Abstract
Patients with mild/moderate hemophilia (H)A, acquired HA (AHA) and lupus anticoagulants (LA), have prolonged aPTTs with low levels of factor (F)VIII activity, but the differentiation of these disorders is complex and time consuming. We established an approach to quickly differentiate these disorders using comprehensive coagulation tests. Patients’ plasmas with mild/moderate HA, AHA, LA without anti-phospholipid syndrome [LA-APS(−)], and LA with APS [LA-APS(+)] were examined using clot waveform analysis (CWA) and thrombin generation test (TGT). Activated protein C (APC) sensitivity was assessed by TGT. CWA revealed similarly prolonged clot times in all groups [NP/mild/moderate HA/AHA/LA-APS(−)/LA-APS(+); 33 ± 1/82 ± 12/116 ± 44/90 ± 29/96 ± 15 s] but significantly different decreased maximal coagulation velocity (3.1 ± 0.1/1.3 ± 0.3/0.9 ± 0.5/1.6 ± 0.3/2.2 ± 0.5). In TGT, AHA group demonstrated severely reduced peak-thrombin levels (362 ± 23/170 ± 27/49 ± 21/158 ± 75/158 ± 99 nM), whilst both LA groups markedly prolonged lag times (4.5 ± 0.3/5.0 ± 0.4/4.7 ± 0.8/12.5 ± 7.7/28.8 ± 11.8 min), suggesting that AHA could be readily identified, but the different LA sub-types failed to be classified. An APC sensitivity demonstrated that ‘normalized’ APC-induced levels of peak thrombin in LA-APS(+) were significantly lower relative to LA-APS(−) (normalized %inhibition; 5 ± 7/42 ± 39 %). Our studies confirmed that %inhibition by APC was significantly decreased in NP preincubated with purified IgGs from LA-APS(+) compared to LA-APS(−), facilitating differentiation between LA groups. A combined approach using CWA and TGT could be a useful means of differentiating coagulation disorders with prolonged aPTT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemophilia A (HA) results from a deficiency or defect of factor (F)VIII and is the most common of the severe, inherited bleeding disorders. The clinical phenotype in HA generally correlates with levels of FVIII activity (FVIII:C), and on this basis, patients are classified into three distinct types (severe <1 IU/dl; moderate 1–5 IU/dl; mild >5 IU/dl) [1]. Acquired hemophilia A (AHA) is caused by the development of FVIII inhibitors as autoantibodies against FVIII in previously normal individuals, particularly elderly people, and often results in unexpectedly severe hemorrhage [2]. Patients with lupus anticoagulants (LA) and hypercoagulability associated with anti-phospholipid syndrome (APS) [termed LA-APS(+) in this study] often present with arterial and/or venous thrombosis [3], whilst those with LA in the absence of APS [termed LA-APS(−)] are frequently asymptomatic. In addition, LA-APS(−) sometimes have a hemorrhagic tendency due to the thrombocytopenia or hypoprothrombinemia [4]. Patients with mild/moderate HA, with AHA, and with LA demonstrate the prolonged activated partial thrombin times (aPTT) commonly together with low levels of FVIII:C. FVIII:C levels for cases with LA positive can be particularly evaluated as apparent low levels in a one-stage clotting assay.
Accurate assessment of blood coagulation in vitro is essential for complete clinical diagnosis. Conventional aPTT-based clotting assays are useful for routine laboratory analysis, but they are widely accepted to only partially reflect coagulation in a non-physiological environment and are based on the classical concepts of intrinsic cascade mechanisms. Discrepancies may be evident, therefore, between clinical phenotype and coagulant activity using this type of assay [5, 6]. Additional diagnostic information on the disorders described above is provided by laboratory tests of FVIII inhibitor and LA, but these methods are time consuming and require considerable technical expertise [7].
Interests have recently focused on comprehensive (global) coagulation assays, developed from a better understanding of the clotting mechanism reflecting cell-based models generating thrombin activity on activated platelets [8]. Coagulation assays of this nature, including clot waveform analysis (CWA) and thrombin generation tests (TGT), have now been established [9, 10], and have been applied for the clinical evaluation of bleeding and thrombotic disorders [11–13]. In the present study, we have utilized the rapid techniques of CWA and TGT to differentiate patients with mild/moderate HA, AHA, LA-APS(−), and LA-APS(+) together with a prolonged aPTT and low levels of FVIII:C.
