Abstract
Influenza virus infection can cause fatal complications (e.g., pneumonia) in immunodeficient long-term survivors of allogeneic hematopoietic stem cell transplantation (allo-HSCT). The immune response to the vaccine improves if it is administered at >1 year after allo-HSCT, although the response may vary according to the patient’s immune status. We sought to identify predictors of immune response to trivalent inactivated influenza vaccine (TIV) among patients vaccinated at >1 year after allo-HSCT. We included 27 allo-HSCT recipients, with a median interval of 4.3 years (range 1.0–10.1 years) from transplantation to vaccination. Nineteen patients achieved a response to TIV, although a low immune response to TIV was significantly associated with calcineurin inhibitor treatment, and moderate chronic graft-versus-host disease and IgM levels of <0.5 g/L at the time of vaccination. Multivariate analysis revealed that IgM levels of <0.5 g/L at the vaccination were an independent predictor of a low immune response to TIV. These results indicate that a more effective approach is needed to induce a vaccine-specific immune response among long-term survivors of allo-HSCT who have low serum IgM levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Influenza virus infections account for ≥30% of all respiratory viral infections among recipients of allogeneic hematopoietic stem cell transplantation (allo-HSCT), and these infections are associated with considerable morbidity and mortality [1,2,3,4,5]. Furthermore, the incidence of influenza pneumonia among allo-HSCT recipients is 26–33%, and the related morality rate is approximately 12–30% [3, 6,7,8]. Vaccination is considered a useful strategy for preventing influenza infection among allo-HSCT recipients [9], and the influenza vaccine is generally recommended for allo-HSCT recipients at >6 months after transplantation, although the vaccine may be given at >4 months after allo-HSCT during an influenza outbreak [10, 11]. However, the humoral immune response to the influenza vaccine is poor among allo-HSCT recipients, compared to that among healthy controls [3]. Several investigators have suggested that the timing of the vaccination may predict the serological response, and have demonstrated that its immunogenicity was improved if the vaccine was administered >1 year after allo-HSCT [12,13,14,15]. In contrast, immune reconstitution is slow among allo-HSCT recipients who receive prolonged immunosuppressive treatment for graft-versus-host disease (GVHD) [16], and the immune response to the influenza vaccine may vary according to the patient’s immune status. Furthermore, most immunodeficient long-term survivors of allo-HSCT visit outpatient clinics, and these patients have a high risk of influenza virus infection during the prevalent season. In fact, severe influenza infections can occur at several years after allo-HSCT, especially among patients with chronic GVHD [17]. Thus, it is important to identify factors that can predict the immune response to the influenza vaccine to optimize long-term outcomes among allo-HSCT recipients. However, there are few studies regarding the immune response to the influenza vaccine among only long-term allo-HSCT survivors, and it remains unclear whether any factors are associated with the serological response in this population. Therefore, the present study evaluated long-term allo-HSCT survivors vaccinated at >1 year after transplantation, to identify factors that might predict their immune response to trivalent inactivated influenza vaccine (TIV).
Patients and methods
Patients
This retrospective study examined consecutive allo-HSCT recipients who received the TIV as outpatients between November 2013 and December 2013. The eligibility criteria were: (a) age of >18 years; (b) Eastern Cooperative Oncology Group performance status of 0–2; (c) sufficient heart, lung, liver, and kidney functions to permit administration of the vaccine; (d) vaccination at >1 year after allo-HSCT; (e) no treatment using intravenous immunoglobulin within the previous 3 months; and (f) no suspected allergies to the ingredients of the vaccine. In accordance with the tenets of the Declaration of Helsinki, all patients provided their informed consent for treatment.
