Abstract
To evaluate the efficacy and feasibility of upfront high-dose chemotherapy (HDCT) and rituximab (R) followed by autologous peripheral blood stem cell transplantation (auto-PBSCT) in patients with newly diagnosed high-intermediate(HI)-, and high(H)-risk diffuse large B-cell lymphoma (DLBCL), we conducted a multicenter prospective phase II trial. In 15–60-year-old patients with H- or HI-risk DLBCL, after three courses of (R-)CHOP14, high-dose etoposide was given prior to peripheral blood stem cell harvesting. After an additional three courses of (R-)CHOP14, auto-PBSCT was performed following HDCT. The primary endpoint of the study is progression-free survival (PFS) at 2 years after registration in eligible patients. The expected PFS and the threshold PFS were estimated to be 70 and 50 %, respectively. Among 40 eligible patients registered, 30 patients completed treatment. With a median observation period in surviving eligible patients of 63 months, the 2- and 4-year PFS after registration were 79.9 and 72.0 %, respectively. The 2- and 4-year overall survival (OS) were 92.5 and 84.6 %, respectively. In 30 patients who completed treatment, the 4-year PFS and OS after auto-PBSCT were 79.2 and 85.9 %, respectively. In conclusion, the results of our study suggest that upfront HDCT and auto-PBSCT combined with rituximab is highly effective as an initial treatment for HI-, and H-risk DLBCL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse large B-cell lymphoma (DLBCL) accounts for 30–40 % of non-Hodgkin’s lymphoma (NHL) [1]. Induction chemotherapy by cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) combined with rituximab (R-CHOP) is considered as a standard for patients with newly diagnosed DLBCL [2–7]. However, in patients with high (H)- or high-intermediate (HI)-risk NHL according to the International Prognostic Index (IPI), disease relapse remains a substantial problems, and the overall survival (OS) is not satisfactory [8].
High-dose chemotherapy (HDCT) combined with autologous hematopoietic stem cell transplantation has been reported as a method to increase the intensity of treatment. Initially, this therapy was conducted in patients with recurrent or refractory lymphoma. Among patients with relapsed aggressive NHL sensitive to chemotherapy, the 5-year event-free survival (EFS) and 5-year OS were both significantly better in the HDCT group [9]. HDCT combined with autologous hematopoietic stem cell transplantation has also been reported as an upfront consolidation treatment. Several randomized studies reported that progression-free survival (PFS) or EFS for the HDCT group was significantly better compared to those for the chemotherapy group in H and HI patients according to IPI [10–12]. In terms of OS, however, only one study showed the superiority of the HDCT in IPI HI patients [13]. Several groups have reported the prospective studies of upfront HDCT combined with rituximab [14–19]. Although two randomized studies HDCT versus chemotherapy in combination with rituximab have recently been reported, the usefulness of upfront HDCT plus autologous hematopoietic stem cell transplantation has not been proved in the rituximab era [20, 21].
There also have been attempts to increase the intensity of chemotherapy by shortening the interval between the cycles of CHOP by the administration of granulocyte colony-stimulating factor (G-CSF). Although biweekly CHOP (CHOP14) is not revealed to be superior to triweekly CHOP (CHOP-21) chemotherapy for previously untreated DLBCL and CHOP21 remains the standard first-line treatment in patients with DLBCL [22], it is noteworthy that CHOP14 might increase the efficacy of HDCT by shortening the interval from diagnosis to HDCT.
Therefore, to evaluate the effectiveness of CHOP14 with rituximab (R-CHOP14) and HDCT including rituximab combined with autologous peripheral blood stem cell transplantation (auto-PBSCT) in patients with newly diagnosed HI-, and H-risk (aa-IPI-H, HI) DLBCL, we conducted a collaborative multicenter phase II trial.