Materials and methods
This study was approved by the Medical Research Ethics Committee of Nara Medical University, and blood samples were obtained after informed consent following local ethical guidelines.
Reagents
Recombinant human tissue factor (rTF; Innovin®, Dade, Marburg, Germany), ellagic acid (Elg; Sysmex, Kobe, Japan), and recombinant activated protein C (rAPC; Hematologic Technologies Inc., Essex Junction, VT, USA) were purchased from the indicated venders. Phospholipid (PL) vesicles containing 10 % phosphatidylserine, 60 % phosphatidylcholine, and 30 % phosphatidylethanolamine were prepared as previously described [14]. Seven patients’ plasmas with LA-APS(+) from different batches were purchased from Trina Bioreactive (Zurich, Switzerland).
Diagnosis and patients’ profiles (Table 1)
Patients’ plasmas with mild/moderate HA (n = 10), AHA (n = 10), LA-APS(−) (n = 10), and LA-APS(+) (n = 3, and additional 7 cases were commercially purchased as described above) and were investigated. As shown in Table 1, in aPTT values, all the cases were markedly prolonged (see the “Results”). PT values in each group were 11.7 ± 0.4, 11.7 ± 0.7, 12.3 ± 0.7, and 13.4 ± 1.2, respectively, showing the prolongation with somewhat difference between LA groups and other groups. However, it appeared likely to be difficult to discriminate using aPTT and PT alone because of the presence of some cases with overlapped PT values in all groups. AHA was diagnosed by the detection of FVIII inhibitor for non-hemophiliac individuals complicated with sudden hemorrhagic symptoms. The FVIII inhibitors in AHA groups were 42.5 ± 47.9 BU/ml in a Bethesda assay [15], and three cases showed the type 1 kinetic inhibition, and seven cases showed the type 2 pattern. The FVIII epitopes were located on the C2 domain in six cases, the A2 domain in one case, and both domains in three cases. APS was diagnosed according to the Sapporo criteria with Sydney revision [16]. LA test was diagnosed as the positive a cut-off level >1.2-fold of control using an excess PL-added clotting time, and LA-APS(−) (1.70 ± 0.36) and LA-APS(+) (1.71 ± 0.21) showed any little significant difference. All the cases in LA-APS(−) were diagnosed by further examination because of ecchymosis or subcutaneous hemorrhage or by chance examination of pre-operation screening test. All the cases in LA-APS(+) showed the thrombotic symptoms. In this study, we focused only an LA test in LA-APS(+) as an anti-PL antibody, because all the cases with LA-APS(−) did not show either anti-cardiolipin antibody or β2-glycoprotein I. FVIII inhibitors were negative for all cases in both LA groups in a Bethesda assay. Protein C, protein S, and factor V activity levels were within normal ranges for all the cases in both the LA groups (data not shown).
Blood samples and purified IgG
The blood samples in all patients and healthy individuals were collected into plastic tubes containing 3.2 % sodium citrate at a 9:1 ratio. Pooled normal plasma (PNP) was prepared from normal healthy individuals (n = 20). The study subjects had not taken any medication that may have affected coagulation at the time of blood sampling. Platelet-poor plasma was recovered after centrifugation of citrated whole blood for 15 min at 1,500g. All plasmas were stored at −80 °C, and thawed at 37 °C immediately prior to the assays. IgG fractions of patient’s plasmas were prepared and purified by protein G-Sepharose.
FVIII:C measurement
FVIII:C in test samples were measured by a one-stage clotting assay. FVIII:C levels in mild/moderate HA, AHA, LA-APS(−), and LA-APS(+) were 2.2 ± 0.9, 2.1 ± 2.2, 30.3 ± 21.7, and 37.7 ± 24.3 IU/dl, respectively. Since the FVIII:C level ranges from 70 to 130 IU/dl as a normal reference in our laboratory, the low FVIII:C was defied as less than 70 IU/dl in this study.