Study design
The design of this retrospective study was approved by the institutional review board of the Hamamatsu University School of Medicine. The patients received a single 0.5-mL dose of the TIV (KAKETSUKEN, Kumamoto, Japan) via hypodermic injection. The TIV contained 15 μg of hemagglutinin protein from each strain: A/California/7/2009 (H1N1) pdm09, A/Texas/50/2012 (H3N2), and B/Massachusetts/02/2012. Each milliliter of the vaccine included formalin (0.01 w/v%), thimerosal (0.005 mg), sodium chloride (8.1 mg), hydrogen phosphate sodium hydrate (2.5 mg), and potassium dihydrogen phosphate (0.4 mg). A baseline hemagglutination inhibition (HAI) test was performed before the vaccination, and the humoral responses to the three antigens (H1N1, H3N2, and B strain) were evaluated 28 days later, as previously described [18]. Laboratory data regarding immunological markers were also collected before the vaccination. Blood samples were obtained before the vaccination, and standard immunofluorescence tests were performed in a reference laboratory to obtain counts of CD3+, CD4+, and CD8+ T cells, as well as counts of CD19+ and CD20+ B cells. The cut-off immunoglobulin levels for comparing patients with or without a response to the TIV were selected based on a previous study’s findings [12]. All patients were monitored for safety and adverse events, and any injection site or systemic reactions during 28 days after the vaccination were recorded. We also evaluated patients for a new diagnosis or worsening of GVHD after the vaccination. Chronic GVHD was diagnosed using clinical and/or histological evidence, based on the standard criteria [19].
Definitions of the TIV response and responders
Serological response was assessed using seroconversion rates and seroprotection rates. Seroconversion rates were defined as the percentages of patients with a 4-fold increase in their post-vaccination titers, compared to their baseline titers. Seroprotection rates were defined as the percentages of patients with a post-vaccination titer of at least 1:40. In this study, “responders” were defined as patients with a serological response to the influenza vaccine, which was based on the achievement of seroconversion and/or seroprotection for at least one influenza antigen at 28 days after the TIV vaccination.
Statistical analysis
Categorical data were compared using the χ 2 test or Fisher’s exact test. Continuous data were compared using Wilcoxon’s rank-sum test. Factors that exhibited a P value of <0.05 in the univariate analysis were included in the multivariate logistic regression model. All statistical analyses were performed using the SPSS software (version 21.0; SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Twenty-seven allo-HSCT recipients (13 patients with acute myeloid leukemia, 12 patients with acute lymphoblastic leukemia, and 2 patients with myelodysplastic syndrome) were vaccinated between November 2013 and December 2013. The clinical characteristics of the patients are shown in Table 1. The median patient age was 42 years (range 19–60 years), and the median time from transplantation to vaccination was 4.3 years (range 1.0–10.1 years). Six patients (23%) received cord blood transplantation. Twelve patients (44% of all patients) had chronic GVHD at the vaccination. Among these patients, five patients had mild chronic GVHD and seven patients had moderate chronic GVHD. No patients had severe chronic GVHD. Seven patients (26%) were receiving calcineurin inhibitors at the vaccination (three patients received a calcineurin inhibitor and mycophenolate mofetil, and four patients received a calcineurin inhibitor and corticosteroids). A prednisone dose of 5–10 mg/day was added to the calcineurin inhibitor treatment for chronic GVHD. We did not detect any patients with uncontrollable active chronic GVHD despite immunosuppressant treatment. Twenty-five patients (93%) had already received at least one influenza vaccine after allo-HSCT. At the vaccination, five patients exhibited lymphocyte counts of <1000/μL. Eleven patients exhibited a CD4+ T-cell count of <500/μL, and 10 patients exhibited a CD8+ T-cell count of <500/μL. Three patients exhibited IgG levels of <4 g/L, 3 patients exhibited IgA levels of <0.5 g/L, and 8 patients exhibited IgM levels of <0.5 g/L.
Serological response to the TIV
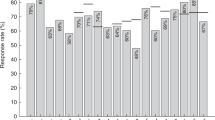
Nineteen patients (70%) achieved a serological response (seroconversion and/or seroprotection for at least one influenza antigen at 28 days after the TIV vaccination). The seroconversion and seroprotection rates for A(H1N1), A(H3N2), B, and for all three influenza antigens after the vaccination are summarized in Table 2.