Patients and methods
Eligibility criteria
Patients with newly diagnosed DLBCL according to the WHO classification were eligible to this study. The major eligibility criteria were as follows: age 15–60 years; CD20-positive histologically confirmed DLBCL (central pathological review was not done in this study) with at least one measurable lesion; aa-IPI of H- or HI-risk; Ann Arbor Clinical Stage (CS) of II to IV; Eastern Cooperative Oncology Group Performance Status (PS) of 0–3; and no serious organ disorder. In addition, patients need to have neutrophil count ≥1.0 × 109/L, platelet count ≥75 × 109/L, AST/ALT within 3 times the upper limit of the normal range; total bilirubin ≤2.0 mg/dL; serum creatinine level ≤2.0 mg/dL; cardiac ejection fraction ≥50 %; PaO2 ≥60 mmHg or SaO2 ≥90 %. All patients needed to give written informed consent before registration to the study. Patients were excluded if they had active double cancer, active tuberculosis, a history of ischemic heart disease, cardiomyopathy, arrhythmia being treated with an antiarrhythmic drug, a history of drug-induced anaphylactic shock, and severe psychiatric disorders. Patients with lymphoma in the central nervous system, women who are or may be pregnant or breast-feeding, those with positive for HBs antigen, HCV antibody, HTLV-1, or HIV antibody. This protocol was approved by the institutional review boards of Kyushu University and all other participating centers.
Treatment protocol
Figure 1 shows an outline of the treatment protocol of the study. As an induction chemotherapy course (3 cycles), 1 course of CHOP14 (cyclophosphamide at 750 mg/m2/day on Day 1, doxorubicin at 50 mg/m2/day on Day 1, vincristine at 1.4 mg/m2/day on Day 1, and prednisolone at 100 mg/body/day on Days 1–5, that were repeated at 14-day interval with G-CSF support) was administered, followed by 2 courses of CHOP14 combined with rituximab (R-CHOP14). After the first 3-cycle CHOP14, the response was assessed by a CT scan with contrast, and those who showed complete response (CR), complete response unconfirmed (CRu), or partial response (PR) were eligible for the subsequent study protocol. The therapeutic effect was evaluated according to the International Workshop Criteria [23].
For mobilization of the autologous peripheral blood stem cells (PBSC), high-dose etopisoide (HD-VP-16, etoposide at 500 mg/m2/day on Days 1–3) combined with rituximab were administered, followed by G-CSF (filgrastim at 600 μg/body/day, daily). In the hematopoietic recovery period after HD-VP-16 combined with rituximab, collection of PBSC by apheresis was started when the white blood cell count recovered to 5000/m3 or above. PBSC collection was continued until achieving the minimum number of PBSCs (1 × 106 CD34+ cells/kg). Harvested PBSC were stored by freezing until the day of transplantation [24, 25].
As a consolidation chemotherapy (3 cycles), an additional 1 course of CHOP14 and 2 courses of R-CHOP14 were administered. After the second 3-cycle CHOP14, patients were evaluated for eligibility of autologous peripheral blood stem cell transplantation (PBSCT). The response was assessed by a CT scan with contrast, and those who showed CR, CRu, or PR were eligible for the auto-PBSCT. The other major eligibility criteria for auto-PBSCT were Eastern Cooperative Oncology Group Performance Status (PS) of 0–1; and no serious organ dysfunction as mentioned previously.
The MCEC conditioning regimen for auto-PBSCT consisted of ranimustine at 200 mg/m2/1 h on Days −8 and −3; carboplatin at 300 mg/m2/1 h, Days −7, −6, −5, and −4; etoposide at 500 mg/m2/6–8 h, Days −6, −5, and 4; cyclophosphamide at 50 mg/kg/3 h on Days −3 and −2 combined with rituximab [26, 27], and thawed PBSCs were transplanted (on Day 0). Rituximab was administered at 375 mg/m2 once on the day before beginning the 2nd, 3rd, 5th, and 6th courses of CHOP14. It was also administered on the day before beginning HD-VP-16 and 2 days before the expected date of PBSC harvesting. It was administered on the day before beginning MCEC and on the day after transplantation. The response was assessed by a CT scan with contrast at 3, 6, 12, 18, and 24 months after auto-PBSCT. Adverse events were evaluated using the NCI-CTC version 2.0.