Clot waveform analysis (CWA)
CWA was performed on the CS2000i® instrument (Sysmex) using Thrombocheck APTT-SLA (Sysmex) as a trigger reagent [11]. In patients’ plasmas, clot formation was initiated by the addition of CaCl2 (20 mM). The clot waveforms obtained were computer-processed using the commercial kinetic algorithm. The minimum value of the first derivative (min1) was calculated as an indicator of the maximum velocity of coagulation achieved. Since the minimum of min1 was derived from negative changes, the data were expressed as |min1|. The clot time (CT) was defined as the time until the start of coagulation.
Thrombin generation test (TGT)
TGT was assayed using our previously established protocol [17]. Briefly, 20 μl trigger mixture reagent (TF/PL/Elg; f.c. 0.5 pM/4.0 μM/0.3 μM) and 80 μl test plasmas were mixed in microtiter plates (ThermoLab System, Helsinki, Finland). The plate was placed in the fluorometer, and at the initiation of the assay, 20 μl of 100 mM CaCl2 and 5 mM fluorogenic substrate Z-Gly-Gly-Arg-AMC was dispensed to all wells. The development of fluorescent signals was monitored at 8-s intervals using Fluoroskan Ascent microplate reader (Thermo Electron Co., Waltham, MA) with 390 (excitation) and 460 nm (emission) filter set. Thrombin generation (nM) was calculated from fluorescent signals corrected by reference to the thrombin calibrator samples. Data analyses were performed using the Thrombinoscope software. The parameters, lag time, and peak thrombin (peak-Th), were recorded.
APC-sensitivity assay
The sensitivity to APC was investigated for patients’ plasmas and for PNP reacted with purified IgG. The peak-Th of each test sample was determined by TGT triggered by TF and PL (1 pM and 4 μM) with and without the addition of rAPC (20 nM) [18]. PNP was run in parallel on each plate. Data were expressed as normalized peak-Th and as normalized percent (%)inhibition of peak-Th by dividing the %inhibition of peak-Th in test plasma by %inhibition of peak-Th determined in PNP alone [18] using the following formula:
Data analysis
Data are presented as the average and standard deviation. Data analysis was performed using the Microsoft Excel software. Significant differences were determined by the Wilcoxon t test and the Mann–Whitney U test. p values <0.05 were considered as statistically significant.
Results
CWA in patients’ plasmas with the mild/moderate HA, AHA, and LA groups
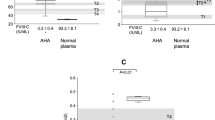
Coagulation function in patients with mild/moderate HA, AHA, LA-APS(−), and LA-APS(+) was initially assessed using CWA. The CWA parameters reflect the dynamic process of fibrin formation. Representative curves of the aPTT-based CWA are illustrated in Fig. 1a. The initiation of coagulation observed in all groups was markedly delayed compared with those in normal healthy controls. The clot times in mild/moderate HA, AHA, LA-APS(−), and LA-APS(+) (82 ± 12, 116 ± 44, 90 ± 29, and 96 ± 15 s, respectively) were significantly prolonged compared to controls (33 ± 1 s), but there were little significant differences between the groups (Fig. 1c). The coagulation maximum velocity (|min1|) levels in all groups were decreased relative to control plasma, however, and both LA groups were mildly higher than those in MHA and AHA (Fig. 1b). The |min1| levels were decreased in AHA, mild/moderate HA, LA-APS(−), and LA-APS(+) in the order (0.9 ± 0.5, 1.3 ± 0.2, 1.6 ± 0.2, and 2.2 ± 0.5 vs NP 3.1 ± 0.1), consistent with depressed coagulation function in vitro in all groups (Fig. 1c). The |min1| values were significantly different amongst the groups.
