. 2013;121:2596–606.
Abaeva AA, Canault M, Kotova YN, Obydennyy SI, Yakimenko AO, Podoplelova NA, et al. Procoagulant platelets form an alpha-granule protein-covered “cap” on their surface that promotes their attachment to aggregates. J Biol Chem. 2013;288:29621–32.
Podoplelova NA, Sveshnikova AN, Kotova YN, Eckly A, Receveur N, Nechipurenko DY et al. Coagulation factors bound to procoagulant platelets concentrate in cap structures to promote clotting. Blood. 2016;128:1745–1755.
Acknowledgements
We thank Prof. R.W. Farndale for providing CRP. The study was supported by the Grant from the endowment fund “Doctors, Innovations, Science for Children”, and by the Russian President Grant MD-229.2017.4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
E. V. Suntsova, I. M. Demina, A. A. Ignatova contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Suntsova, E.V., Demina, I.M., Ignatova, A.A. et al. Bleeding tendency and platelet function during treatment with romiplostim in children with severe immune thrombocytopenic purpura. Int J Hematol 105, 841–848 (2017). https://doi.org/10.1007/s12185-017-2207-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-017-2207-3