Statistical analysis
The primary endpoint of the study was progression-free survival (PFS) at 2 years after registration in eligible patients. The secondary endpoints were overall survival (OS) at 2 years after registration in eligible patients, PFS at 2 years after transplantation in patients who completed the treatment, and safety of auto-PBSCT. The expected PFS at 2 years was estimated to be 70 %, and the threshold PFS at 2 years was estimated to be 50 % on the basis of previous studies [2, 13, 28, 29]. With a statistical power of 80 % and one-sided type I error (α = 0.05), the number of patients required for this study was calculated to be 43 patients. The target number of patients for registration was set at 54 by anticipating the rate of ineligible patients to be 20 %.
The OS was evaluated in all eligible patients from the day of registration to the day of death due to all causes. In patients who discontinued the study protocol, evaluation was ended on the last day of confirmation of survival. The PFS was evaluated in all eligible patients from the day of registration to the earlier part of the day when a judgment of exacerbation (PD, recurrence, clinical exacerbation) was made for the first time after the beginning of treatment and the day of death due to all causes. In survivors who showed no exacerbation or recurrence, evaluation was ended on the last day of confirmation of the absence of exacerbation or recurrence (last day of confirmation of PFS). All statistical analyzes were performed with EZR (Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R (The R Foundation for Statistical Computing, version 2.13.0). More precisely, it is a modified version of R commander (version 1.6-3) designed to add statistical functions frequently used in biostatistics [30].
Results
Patient characteristics
Between December 1, 2004 and March 31, 2010, 46 patients were enrolled from 15 institutions, and 40 of them were finally eligible for registration to the study. Six patients were ineligible for the study because of incorrect pathological diagnosis after reviewing by pathologists (n = 2), gastrointestinal tract bleeding requiring transfusion (n = 1), and patient’s refusal (n = 3). Table 1 summarizes the characteristics of 40 eligible patients. The median age was 54 years (range, 23–60 years), and 19 patients (48 %) were male. The PS at registration was 2 in 4 patients (10 %), and 3 in 7 patients (18 %). The aa-IPI was HI in 32 patients (80 %) and H in 8 patients (20 %).
Patient flow and treatment response
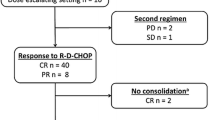
Patient flow and treatment response are summarized in Fig. 2. Among the 40 eligible patients, 39 received the planned 3-cycles of (R-)CHOP14, and 1 patient was unable to complete the study treatment due to herpes zoster infection. In the 39 patients who were evaluated with CT scans after 3-cycles of (R-)CHOP14, the response was CR in 19, CRu in 3, PR in 15, and PD in 2. The treatment was discontinued in these 2 patients due to PD, and 2 other patients discontinued the study treatment before the PBSC collection (drug-induced lung disease, n = 1; patient’s refusal, n = 1).
In the 35 patients evaluable at the efficacy evaluation before HDCT plus PBSCT, the response was CR in 25, CRu in 2, PR in 6, and PD in 2. The planned HDCT was not performed in 5 patients due to the following reasons: PD before auto-PBSCT (n = 2), vesicointestinal fistula (n = 1), withdraw of the consent (n = 1), and liver dysfunction (grade 3) during conditioning regimen of MCEC (n = 1). Therefore, a total of 30 patients (75 % of the eligible patients at registration) completed the study treatment including HDCT plus auto-PBSCT, and 10 patients failed to complete the study due to the following reasons: PD in 5, complications in 4, and patients’ refusal in 2. In 30 patients who completed the study treatment, there was no severe adverse effect by which the study treatment was stopped or anti-cancer drug was reduced before HDCT. The median interval from registration to auto-PBSCT was 5.2 months (range 3.9–7.9 months). At the efficacy evaluation 3 months after auto-PBSCT in 30 evaluable patients, the response was CR in 26, CRu in 2, PR in 1, and PD in 1. Thereafter, recurrence was observed in 4 of the 26 CR patients and in 1 of the 2 CRu patients.