Factors associated with a serological response
The associations of the clinical and biological characteristics with a low serological response are shown in Table 3. Low serological response to the TIV was significantly associated with calcineurin inhibitor treatment (P = 0.01), moderate chronic GVHD at the vaccination (P = 0.01), and IgM levels of <0.5 g/L (P = 0.002) (Table 3). The patients without a serological response to the TIV tended to have all grades of chronic GVHD at the vaccination (P = 0.09). The donor source for the allo-HSCT, patient age at the vaccination, time from allo-HCT to vaccination (1–2 years vs. ≥2 years), lymphocyte counts, IgG levels, and IgA levels were not significantly associated with the serological response to the vaccination. Multivariate analysis revealed that IgM levels of <0.5 g/L at the vaccination were an independent predictor of a low response to the TIV at >1 year after the transplantation (hazard ratio 19.6; 95% confidence interval: 1.16–330.8, P = 0.04) (Table 4). None of the patients experienced an influenza infection during the 6 months after the vaccination.
Safety
Three patients experienced a localized reaction at the injection site. We did not observe any cases of systemic reactions (e.g., myalgia, fever, headache, or fatigue). None of the patients showed a new diagnosis or worsening of chronic GVHD after the vaccination.
Discussion
Improvements in the best practices and supportive care for allo-HSCT recipients have contributed to better outcomes and an increasing number of long-term allo-HSCT survivors. However, long-term survivors who have chronic GVHD and/or are receiving immunosuppressive therapy experience delayed immune reconstitution, and influenza virus infection can lead to fatal complications (e.g., pneumonia) in these patients. Therefore, vaccination against influenza infection is an important method for optimizing the long-term outcomes after allo-HSCT. Previous studies have revealed that a shorter interval from transplantation to vaccination, rituximab administration during the year before vaccination, lower absolute CD19-positive cell counts, and the existence of active GVHD with immunosuppressive treatment or chronic GVHD at baseline were associated with a reduced response to the influenza vaccine [12,13,14,15]. However, 23–26.8% of the patients enrolled in the previous studies were vaccinated at <1 year after transplantation [12, 13]. Therefore, the factors that influence the immunogenicity of the influenza vaccine among only long-term survivors have not been clearly elucidated. In the present study, we found that serum IgM levels of <0.5 g/L at the time of vaccination were a significant independent predictor of a lower response to TIV among long-term survivors vaccinated at >1 year after allo-HSCT.
Unfortunately, it is difficult to compare our results with rates of serological response to the seasonal influenza vaccine from previous reports, based on differences in the definition of “response” and the use of different vaccine types (e.g., different strains of the influenza virus and adjuvant or no adjuvant) and administered doses (one dose vs. two doses or standard dose vs. high dose). Issa et al. recently assessed the response rates after one dose of an unadjuvanted H1N1 influenza vaccine, based on the percentages of patients with a post-vaccination titer of at least 1:40 (seroprotection rate) [13]. In that study, the response rate was 51.2% among all patients, compared to 38.7 and 69% among patients vaccinated at 1–2 years and >2 years after allo-HSCT, respectively. We observed similar seroprotective rates for the H1N1 antigen among all patients and patients vaccinated at >2 years after allo-HSCT (59.3 and 54.5%, respectively). However, our seroprotective rate for the H1N1 antigen was noticeably higher for patients vaccinated at 1–2 years after allo-HSCT, compared to that from Issa et al.’s study (80 vs. 38.7%) [13]. This discrepancy may be related to the relatively small proportion of patients vaccinated at 1–2 years after allo-HSCT in the present study (19.0 vs. 37.8% in Issa et al.’s study).
