Abstract
An 80-year-old man was diagnosed with disseminated intravascular coagulation (DIC) and thrombotic microangiopathy (TMA) associated with mucin-producing gastric cancer with bone marrow metastasis. He died suddenly on the third day of hospitalization before chemotherapy. Microscopic autopsy findings revealed fibrin thrombi by phosphotungstic acid hematoxylin (PTAH) staining of the renal glomeruli, and platelet thrombi by von Willebrand Factor (Factor VIII Antigen) staining of the microvessels of the bleeding intestine. Tumor cells were negative for both stains. Staining of endothelial cells (EC) of the small vessels with thrombomodulin (TM) stain revealed destruction of EC structure. This patient was thought to have had systemic dissemination of solid tumor cells associated with DIC and TMA, the clinical course of which is extremely aggressive. Different types of thrombi were observed in different organs, such as the kidneys and small intestine, which supported the co-occurrence of DIC and TMA by microscopic pathological findings. These findings provide pathological evidence for the pathology of the concurrent development of DIC and TMA and show differences in the types of thrombi according to the blood vessel localization. Furthermore, the findings were highly suggestive of the mechanisms causing organ dysfunction, such as renal dysfunction, and gastrointestinal bleeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Disseminated intravascular coagulation (DIC) is defined as a condition in which systemic intravascular coagulation is activated in the absence of local or compensatory control due to various underlying diseases [1–4]. In addition to DIC associated with hematopoietic malignancy and infection, refractory DIC in internal medicine includes chronic DIC, such as DIC caused by vascular lesions (aneurysms and angiomas), DIC in cancer patients, and DIC caused by systemic dissemination of solid cancers. Although cases with DIC caused by systemic dissemination of solid cancers are often reported, there are few reports on the pathological and other findings regarding the coagulation pathology of DIC and other conditions except for cancer cells arising from underlying diseases.
We recently experienced a patient with bone marrow carcinosis due to gastric cancer, a type of systemic dissemination of solid cancer, which was complicated by DIC and thrombotic microangiopathy (TMA). Although these complications were diagnosed before his death, we were unable to save him. After his death, we received permission by his family with an informed consent to perform an autopsy to obtain detailed pathological findings regarding the complications, which are reported here.
Case
The patient was a Japanese man 80 years of age. He had a history of vertebrobasilar insufficiency, pulmonary emphysema, anemia, hypertension, and cerebral infarction. His regular medications included an angiotensin-converting enzyme (ACE) inhibitor imidapril, ifenprodil for treatment of cerebral infarction, and aspirin. On December 12, 20XX, he visited our emergency medical care center for epistaxis. Because the epistaxis had resolved by the time of his arrival, he was instructed to discontinue oral administration of ifenprodil and aspirin, and was sent home. Four days later, on December 16, he visited our center for the recurrence of epistaxis. Although the blood test results from this visit showed a platelet count of 177,000/μL, a fibrinogen level of less than 50 mg/dL, a fibrinogen and fibrin degradation products (FDPs) level of 32.8 μg/mL, and a D-dimer level of 30.5 μg/mL, he was again allowed to go home. Abdominal pain occurred on December 19, and he was admitted on December 20 with chief complaints of abdominal pain, achalasia, and frequent epistaxis. The physical findings on admission were as follows: height of 152 cm, weight of 37.4 kg, temperature of 37.4°C, blood pressure of 132/70 mmHg, pulse of 108/min, and respiratory rate of 25/min. He was lucid and able to carry out a normal conversation. His skin appeared yellowish. Finger clubbing and subcutaneous hematoma at the site of infusion were observed. Anemia in the palpebral conjunctiva and jaundice in the bulbar conjunctiva were observed. Cardiopulmonary auscultation detected a wheeze in the right main bronchus area, and respiratory sounds were decreased in both lower lung fields. Tenderness was detected in the epigastric area. No edema was found. The laboratory test results on admission are shown in Table 1. Marked anemia, thrombocytopenia, hypercoagulability, hyperfibrinolysis, marked increase in schizocytes by 11 %, and increased indirect bilirubin levels were observed. The activity of a disintegrin-like and metalloproteinase with thrombospondin type 1 motifs 13 (ADAMTS13) remained mildly decreased. Among tumor markers, both carbohydrate antigen (CA) 19–9 and carcinoembryonic antigen (CEA) levels were elevated. Computed tomography performed on the day of admission revealed shadows suggestive of thickening of the lower esophageal wall and lymphadenopathy (Fig. 1). Similarly, on the day of admission, May-Giemsa staining of bone marrow smears and bone marrow aspirate clot preparations revealed aggregation of large tumor cells, which were found to contain mucins by periodic acid-Schiff (PAS) staining (Fig. 2). On the day following admission, upper gastrointestinal endoscopy revealed submucosal swelling, redness, and a concave surface in the junction between the esophageal and gastric mucosa, leading to a diagnosis of cancer of the gastric cardia. We consider this case to be bone marrow carcinosis from undifferentiated gastric cancer on the basis of clinical course and bone marrow findings.