PBSC harvest
In 35 patients, PBSC harvest (PBSCH) was performed after HD-VP-16. The median number of CD34-positive cells harvested was 19.0 × 106/kg (range 2.0–60.8 × 106/kg). The median number of days required for PBSCH was 1 (range 1–3). In all 35 patients, planned number of CD34-positive cells was collected during the one course of HD-VP-16. The median duration of G-CSF administration was 7 days (range 3–14 days). The median interval from beginning HD-VP-16 plus rituximab to the first day of harvest was 16 days (range 13–21 days), and that from the last rituximab administration before harvest to the first day of harvest was 2 days (range 0–5 days). No grade 3 or more severe adverse events due to HD-VP-16 were observed.
Survival
The median observation period in surviving eligible patients was 63.9 months (range 12.6–101.7 months) after registration. The Kaplan–Meier estimates of PFS at 2 and 4 years after registration were 79.9 % (95 % CI 63.8–89.4 %) and 72.0 % (95 % CI 55.1–83.4 %), respectively (Fig. 3a). Therefore, our study fulfilled the primary endpoint, the PFS at 2 years after registration, as we estimated the threshold PFS at 2 years to be 50 %. The Kaplan–Meier estimates of OS at 2 and 4 years after registration were 92.5 % (95 % CI 78.5–97.5 %) and 84.6 % (95 % CI 68.8–92.8 %), respectively (Fig. 3b). The PFS and OS were not significantly different according to the patient age (50 years or older vs less than 50 years), PS at registration (0 vs 1 vs 2 vs 3), and aa-IPI at registration (H vs HI) (Supplemental Fig. 1a–c). The recurrence rate was 20.6 % at 2 years after registration in the 40 eligible patients. Only 40 eligible patients were registered due to a delay in patient registration although we originally planned to evaluate 43 eligible patients. However, as the lower limit of the 95 % CI of PFS at 2 years after registration was 64.3 %, which exceeded the threshold success rate (50 %), data obtained were considered to be significant.
Hematologic recovery and survival after auto-PBSCT
Auto-PBSCT was performed in 30 patients. With a median transfused CD34-positive cell count of 9.3 × 106/kg (range 2.0–26.6 × 106/kg), the median day of the neutrophil recovery over 500/mm3 was 9 days (range 7–20 days), and the median day of the platelet recovery over 50 000/mm3 was 13 days (9–100 days). No patients failed to achieve engraftment.
The median observation period in surviving patients who completed treatment was 56.4 months (range 8.7–97.3 months) after auto-PBSCT. The Kaplan–Meier estimates of PFS at 2 and 4 years after auto-PBSCT were 86.4 % (95 % CI 67.7–94.7 %) and 79.2 % (95 % CI 59.4–90.1 %), respectively (Fig. 4a). The Kaplan–Meier estimates of OS at 2 and 4 years after auto-PBSCT were 96.7 % (95 % CI 78.6–99.5 %) and 85.9 % (95 % CI 66.7–94.5 %), respectively (Fig. 4b). The OS was significantly better in patients who achieved CR or CRu than in those who achieved PR before HDT (Supplemental Fig. 2b). The recurrence rate was 10.4 % at 2 years after auto-PBSCT in the 30 patients who completed the study treatment.
Toxicity of induction therapy and HDCT with auto-PBSCT
During a chemotherapy period before auto-PBSCT, hematological adverse effects more than Grade 3 were observed but recovered to lower than Grade 2 until next courses by appropriate supportive therapy in the all patients who received auto-PBSCT. As adverse effects of Grade 3, bacterial infection (5 cases), peripheral neuropathy (3 cases), herpes zoster (2 cases), abdominal pain (1 case), drug-induced lung disease (1 case), intestinovesical fistula (1 case), febrile neutropenia (1 case) and hepatic disorder (1 case) were observed. As that less than Grade 2, skin disorder (2 cases), hypertension (1 case) stomach varix (1 case), tachycardia (1 case), deep vein thrombosis (1 case), adrenal malfunction (1 case), diabetes mellitus (1 case) and gingivitis (1 case) were observed. Adverse events observed before the HDC plus auto-PBSCT were herpes zoster in 1, deep vein thrombosis in 1, diabetes mellitus in 1, hypertension in 1, abdominal pain (grade 3) in 1, tachycardia in 1, atopic dermatitis in 1, adrenal failure in 1, right gingivitis in 1, drug-induced lung disease in 1, and intestinovesical fistula in 1. Treatment was discontinued due to toxicity in 3 patients (1 with herpes zoster, 1 with drug-induced lung disease, and 1 with intestinovesical fistula). Treatment was also discontinued in 1 patient who developed liver dysfunction (grade 3) during the conditioning with MCEC (Table 2).