We observed that serum IgM levels of <0.5 g/L were a significant predictor of a poor serological response to the influenza vaccine among patients vaccinated at >1 year after allo-HSCT. Given that the data regarding IgM levels are easily accessible in the clinical setting, this marker may help predict individual patients’ immune responses to the influenza vaccine. Interestingly, serum IgG and IgA levels were not associated with the response rate to the vaccine in the present study. Mohty et al. reported a significant relationship between serum immunoglobulin levels and a serological response in their univariate analysis [12], as well as a trend towards a lower response rate among patients with serum IgM levels of <0.5 g/L in their multivariate analysis; these results support the findings of the present study. In this context, circulating B-cell counts reach their normal levels at 1–2 years after allo-HSCT [20], donor B cells initially emerge with a naïve phenotype (IgM+ and IgD+), and memory B cells can take up to 5 years to mature [21, 22]. IgM levels typically recover during the first 2–6 months, while IgG levels become normalized at 3–18 months after allo-HSCT, and the reconstitution of IgA may be delayed for up to 3 years [23, 24]. Furthermore, most B cells produce IgM, rather than IgG or IgA, during the first 2 years after allo-HSCT [25]. On the other hand, serum IgG levels may also reflect production from long-lived host plasma cells, which can persist for up to 2 years because of their resistance to the preparative regimen and radiation [26, 27], and IgG levels may not be correlated with B-cell reconstitution after allo-HSCT. Based on these factors, and the findings of the present study, lower serum IgM levels at the vaccination may be a more accurate marker of B-cell reconstitution after allo-HSCT, compared to serum levels of IgG or IgA.
The present study also revealed that moderate chronic GVHD and concurrent calcineurin inhibitor therapy were associated with a lower serological response in the univariate analysis, although we did not observe any significant associations in the multivariate analysis. In contrast, previous studies have reported that these factors influenced immunogenicity after vaccination among allo-HSCT recipients [9, 12, 15, 28]. This relationship remains plausible, as these factors could be attributed to the prolonged reconstitution of B cells. This difference may also be related to the small sample size and absence of severe chronic GVHD in the present study, which could limit the accuracy of our analysis. For example, the number of patients with moderate/severe chronic GVHD and immunosuppressive treatment may affect the apparent response to the TIV. Additional large-scale studies are needed to clarify the relationship between these factors and the immunogenicity of the influenza vaccine.
Various strategies have been considered to enhance immunogenicity after vaccination among allo-HSCT recipients. Although several investigators have evaluated a two-dose influenza vaccine with an adjuvant among allo-HSCT recipients, this approach remains controversial [12, 28, 29]. Nevertheless, guidelines from the collaboration of several international organizations suggest that two doses of the influenza vaccine could be contemplated if the vaccine is administered at <6 months after allo-HSCT [30]. In contrast, Halasa et al. have recently reported that allo-HSCT recipients achieved a better immunogenicity after receiving a high-dose TIV, compared to the standard vaccination [31]. However, the clinical efficacies of these novel strategies might not be conclusive, based on the small number of enrolled patients. Therefore, large randomized trials are needed to confirm the efficacies of theses novel strategies.
This study has several limitations. First, as previously described, the small sample size may limit the accuracy of our analysis. The effect of chronic GVHD on the apparent immunogenicity of the vaccine might also have been stronger if the cohort was larger and included more patients with moderate or severe chronic GVHD. In addition, the median time from allo-HSCT to vaccination was very long, with only five patients being vaccinated at 1–2 years. Furthermore, only one patient was >60 years at vaccination and majority of patients (93%) had already received at least one influenza vaccine after allo-HSCT. Thus, these patients’ characteristics could have introduced unrecognized bias that might have influenced the TIV response rates. Second, as with previous studies, we included patients who received a transplant from various stem cell sources. However, because immune reconstitution after allo-HSCT and response to the vaccine might vary according to the stem cell source, further studies are needed to identify any source-specific differences. Third, the existing studies (including ours) have only evaluated responses to the influenza vaccine using the humoral response to one or more vaccine strains at various endpoints. However, no studies have focused on vaccine efficacy among allo-HSCT recipients. Therefore, we should clarify the vaccine’s effects on the incidences of influenza infection, morbidity, and mortality in clinical trials.