Clot waveform analysis on patients with mild/moderate HA, AHA, and LA groups. a, b Patients’ plasmas from mild/moderate HA, AHA, LA-APS(−), and LA-APS(+), and NP as a control were incubated with aPTT reagent, followed by the addition of with CaCl2 at the start of the assay as described in “Materials and methods”. Representative clot waveforms and the first derivative |min1| in all groups are illustrated in (a) and (b), respectively. The arrows show the clot time (a) and |min1| (b) in NP. c Parameters obtained from waveforms in (a) and (b) are shown (clot time, |min1|). All experiments were performed at least three separate times, and the average values and standard errors calculated. The gray bars show the range obtained from NP. Significant differences were expressed as p < 0.05. MMHA; the mild/moderate HA
TGT in patients’ plasmas with the mild/moderate HA, AHA, and LA groups
Subsequently, we also evaluated global coagulation function in these groups using the TGT triggered by TF/PL/Elg. Figure 2a, b illustrates the thrombograms and parameters, including lag time and peak thrombin, that were obtained as described in “Materials and methods”. Relative to normal controls (4.5 ± 0.3 min), the lag time in mild/moderate HA and AHA was similar (5.0 ± 0.4 and 4.7 ± 0.8 min, respectively), whilst in LA-APS(−) and LA-APS(+), this was significantly delayed (12.5 ± 7.7 and 28.8 ± 11.8 min, respectively, p < 0.01). In particular, the lag time in LA-APS(+) was more significantly delayed than in LA-APS(−) (p < 0.01). In five cases from the LA groups, thrombin generation was not recorded within 60 min due to the presence of potent anti-phospholipid antibodies. In contrast, the peak-thrombin parameter in AHA was severely decreased (49 ± 21 nM; p < 0.01) relative to the other groups, each with similar levels [mild/moderate HA, LA-APS(−), LA-APS(+); 170 ± 27, 158 ± 75, and 158 ± 99 nM, respectively]. In all patients, the peak-thrombin levels were lower than that in normal controls (362 ± 23 nM). These findings were in keeping with our earlier report of severely impaired coagulation function in AHA [13]. The data indicated that the TGT might help to differentiate between AHA, mild/moderate HA, and LA, but would not distinguish the LA sub-types.
Thrombin generation on patients with mild/moderate HA, AHA, and LA groups. a Patients’ plasmas and NP were incubated with TF (0.5 pM), PL (4 μM), and Elg (0.3 μM), followed by the addition of fluorogenic substrate and CaCl2, as described in “Materials and methods”. Representative thrombograms in all groups are illustrated in (a). b Parameters obtained from TGT in (a) are shown (lag time, peak-Th). All experiments were performed at least three separate times, and the average values and standard errors calculated. The gray bars show the range obtained from NP. Significant differences were expressed as p < 0.05. MMHA; the mild/moderate HA
Evaluation of APC sensitivity in LA-APS(+) and LA-APS(−)
A previous report had indicated that sensitivity to APC was impaired in patients with LA complicated with thrombosis [18]. To further investigate APC resistance in our cases with LA-APS(−) and LA-APS(+), peak-Th was measured using the TF/PL-TGT with and without the addition of rAPC (20 nM). Data were calculated as normalized peak thrombin, and expressed as normalized percent (%)inhibition of peak-Th by dividing the %inhibition of peak-Th in patient’s plasma by that in PNP alone. Representative results are shown in Fig. 3a, and summarized in Fig. 3b. In both LA groups, normalized %inhibition levels of peak-Th by APC were significantly reduced compared to normal plasma (p < 0.01), consistent with the concept of APC resistance in patients with LA. Normalized %inhibition in LA-APS(+) was significantly lower, however, than in LA-APS(−) [5 ± 7 % and 42 ± 39 %, respectively (p < 0.05)], indicating that APC resistance in LA-APS(+) could be more clinically relevant than in LA-APS(−). In patients with AHA, thrombin generation was severely impaired even without the addition of rAPC, and measurements of APC resistance were not possible in these circumstances.