Table 3 summarizes grade 3 or grade 4 adverse events observed after auto-PBSCT in the 30 patients who completed study treatment. Grade 3 febrile neutropenia was observed in 17 patients (57 %) and grade 4 in 2 (7 %). Frequently observed grade 3 non-hematologic toxicities included infections without neutropenia (10 patients, 33 %), diarrhea (9, 30 %), and nausea/vomiting (7, 23 %). No grade 4 non-hematologic toxicity was noted after neutrophil recovery. One patient (3 %) died of sepsis at 2 years and 5 months after transplantation. One patient (3 %) developed pancreas cancer at 609 days after auto-PBSCT, and died at 1375 days.
Discussion
In a multicenter collaborative phase II clinical trial, we have shown the feasibility and effectiveness of R-CHOP14 and HDCT combined with rituximab plus auto-PBSCT as the initial treatment for HI-, and H-risk DLBCL. Phase II clinical trials of HDCT plus auto-PBSCT as the initial treatment for HI-, and H-risk DLBCL have been carried out, and Table 4 compares our results with those of recent phase II studies using upfront HDCT plus autologous stem cell transplantation with rituximab [17–19]. Given the feature of aa-IPI-H/HI DLBCL with relatively older population (median age, 54 years) and poor PS at registration (ECOG PS 2 or more, 27 %) in the present study, our results were satisfactory with a 4-year PFS of 72.0 % and a 4-year OS of 84.6 % compared to other phase II clinical trials. However, recent phase III trials failed to show superior OS in patients who received HDCT combined with auto-PBSCT and rituximab compared to those who received rituximab-containing chemotherapy alone [20, 21]. Although meta-analysis showed that HDCT plus auto-PBSCT was not considered as a standard consolidation treatment for patients with newly diagnosed DLBCL [31], the MCEC regimen and auto-PBSCT combined with rituximab and R-CHOP14 might be one of candidates for future prospective study for patients with newly diagnosed HI-, and H-risk DLBCL.
In the present study, 74 % of the eligible patients completed the planned treatment including HDCT and autologous PBSCT, which is comparable to the other phase II studies of upfront HDCT combined with rituximab [14–19]. Even though we utilized full 6 courses of standard dose CHOP14 before PBSCT, only 10 % of the patients discontinued the study treatment due to progression of DLBCL. In addition to the dose intensification by shortening the interval with G-CSF support, HD-VP16, the chemotherapy for PBSCH, might have contributed the low PD rate before PBSCT. Notably, only 10 % of the patients discontinued the study treatment due to toxicities, and only one (2.5 %) died of complications until the PBSCT. In contrast, the previous studies reported 4–7 % early death before PBSCT, probably due to the more aggressive chemotherapy used for induction and consolidation [16–19].
Although the BEAM regimen is commonly used for conditioning of auto-PBSCT, we previously reported feasibility and high anti-lymphoma effect of MCEC regimen [27]. In cases of unavailability of BCNU, we believe that MCNU can be safely used as a substitute drug for HDCT in HI-, and H-risk DLBCL patients. It was reported that rituximab administration before harvesting peripheral stem cells could reduce lymphoma cells in PBSCH graft [32, 33]. Although in the present study, we did not measure MRD in PBSCH graft, rituximab administration immediately before harvesting peripheral stem cells and immediately after PBSCT may have removed minimal residual disease with suppression of the recurrence rate 2 years after PBSCT to 10.4 %. The effectiveness and safety of PBSCH using HD-VP16 was previously confirmed by a multicenter collaborative clinical study conducted by the JSCT [29]. In this study, peripheral blood stem cells were harvested from all 35 patients using rituximab and HD-VP16 without severe adverse effects. Concerning engraftment after auto-PBSCT, the possibility that rituximab affects the engraftment of cells has been suggested in some reports [34, 35] but has been denied in another report [26]. In this study, the recovery of the neutrophil and platelet was quick, showing no clear effect of rituximab on engraftment.