Bone marrow smear and clot findings. May-Giemsa (MG) staining of bone marrow smears and Hematoxylin and eosin (HE) staining of bone marrow aspirate clot preparations revealed aggregation of large tumor cells, which were found to contain mucins by periodic acid-Schiff (PAS) staining. Black arrows large tumor cells containing mucin in PAS staining
Because the patient had concurrent DIC associated with marked coagulation and fibrinolytic activity and already showed a bleeding tendency, treatment for DIC including 180 mg/day of nafamostat mesilate, and 1500 U/day of antithrombin (AT) was started immediately after admission. While chemotherapy was considered for treatment of his primary disease, the patient suffered cardiopulmonary arrest and died on the third day after admission.
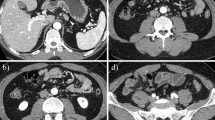
The autopsy revealed macroscopic findings including submucosal swelling, redness, and a concave surface in the junction between the esophageal and gastric mucosa, which were consistent with findings of cancer of the gastric cardia (Fig. 3). The microscopic findings of each organ included diffuse phosphotungstic acid hematoxylin (PTAH) staining, which indicated the presence of fibrin in renal glomeruli. Microvessels of the bleeding small intestine were extremely well stained by factor VIII (FVIII, von Willebrand factor [VWF]), which is platelet-associated staining (Fig. 4). No infiltration of inflammatory cells, such as neutrophil, was observed around microvessels with thrombi under the mucosa of the small intestine. The blood vessels of the other organs, including the great vessels, were predominantly stained by PTAH. There were no apparent findings suggestive of either thrombi or infiltration of inflammatory cells around the aggregation of tumor cells (Fig. 5). Thrombomodulin staining by using anti-thrombomodulin antibody was performed in order to assess the severity of vascular endothelial damage in the microvessels of the bleeding small intestine. Compared to findings from control patients without concurrent DIC, there was little or no thrombomodulin staining in the microvessels of our patient (Fig. 6).
Macroscopic findings of autopsy. The autopsy revealed macroscopic findings including submucosal swelling, redness, and a concave surface in the junction between the esophageal and gastric mucosa, which were consistent with findings of cancer of the gastric cardia. The lesion is showed by white arrow
Location of thrombi by autopsy. The microscopic findings of each organ included diffuse phosphotungstic acid hematoxylin (PTAH) staining, which indicated the presence of fibrin in renal glomeruli (a). Microvessels of the bleeding small intestine were extremely well stained by factor VIII [FVIII, von Willebrand factor (VWF)], which is platelet-associated staining (d). Strong stain of glomeruli by factor VIII staining and small intestine by PTAH staining were not observed (b, c)
Discussion
One of the typical pathological conditions for which hematologists are consulted is infiltration of malignant tumors into the bone marrow (i.e., bone marrow carcinosis). Among the cases experienced in our department, those of bone marrow carcinosis from gastrointestinal cancer, such as gastric cancer, have particularly poor prognosis as previous other report [5]. Many of these cases resulted in death in a short period of time, as was also true in the present case.
In terms of pathological cytology, there are many reported cases of undifferentiated or mucin-producing adenocarcinomas, as in the present case, as well as other types [6]. Analysis from a clinical perspective revealed DIC associated with substantial hypercoagulability and hyperfibrinolysis in terms of coagulation and concurrent TMA associated with marked red cell fragmentation (RCF) and increased levels of indirect bilirubin and lactate dehydrogenase (LDH). The mean survival time after diagnosis ranges from 23 to 67 days according to the presence or absence of hyponatremia, lung metastasis, peritoneal metastasis, etc. [7] and from 11 to 121 days with chemotherapy [8]. During the clinical course of our case, no resolution of DIC was observed, and his anemia progressed despite daily administration of red blood cell transfusions. Bleeding was assumed to be the cause of death.
The autopsy of this patient revealed new microscopic pathological findings. Specifically, we obtained samples positive for PTAH staining that suggested the presence of diffuse fibrin thrombi in the renal glomeruli. Samples were also positive for FVIII (VWF) staining, suggesting concurrent platelet thrombi in microvessels of the bleeding small intestine (Fig. 4). These findings were consistent with the results of the coagulation test performed before the death of the patient, which indicated the concurrence of DIC and TMA. Interestingly, the autopsy findings revealed fibrin and platelet thrombi mainly in the renal glomeruli and microvessels of the small intestine, respectively. To our knowledge, this is the first case report of the concurrence of these conditions. The findings in the present case may be helpful for exploring the mechanisms of thrombus formation that separately cause organ dysfunction, such as renal dysfunction, and bleeding symptoms, such as gastrointestinal bleeding, in DIC.
Thrombomodulin is membrane glycoprotein with a molecular weight of approximately 100,000 that is expressed on vascular endothelial cells. It binds with thrombin to convert protein C to activated protein C; consequently, the procoagulant activity of thrombin is converted to anticoagulant activity [9]. Thus, the evidence and lack of thrombomodulin suggested that the epithelia of the microvessels of the bleeding small intestine had severe vascular endothelial damage.