APC sensitivity in cases with AHA, LA-APS(−), and LA-APS(+). a Patients’ plasmas and NP were incubated with TF (1 pM) and PL (4 μM) in the absence or presence of rAPC (20 nM), followed by the addition of fluorogenic substrate and CaCl2, as described in “Materials and methods”. Representative thrombograms in the absence (solid line) or presence (dot line) of APC are shown. b Parameter of normalized %inhibition of peak-Th obtained from TGT in (a) is shown. The normalized %inhibition of peak-Th was calculated by dividing the %inhibition of peak-Th in patient’s plasma caused by APC by %inhibition of peak-Th determined in NP alone, as described in “Materials and methods”. All experiments were performed at least three separate times, and the average values and standard errors calculated. Significant differences were expressed as p < 0.05
We attempted to confirm whether the discrepancy in the effects of APC sensitivity between LA-APS(+) and LA-APS(−) could be attributed to a direct reaction induced by LA-IgG. IgG samples were prepared from patients’ plasma, and various amounts of purified IgG were preincubated with PNP, prior to estimating APC sensitivity. The normalized %inhibition levels of peak-Th induced by rAPC in PNP in the presence of IgG (150 μg/ml) from LA-APS(+) to LA-APS(−) were 39 ± 18 and 86 ± 18 % (p < 0.05), respectively. In addition, similar results (38 ± 10 and 68 ± 29 %, respectively) were obtained at the maximum concentration of IgG employed (300 μg/ml, Fig. 4). These results confirmed that the APC resistance in these cases was due to direct interaction with LA-IgG, and further demonstrated that the clinical symptoms in the two LA groups reflected APC sensitivity.
APC sensitivity in normal plasma mixed with purified IgG from LA cases. IgG was purified from AHA, LA-APS(−), and LA-APS(+) and were preincubated with NP, and then were mixed with TF (1 pM) and PL (4 μM) in the absence or presence of rAPC (20 nM), followed by the addition of fluorogenic substrate and CaCl2, as described in “Materials and methods”. The parameter of normalized %inhibition of peak-Th obtained from TGT is shown. The normalized %inhibition of peak-Th was plotted as a function of IgG concentration. All experiments were performed at least three separate times, and the average values calculated
Discussion
The differential diagnosis of coagulation disorders with a prolonged aPTT and low levels of FVIII:C include mild/moderate HA, AHA, and LA-positive cases. Classification of these patients on the basis of the conventional aPTT assays is not straightforward, however. In this context, Solano et al. [19] reported contrasting shape patterns in aPTT-based clot waveform analysis triggered by different contact activators in LA and clotting factor deficiencies. In the present study, it seemed to be possible to some extent to distinguish LA from mild/moderate HA and AHA by assessing the |min1| obtained from the first-differential waveform in this technique. The cut-off value of |min1| between the LA groups and other cases appeared likely to be approximately 1.5 as a measure, with the LA patients >1.5. In addition, significant differences were observed between the |min1| in LA-APS(−) and LA-APS(+) and between mild/moderate HA and AHA. The results overlapped, however, and this measurement alone appeared to be insufficient to differentiate the defects.
Further studies were undertaken, therefore, using the TGT. The presence of anti-PL antibodies in LA directly inhibits thrombin generation on PL surfaces, and previous studies have indicated that the TGT lag time is prolonged in LA patients [20]. Our findings were in keeping with the earlier data, and confirmed that this measurement was similar to normal in patients with AHA and mild/moderate HA but was significantly abnormal in patients with LA. Moreover, the lag time in LA-APS(+) appeared to be more prolonged relative to LA-APS(−), and a lag time of >20 min seemed to identify the LA-APS(+) category. A recent report has suggested that the risk of thrombosis associated with LA might be influenced by the ratio of lag time to peak-Th (peak-Th/lag-time index) in the TGT [18, 20]. In our cases, the index in AHA showed little significant difference with those in both LA subgroups, but that in LA-APS(−) showed the significant difference compared to LA-APS(+) (p; 0.047; data shown). However, cases with overlapped values of index between LA subgroups were observed, suggesting that it might be somewhat difficult to differentiate clearly the disorders by the index.
In the present analyses, peak-Th values were decreased in all groups, and, in particular, were markedly depressed in AHA (approximately <50 nM) compared with mild/moderate HA, indicating the sufficient differentiation of mild/moderate HA and AHA by the TGT. These results were consistent with our earlier investigations that had confirmed that a pivotal inhibitory mechanism in AHA was related to indirect inhibition of FIXa-mediated FX activation on the tenase assembly by FVIII(a) and anti-FVIII autoantibody complexes [13]. The curves in AHA may not be possibly differentiated from those in congenital severe HA if the clinical history and FVIII:C levels are not shown. However, the differentiation with AHA and severe HA seems to be important to interpret the TGA and CWA data with consideration for individual patients profiles.