Regarding safety, the treatment-related mortality rate associated with autologous hematopoietic stem cell transplantation plus HDCT was reported to be 5.7 % [36]. Although the MCEC regimen caused considerable non-hematologic toxicities, none died of complications within 1 year after auto-PBSCT in this study. However, 2 patients (5 %) died without progression of DLBCL beyond the 2 years after auto-PBSCT, one due to infection while receiving corticosteroid, and one due to secondary cancer. In addition, none developed myelodysplastic syndrome/leukemia or late pulmonary toxicity. Of the 7 patients with poor PS graded as 3 at registration, the 6 patients were able to complete the therapy, so this treatment is also considered to be applicable to patients with PS3 at diagnosis. These results suggest that auto-PBSCT plus HDCT using MCEC is tolerable in safety and applicable to a wide range of patients.
Although our prospective study showed promising results, there are several limitations. First, we used contrast-enhanced CT rather than FDG/PET-CT for the evaluation of therapeutic effects, because access to FDG/PET-CT was difficult at the start of the study. Second, we did not use molecular biology technique for stratification of high-risk population. Thirdly, a longer follow-up is needed for actual estimation of secondary malignancies after HDCT and auto-PBSCT. Forthly, only 40 eligible patients were registered due to a delay in patient registration although we originally planned to evaluate 43 eligible patients. However, as the lower limit of the 95 % CI of PFS at 2 years after registration was 64.3 %, which exceeded the threshold success rate (50 %), data obtained were considered to be significant. Consequently, we may consider that this regimen is adopted for subsequent clinical trials. Lastly, OS of the 40 eligible patients is favorable comparing with OS of the 30 patients who completed the scheduled treatment (Figs. 3, 4). In the 10 patients who did not completed the treatment, 8 patients are alive and five keep CR state. One of 5 CR patients reviewed auto-PBSCT. In addition, it might influence a treatment result that there were more ratios of patient of HI-risk than the ratio of H-risk patients for whom HDCT following auto-PBSCT demonstrated to be more effective than standard chemotherapy in S9704 study [21]. Although patients of H- or HI-risk were eligible, good-prognosis patients might be included. We are enforcing the clinical trial (NHL10) to decide adaptation of HDCT following auto-PBSCT using interin PET in the first treatment of DLBCL. We thereby expect to decide appropriate adaptation of HDCT following auto-PBSCT.
In conclusion, our study showed that upfront HDCT and auto-PBSCT combined with rituximab and R-CHOP14 achieved high treatment completion rates, low treatment-related mortality rates, and low recurrence rates for primary HI-, and H-risk CD20-positive DLBCL. However pathological diagnosis of DLBCL was not confirmed by central pathological review, this treatment is thought to be a promising treatment for untreated patients with H- and HI-risk DLBCL. But on the other hand, there might be some good-prognosis patients who were unnecessary of HDCT following auto-PBSCT. Strict evaluation of response by FDG/PET-CT at early timing should be explored for stratification of the candidates who require upfront HDCT and auto-PBSCT. Currently, we are conducting a prospective study of early FDG/PET-CT-guided post-remission therapy including HDCT with Auto-PBSCT plus rituximab, which was based on the present study.
References
The Non-Hodgkin’s Lymphoma Classification Project. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood. 1997;89:3909–18.
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42.
Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Fermé C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de L’Adulte. J Clin Oncol. 2005;23:4117–26.
Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good prognosis diffuse large B-cell lymphoma: a randomized controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–91.
Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–7.
Sehn L, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–33.
Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomized controlled trial (RICOVER-60). Lancet Oncol. 2008;9:105–16.
Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–61.
Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333:1540–5.