In conclusion, our study revealed that low serum IgM levels at the vaccination were associated with a poor serological response to the TIV among long-term survivors vaccinated at >1 year after transplantation. Despite the study’s limitations, our findings suggest that serum IgM levels may be a clinically useful predictor of immune response, given that these data are easily accessed, even in the outpatient setting. However, additional steps are needed to improve the immunogenicity of the influenza vaccine, and other effective approaches are needed to optimize the vaccine-specific immune response among long-term allo-HSCT survivors who have low serum IgM levels.
References
Ljungman P, Ward KN, Crooks BN, Parker A, Martino R, Shaw PJ, et al. Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2001;28:479–84.
Martino R, Ramila E, Rabella N, Munoz JM, Peyret M, Portos JM, et al. Respiratory virus infections in adults with hematologic malignancies: a prospective study. Clin Infect Dis. 2003;36:1–8.
Nichols WG, Guthrie KA, Corey L, Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39:1300–6.
Martino R, Porras RP, Rabella N, Williams JV, Ramila E, Margall N, et al. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant. 2005;11:781–96.
Khanna N, Steffen I, Studt JD, Schreiber A, Lehmann T, Weisser M, et al. Outcome of influenza infections in outpatients after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2009;11:100–5.
Johny AA, Clark A, Price N, Carrington D, Oakhill A, Marks DI. The use of zanamivir to treat influenza A and B infection after allogeneic stem cell transplantation. Bone Marrow Transplant. 2002;29:113–5.
Choi SM, Boudreault AA, Xie H, Englund JA, Corey L, Boeckh M. Differences in clinical outcomes after 2009 influenza A/H1N1 and seasonal influenza among hematopoietic cell transplant recipients. Blood. 2011;117:5050–6.
Reid G, Huprikar S, Patel G, Razonable RR, Mossad S, Levi M, et al. A multicenter evaluation of pandemic influenza A/H1N1 in hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2013;15:487–92.
Machado CM, Cardoso MR, da Rocha IF, Boas LS, Dulley FL, Pannuti CS. The benefit of influenza vaccination after bone marrow transplantation. Bone Marrow Transplant. 2005;36:897–900.
Ljungman P, Engelhard D, de la Camara R, Einsele H, Locasciulli A, Martino R, et al. Vaccination of stem cell transplant recipients: recommendations of the Infectious Diseases Working Party of the EBMT. Bone Marrow Transplant. 2005;35:737–46.
Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:309–18.
Mohty B, Bel M, Vukicevic M, Nagy M, Levrat E, Meier S, et al. Graft-versus-host disease is the major determinant of humoral responses to the AS03-adjuvanted influenza A/09/H1N1 vaccine in allogeneic hematopoietic stem cell transplant recipients. Haematologica. 2011;96:896–904.
Issa NC, Marty FM, Gagne LS, Koo S, Verrill KA, Alyea EP, et al. Seroprotective titers against 2009 H1N1 influenza A virus after vaccination in allogeneic hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2011;17:434–8.
Karras NA, Weeres M, Sessions W, Xu X, Defor T, Young JA, et al. A randomized trial of one versus two doses of influenza vaccine after allogeneic transplantation. Biol Blood Marrow Transplant. 2013;19:109–16.
Dhedin N, Krivine A, Le Corre N, Mallet A, Lioure B, Bay JO, et al. Comparable humoral response after two doses of adjuvanted influenza A/H1N1pdm2009 vaccine or natural infection in allogeneic stem cell transplant recipients. Vaccine. 2014;32:585–91.
Socie G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124:374–84.