APC sensitivity is commonly considered to be associated with the cause of thrombosis in LA-APS(+) [21], and is usually assessed by directly adding APC to plasma samples in traditional aPTT-clotting assays. Liestol et al. [18] also reported, however, that APC sensitivity could be quantitatively measured in LA using the TGT. In their assay, APC sensitivity was evaluated using the ETP parameter (endogenous thrombin potential) derived from thrombin generation triggered by a high concentration of TF (5 pM) in the presence of APC added in vitro. We have previously utilized a low concentration of TF (1 pM) mixed with ellagic acid (Elg) as a trigger in a modified TGT to reflect thrombin potential in both intrinsic and extrinsic coagulation mechanisms [17]. In the present study, peak-Th measurements in this modified assay appeared to enable the reliable assessment of APC sensitivity, and indicated that this index was significantly lower (approximately <5 %) in LA-APS(+) compared to LA-APS(−). These data were confirmed by the ex vivo addition of purified antibody IgGs from these patients to NP, and enabled classification of the LA subgroups. The findings again indicated that anti-PL antibodies affected APC sensitivity directly, and provided additional evidence that the differences in the clinical symptoms in LA were associated with APC sensitivity and the presence of anti-PL antibodies.
AHA frequently presents with life-threatening hemorrhage, and rapid diagnosis is required to confirm low levels of FVIII:C in the presence of FVIII inhibitors. Since FVIII:C in both groups possessing LA positive can be evaluated as apparent low levels, it is also necessary to exclude LA, and the possibility that the low levels of FVIII:C are caused by the inhibition of PL binding in tenase assembly [3, 22]. Conventional laboratory tests for these purposes are technically demanding and are usually available only in specialized centers. Commercial facilities may be available, but are likely to involve considerable delay. We have shown that clinically useful data can be obtained relatively rapidly in these patients using automatic coagulation analyzers in conjunction with so-called ‘comprehensive’ or ‘global’ coagulation assays, although these are now available only in specialized centers.
However, there may be still limitation to differentiate these disorders clearly using a series of approach in the clinical setting. First, the cases enrolled in the present study were small numbers. Further investigation with large numbers of patients, therefore, would be required. Second, the heterogeneity of LA may make it difficult to discriminate from other diseases. In this study, the APC-sensitivity test using TGT could discriminate clearly between the LA-APS(−) and LA-APS(+), but it would be not completely excluded that the results may fluctuate due to the heterogeneity of LA. Finally, the clinical use of CWA and/or TGT has been recently increasing and gradually widespread, but remains still total insufficient. We hope that these comprehensive coagulation assays with broad utility could be widely utilized in the clinical setting in the future.
References
White GC 2nd, Rosendaal F, Aledort LM, Lusher JM, Rothschild C, Ingerslev J, et al. Definitions in Hemophilia. Recommendation of the Scientific Subcommittee on Factor VIII and Factor IX of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 2001;85:560.
Kessler CM, Knöbl P. Acquired haemophilia: an overview for clinical practice. Eur J Haematol. 2015;95(Suppl 81):36–44.
Robertson B, Greaves M. Antiphospholipid syndrome: an evolving story. Blood Rev. 2006;20:201–12.
Baca V, Montiel G, Meillón L, Pizzuto J, Catalan T, Juan-Shum L, et al. Diagnosis of lupus anticoagulant in the lupus anticoagulant hypoprothrombinemia syndrome: report of two cases and review of the literature. Am J Hematol. 2002;71:200–7.
Oldenburg J, Pavlova A. Discrepancy between one-stage and chromogenic factor VIII activity assay results can lead to misdiagnosis of haemophilia A phenotype. Hamostaseologie. 2010;30:207–11.