The hypercoagulability of solid cancers is attributed to tissue factors (TFs) expressed by tumor cells and various surrounding cells, enhanced production of cancer procoagulant (CP), and circulatory release of microparticles expressing these substances [10]. The fibrinolytic system has also been shown to be activated by enhanced expression of annexin II which are receptors of tissue plasminogen activator (t-PA) and plasminogen, and urokinase-type plasminogen activator (u-PA) receptors in various tumor cells [11, 12]. The present case also indicates that the disease type of DIC in carcinoma, such as undifferentiated adenocarcinoma and mucin-producing adenocarcinoma, is characterized by both hypercoagulability and hyperfibrinolysis. Sallah et al. reported that bleeding symptoms make up a large proportion (54 %) of clinical symptoms, followed by thrombotic symptoms alone (34 %), and the combination of both (7 %); however, various symptoms may occur [13]. Moreover, in Trousseau syndrome, which is frequently associated with mucin-producing cancer, the expression of L-selectin and P-selectin ligands on the surface of mucin is a main cause of platelet aggregation and leads to thrombus formation independent of thrombin [14]. This mechanism is thought how the mucin producing or undifferentiated gastric cancer impacts on the development of TMA. In the present case, the lesion in the small intestine also suggested the presence of these mechanisms of thrombus formation. The reason why some organs developed fibrin thrombi while others developed platelet thrombi remains unknown. However, a pathological condition in which bone marrow carcinosis from solid cancer is simultaneously complicated by DIC and TMA is frequently observed. We were able to confirm the pathological findings of this condition in the current case.
Conclusion
We experienced a case of bone marrow carcinosis due to gastric cancer. While the findings at the time of diagnosis suggested a pathological condition simultaneously complicated by DIC and TMA, postmortem autopsy revealed fibrin thrombi mainly in the kidneys and platelet thrombi mainly in the small intestine. These findings are of note because they provide evidence for the pathology of the concurrent development of DIC and TMA and showed differences in the types of thrombi according to blood vessel localization. Furthermore, the findings were highly suggestive of the mechanisms of organ dysfunction, including renal dysfunction, and gastrointestinal bleeding.
References
Carey MJ, Rodgers GM. Disseminated intravascular coagulation: clinical and laboratory aspects. Am J Hematol. 1998;59:65–73.
Taylor FB Jr, Toh CH, Hoots WK, Wada H, Levi M. Towards a definition, clinical and laboratory criteria and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2000;86:1327–30.
Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24–33.
Toh CH, Alhamdi Y. Current consideration and management of disseminated intravascular coagulation. Hematology Am Soc Hematol Educ Program. 2013;2013:286–91.
Ekinci AŞ, Bal Ö, Özatlı T, Türker İ, Eşbah O, Demirci A, et al. Gastric carcinoma with bone marrow metastasis: a case series. J Gastric Cancer. 2014;14:54–7.
Brain MC, Azzopardi JG, Baker LR, Pineo GF, Roberts PD, Dacie JV. Microangiopathic haemolytic anaemia and mucin-forming adenocarcinoma. Br J Haematol. 1970;18:183–93.
Kim HS, Yi SY, Jun HJ, Lee J, Park JO, Park YS, et al. Clinical outcome of gastric cancer patients with bone marrow metastases. Oncology. 2007;73:192–7.
Kwon JY, Yun J, Kim HJ, Kim KH, Kim SH, Lee SC, et al. Clinical outcome of gastric cancer patients with bone marrow metastases. Cancer Res Treat. 2011;43:244–9.
Esmon NL, Owen WG, Esmon CT. Isolation of a membrane-bound cofactor for thrombin-catalyzed activation of protein C. J Biol Chem. 1982;257:859–64.
Levi M. Disseminated intravascular coagulation in cancer patients. Best Pract Res Clin Haematol. 2009;22:129–36.
Vishwanatha JK, Chiang Y, Kumble KD, Hollingsworth MA, Pour PM. Enhanced expression of annexin II in hyman pancreatic carcinoma cells and primary pancreatic cancers. Carcinogenesis. 1993;14:2575–9.
Menell JS, Cesarman GM, Jacovina AT, McLaughlin MA, Lev EA, Hajjar KA. Annexin II and bleeding in acute promyelocytic leukemia. New Engl J Med. 1999;340:994–1004.
Sallah S, Wan JY, Nguyen NP, Hanrahan LR, Sigounas G. Disseminated intravascular coagulation in solid tumors: clinical and pathologic study. Thromb Haemost. 2001;86:828–33.
Wahrenbrock M, Borsig L, Le D, Varki N, Varki A. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J Clin Invest. 2003;112:853–62.
Acknowledgments
We express our sincere appreciation to Dr. Koji Okamoto at the Department of Surgery, Kitakyushu Municipal Yahata Hospital (formerly the Department of Surgery, University of Occupational and Environmental Health, Japan) for performing thrombomodulin staining of the blood vessels from the bleeding small intestine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
About this article
Cite this article
Seki, Y., Wakaki, K. Pathological findings in a case of bone marrow carcinosis due to gastric cancer complicated by disseminated intravascular coagulation and thrombotic microangiopathy. Int J Hematol 104, 506–511 (2016). https://doi.org/10.1007/s12185-016-2051-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-016-2051-x