Ljungman P, de la Camara R, Perez-Bercoff L, Abecasis M, Nieto Campuzano JB, Cannata-Ortiz MJ, et al. Outcome of pandemic H1N1 infections in hematopoietic stem cell transplant recipients. Haematologica. 2011;96:1231–5.
Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937–43.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–56.
Storek J, Ferrara S, Ku N, Giorgi JV, Champlin RE, Saxon A. B cell reconstitution after human bone marrow transplantation: recapitulation of ontogeny? Bone Marrow Transplant. 1993;12:387–98.
Suzuki I, Milner EC, Glas AM, Hufnagle WO, Rao SP, Pfister L, et al. Immunoglobulin heavy chain variable region gene usage in bone marrow transplant recipients: lack of somatic mutation indicates a maturational arrest. Blood. 1996;87:1873–80.
Storek J, Witherspoon RP, Storb R. Reconstitution of membrane IgD- (mIgD-) B cells after marrow transplantation lags behind the reconstitution of mIgD + B cells. Blood. 1997;89:350–1.
Kalwak K, Gorczynska E, Toporski J, Turkiewicz D, Slociak M, Ussowicz M, et al. Immune reconstitution after haematopoietic cell transplantation in children: immunophenotype analysis with regard to factors affecting the speed of recovery. Br J Haematol. 2002;118:74–89.
Hajdu M, Puskas E, Sipos A, Barta A, Paloczi K, Uher F. Homogeneous immunoglobulins following allogeneic bone marrow transplantation. Acta Haematol. 2003;109:124–8.
Storek J, Witherspoon RP, Luthy D, Storb R. Low IgG production by mononuclear cells from marrow transplant survivors and from normal neonates is due to a defect of B cells. Bone Marrow Transplant. 1995;15:679–84.
Griffith LM, McCoy JP Jr, Bolan CD, Stroncek DF, Pickett AC, Linton GF, et al. Persistence of recipient plasma cells and anti-donor isohaemagglutinins in patients with delayed donor erythropoiesis after major ABO incompatible non-myeloablative haematopoietic cell transplantation. Br J Haematol. 2005;128:668–75.
van Oosterhout M, Verburg RJ, Levarht EW, Moolenburgh JD, Barge RM, Fibbe WE, et al. High dose chemotherapy and syngeneic stem cell transplantation in a patient with refractory rheumatoid arthritis: poor response associated with persistence of host autoantibodies and synovial abnormalities. Ann Rheum Dis. 2005;64:1783–5.
Engelhard D, Nagler A, Hardan I, Morag A, Aker M, Baciu H, et al. Antibody response to a two-dose regimen of influenza vaccine in allogeneic T cell-depleted and autologous BMT recipients. Bone Marrow Transplant. 1993;11:1–5.
Karras NA, Weeres M, Sessions W, Xu X, Defor T, Young JA, et al. A randomized trial of one versus two doses of influenza vaccine after allogeneic transplantation. Biol Blood Marrow Transplant. 2013;19:109–16.
Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–238.
Halasa NB, Savani BN, Asokan I, Kassim A, Simons R, Summers C, et al. Randomized Double-Blind Study of the Safety and Immunogenicity of Standard-Dose Trivalent Inactivated Influenza Vaccine versus High-Dose Trivalent Inactivated Influenza Vaccine in Adult Hematopoietic Stem Cell Transplantation Patients. Biol Blood Marrow Transplant. 2016;22:528–35.
Acknowledgements
We thank the patients for participating in this study, and Dr. Kazunori Ohnishi for reviewing the manuscript. Y.F. is a PhD candidate at the Hamamatsu University School of Medicine and this work is being submitted as a part of the PhD program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
About this article
Cite this article
Fukatsu, Y., Nagata, Y., Adachi, M. et al. Serum IgM levels independently predict immune response to influenza vaccine in long-term survivors vaccinated at >1 year after undergoing allogeneic hematopoietic stem cell transplantation. Int J Hematol 105, 638–645 (2017). https://doi.org/10.1007/s12185-016-2163-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-016-2163-3