Haioun C, Lepage E, Gisselbrecht C, Coiffier B, Bosly A, Tilly H, et al. Comparison of autologous bone marrow transplantation with sequential chemotherapy for intermediate-grade and high-grade non-Hodgkin’s lymphoma in first complete remission: a study of 464 patients. J Clin Oncol. 1994;12:2543–51.
Haioun C, Lepage E, Gisselbrecht C, Bastion Y, Coiffier B, Brice P, et al. Benefit of autologous bone marrow transplantation over sequential chemotherapy in poor-risk aggressive non-Hodgkin’s lymphoma: updated results of the prospective study LNH87-2. Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 1997;15:1131–7.
Santini G, Salvagno L, Leoni P, Chisesi T, De Souza C, Sertoli MR, et al. VACOP-B versus VACOP-B plus autologous bone marrow transplantation for advanced diffuse non-Hodgkin’s lymphoma: results of a prospective randomized trial by the non-Hodgkin’s Lymphoma Cooperative Study Group. J Clin Oncol. 1998;16:2796–802.
Milpied N, Deconinck E, Gaillard F, Delwail V, Foussard C, Berthou C, et al. Initial treatment of aggressive lymphoma with high-dose chemotherapy and autologous stem-cell support. N Engl J Med. 2004;350:1287–95.
Glass B, Ziepert M, Reiser M, Freund M, Trümper L, Metzner B, et al. High-dose therapy followed by autologous stem-cell transplantation with and without rituximab for primary treatment of high-risk diffuse large B-cell lymphoma. Ann Oncol. 2010;21:2255–61.
Schmitz N, Kloess M, Reiser M, Berdel WE, Metzner B, Dorken B, et al. Four versus six courses of a dose-escalated cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) regimen plus etoposide (megaCHOEP) and autologous stem cell transplantation: early dose intensity is crucial in treating younger patients with poor prognosis aggressive lymphoma. Cancer. 2006;106:136–45.
Dilhuydy MS, Lamy T, Foussard C, Gressin R, Casassus P, Deconninck E, et al. Front-line high-dose chemotherapy with rituximab showed excellent long-term survival in adults with aggressive large b-cell lymphoma: final results of a Phase II GOELAMS Study. Biol Blood Marrow Transplant. 2010;16:672–7.
Vitolo U, Chiappella A, Angelucci E, Rossi G, Liberati AM, Cabras MG, et al. Dose-dense and high-dose chemotherapy plus rituximab with autologous stem cell transplantation for primary treatment of diffuse large B-cell lymphoma with a poor prognosis: a phase II multicenter study. Haematologica. 2009;94:1250–8.
Tarella C, Zanni M, Di Nicola M, Patti C, Calvi R, Pescarollo A, et al. Gruppo Italiano Terapie Innovative nei Linfomi. Prolonged survival in poor-risk diffuse large B-cell lymphoma following front-line treatment with rituximab-supplemented, early-intensified chemotherapy with multiple autologous hematopoietic stem cell support: a multicenter study by GITIL (Gruppo Italiano Terapie Innovative nei Linfomi). Leukemia. 2007;21:1802–11.
Fitoussi O, Belhadj K, Mounier N, Parrens M, Tilly H, Salles G, et al. Survival impact of rituximab combined with ACVBP and upfront consolidation autotransplantation in high-risk diffuse large B-cell lymphoma for GELA. Haematologica. 2011;96:1136–43.
Schmitz N, Nickelsen M, Ziepert M, Haenel M, Borchmann P, Schmidt C, et al. German High-Grade Lymphoma Study Group (DSHNHL). Conventional chemotherapy (CHOEP-14) with rituximab or high-dose chemotherapy (MegaCHOEP) with rituximab for young, high-risk patients with aggressive B-cell lymphoma: an open-label, randomised, phase 3 trial (DSHNHL 2002-1). Lancet Oncol. 2012;13:1250–9.
Stiff PJ, Unger JM, Cook JR, Constine LS, Couban S, Stewart DA, et al. Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 2013;369:1681–90.
Cunningham D, Hawkes EA, Jack A, Qian W, Smith P, Mouncey P, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day vs 21-day cycles. Lancet. 2013;381:1817–26.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas: NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244–53.