Yada K, Nogami K, Wakabayashi H, Fay PJ, Shima M. The mild phenotype in severe hemophilia A with Arg1781His mutation is associated with enhanced binding affinity of factor VIII for factor X. Thromb Haemost. 2013;109:1007–15.
Tiede A, Werwitzke S, Scharf RE. Laboratory Diagnosis of acquired hemophilia A: limitations, consequences, and challenges. Semin Thromb Hemost. 2014;40:803–11.
Hoffman M, Monroe DM 3rd. A cell-based model of hemostasis. Thromb Haemost. 2001;85:958–65.
Shima M, Matsumoto T, Fukuda Y, Kubota Y, Tanaka I, Nishiya K, et al. The utility of activated partial thromboplastin time (aPTT) clot waveform analysis in the investigation of hemophilia A patients with very low levels of factor VIII activity (FVIII:C). Thromb Haemost. 2002;87:436–41.
Matsumoto T, Shima M, Takeyama M, Yoshida K, Tanaka I, Sakurai Y, et al. The measurement of low levels of factor VIII or factor IX in hemophilia A and hemophilia B plasma by clot waveform analysis and thrombin generation assay. J Thromb Haemost. 2006;4:377–84.
Shima M, Thachil J, Nair SC, Srivastava A. Towards standardization of clot waveform analysis and recommendations for its clinical applications. J Thromb Haemost. 2013;11:1417–20.
Berntorp E, Salvagno GL. Standardization and clinical utility of thrombin generation assays. Semin Thromb Hemost. 2008;34:670–82.
Matsumoto T, Nogami K, Shima M. A putative inhibitory mechanism in the tenase complex responsible for loss of coagulation function in acquired haemophilia A patients with anti-C2 autoantibodies. Thromb Haemost. 2012;107:288–301.
Okuda M, Yamamoto Y. Usefulness of synthetic phospholipids in measurement of activated partial thromboplastin time: a new preparation procedure to reduce batch difference. Clin Lab Haematol. 2004;26:215–23.
Kasper CK, Aledort LM, Aronson D, Counts R, Edson JR, van Eys J, et al. A more uniform measurement of factor VIII inhibitors. Thromb Diath Haemorrh. 1975;34:869.
Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295–306.
Matsumoto T, Nogami K, Ogiwara K, Shima M. A modified thrombin generation test for investigating very low levels of factor VIII activity in hemophilia A. Int J Hematol. 2009;90:576–82.
Liestøl S, Sandset PM, Mowinckel MC, Wisløff F. Activated protein C resistance determined with a thrombin generation-based test is associated with thrombotic events in patients with lupus anticoagulants. J Thromb Haemost. 2007;5:2204–10.
Solano C, Zerafa P, Bird R. A study of atypical APTT derivative curves on the ACL TOP coagulation analyser. Int J Lab Hematol. 2011;33:67–78.
Devreese K, Peerlinck K, Hoylaerts MF. Thrombotic risk assessment in the antiphospholipid syndrome requires more than the quantification of lupus anticoagulants. Blood. 2010;28(115):870–8.
Nojima J, Iwatani Y, Ichihara K, Tsuneoka H, Ishikawa T, Yanagihara M, et al. Acquired activated protein C resistance is associated with IgG antibodies to protein S in patients with systemic lupus erythematosus. Thromb Res. 2009;124:127–31.
Ames PR, Graf M, Archer J, Scarpato N, Iannaccone L. Prolonged activated partial thromboplastin time: difficulties in discriminating coexistent factor VIII inhibitor and lupus anticoagulant. Clin Appl Thromb Hemost. 2015;21:149–54.
Acknowledgments
We would like to thank Ms. Kana Sasai and Arisa Takenaka for special technical assistant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Funding
This work was partly supported by a Grant-in-Aid for Scientific Research (KAKENHI) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) to K.N (Grant No. 15K09663).
About this article
Cite this article
Matsumoto, T., Nogami, K. & Shima, M. A combined approach using global coagulation assays quickly differentiates coagulation disorders with prolonged aPTT and low levels of FVIII activity. Int J Hematol 105, 174–183 (2017). https://doi.org/10.1007/s12185-016-2108-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-016-2108-x