Makino S, Harada M, Akashi K, Taniguchi S, Shibuya T, Inaba S, et al. A simplified method for cryopreservation of peripheral blood stem cells at −80° C without rate-controlled freezing. Bone Marrow Transplant. 1991;8:239–44.
Katayama Y, Yano T, Bessho A, Deguchi S, Sunami K, Mahmut N, et al. The effects of a simplified method for cryopreservation and thawing procedures on peripheral blood stem cells. Bone Marrow Transplant. 1997;19:283–7.
Kamezaki K, Kikushige Y, Numata A, Miyamoto T, Takase K, Henzan H, et al. Rituximab does not compromise the mobilization and engraftment of autologous peripheral blood stem cells in diffuse-large B-cell lymphoma. Bone Marrow Transplant. 2007;39:523–7.
Numata A, Miyamoto T, Ohno Y, Kamimura T, Kamezaki K, Tanimoto T, et al. Fukuoka Blood and Marrow Transplantation Group. Long-term outcomes of autologous PBSCT for peripheral T-cell lymphoma: retrospective analysis of the experience of the Fukuoka BMT group. Bone Marrow Transplant. 2010;45:311–6.
The International Non-Hodgkin’s Lymphoma Prognosis Factor Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–94.
Chou T, Ishiguro T, Imajo K, Kawano F, Gondo H, Kasai M, et al. A multicenter early phase II study of high-dose chemotherapy with autologous peripheral blood stem cell transplantation for treatment of intermediate-grade non-Hodgkin’s lymphoma. Rinsho Ketsueki. 1999;40:1058–67.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Greb A, Bohlius J, Schiefer D, Schwarzer G, Schulz H, Engert A. High-dose chemotherapy with autologous stem cell transplantation in the first line treatment of aggressive non-Hodgkin lymphoma (NHL) in adults. Cochrane Database Syst Rev. 2008;23:CD004024.
Belhadj K, Delfau-Larue MH, Elgnaoui T, Beaujean F, Beaumont JL, Pautas C, et al. Efficiency of in vivo purging with rituximab prior to autologous peripheral blood progenitor cell transplantation in B-cell non-Hodgkin’s lymphoma: a single institution study. Ann Oncol. 2004;15:504–10.
Arcaini L, Orlandi E, Alessandrino EP, Iacona I, Brusamolino E, Bonfichi M, et al. A model of in vivo purging with rituximab and high-dose AraC in follicular and mantle cell lymphoma. Bone Marrow Transplant. 2004;34(2):175–9.
Hoerr AL, Gao F, Hidalgo J, Tiwari D, Blum KA, Mathews V, et al. Effects of pretransplantation treatment with rituximab on outcomes of autologous stem-cell transplantation for non-Hodgkin’s lymphoma. J Clin Oncol. 2004;22:4561–6.
Khouri IF, Saliba RM, Hosing C, Okoroji GJ, Acholonu S, Anderlini P, et al. Concurrent administration of high-dose rituximab before and after autologous stem-cell transplantation for relapsed aggressive B-cell non-Hodgkin’s lymphomas. J Clin Oncol. 2005;23(10):2240–7.
Greb A, Bohlius J, Trelle S, Schiefer D, De Souza CA, Gisselbrecht C, et al. High-dose chemotherapy with autologous stem cell support in first-line treatment of aggressive non-Hodgkin lymphoma—results of a comprehensive meta-analysis. Cancer Treat Rev. 2007;33:338–46.
Acknowledgments
This work was supported by a Grant from the Regional Medicine Research Foundation (Tochigi, Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Murayama, T., Fukuda, T., Okumura, H. et al. Efficacy of upfront high-dose chemotherapy plus rituximab followed by autologous peripheral blood stem cell transplantation for untreated high-intermediate-, and high-risk diffuse large B-cell lymphoma: a multicenter prospective phase II study (JSCT-NHL04). Int J Hematol 103, 676–685 (2016). https://doi.org/10.1007/s12185-016-1976-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-016-1976-4