Abstract
Mature B-cell acute lymphoblastic leukemia (B-ALL) is typically associated with French-American-British (FAB)-L3 morphology and MYC gene rearrangement. However, rare cases of mature B-ALL with non-L3 morphology and MLL-AF9 fusion have been reported, and such cases are characterized by a rapid and aggressive clinical course. We here report three such cases of pediatric mature B-ALL in female patients respectively aged 15 months, 4 years, and 4 months. Bone marrow smears at diagnosis showed FAB-L1 morphology in all patients. Immunophenotypically, they were positive for cluster of differentiation (CD)10, CD19, CD20 (or CD22), Human Leukocyte Antigen-DR, and surface immunoglobulin λ. No evidence of MYC rearrangement was detected in any of the cases by fluorescent in situ hybridization (FISH) analysis. However, MLL rearrangement was detected by FISH, and MLL-AF9 fusion was confirmed by reverse transcriptase-polymerase chain reaction. All patients achieved complete remission after conventional chemotherapy and subsequently underwent hematopoietic stem cell transplantation as high-risk ALL; patient 3 for infantile ALL with MLL rearrangement and the others for ALL with MLL rearrangement and hyperleukocytosis (white blood cell count at diagnosis >50 × 109/L). At the latest follow-up for each case (12–98 months post-transplantation), complete remission was maintained. Moreover, we discuss the clinical, genetic, and immunophenotypic features of this rare disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute lymphoblastic leukemia (ALL) is a heterogeneous group of hematopoietic neoplasms. B-cell precursor ALL, which is characterized by French-American-British (FAB)-L1 or L2 morphology and a surface immunoglobulin (sIg) negative (−), terminal deoxynucleotidyl transferase (TdT) positive (+), immature B-cell phenotype, comprises the majority of ALL cases [1, 2]. On the other hand, mature B-cell acute lymphoblastic leukemia (B-ALL) is a rare entity, accounting for only 1–2 % of all pediatric ALL cases. Mature B-ALL, characterized by sIg(+), is typically associated with rearrangement of the v-myc avian myelocytomatosis viral oncogene homolog (MYC) gene and FAB-L3 morphology in both adult and pediatric patients [3, 4]. Patients with mature B-ALL have a poor response to and shortened survival with standard ALL therapeutic regimens; however, newer dose-intensive regimens used for Burkitt lymphoma have led to improved cure rates [5].
Chromosomal rearrangements involving the human mixed lineage leukemia or myeloid/lymphoid leukemia (MLL) gene at 11q23 are associated with the development of acute leukemias, and are commonly detected in infantile, as well as in therapy-induced, leukemias [6, 7]. Despite recent technological advances having gradually clarified the molecular mechanisms of MLL fusion-dependent leukemogenesis [8], the presence of certain MLL rearrangements is still an independent dismal prognostic factor, and such patients are usually treated according to high-risk protocols.
Among the reported cases of mature B-ALL with non-L3 morphology and no MYC rearrangement in children, MLL-AF9 chimeric gene positive cases are rare, and this entity is associated with especially poor clinical outcomes [6, 9, 10]. Additionally, the optimal treatment, including the indication of transplantation for ALL with such features, has not yet been established. Herein, we describe three additional cases of childhood ALL with non-FAB-L3 morphology expressing a mature B-cell immunophenotype and showing MLL-AF9 chimeric gene expression without MYC rearrangement. All patients achieved complete remission after conventional chemotherapy and subsequently underwent hematopoietic stem cell transplantation (HSCT). All patients have maintained complete remission. Further, we also discuss the clinical, genetic, and immunophenotypic features of this rare entity.
Case reports
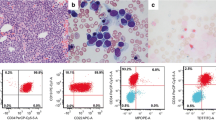
Patient 1 was a 15-month-old female who presented with hepatomegaly and multiple petechiae on the skin covering the trunk. The complete blood count revealed a white blood cell (WBC) count of 54,000/µL with 94 % blasts, a hemoglobin level of 7.8 g/dL, and a platelet count of 41,000/µL. At admission, central nervous system involvement was noted. Radiographic evaluation revealed abdominal paraaortic lymphadenopathy and bilateral renal enlargement. Bone marrow aspirate consisted of 97 % blast cells with FAB-L1 morphology and no myeloperoxidase reaction. However, the blast cells exhibited a mature B-ALL phenotype, with λ sIg(+), cluster of differentiation (CD)10(+), CD19(+), CD20(+), human leukocyte antigen (HLA)-DR(+), and CD34(−). The karyotyping was unable to be confirmed due to no metaphase cell being obtained. Multiplex reverse transcriptase-polymerase chain reaction (RT-PCR) screening detected MLL-AF9 gene fusion, and MLL gene rearrangement was confirmed by fluorescence in situ hybridization (FISH) analysis. The patient was treated according to the Tokyo Children’s Cancer Study Group NHL0105 protocol, which is a Berlin-Frankfurt-Münster (BFM)-derived protocol for non-Hodgkin lymphoma (NHL) or mature B-ALL. Complete remission (CR) was achieved after pre-phase and induction therapy, as demonstrated using bone marrow smears, radiographic evaluation, and lumbar puncture. At this time, the MLL-AF9 fusion gene transcript was negative. After three additional courses of chemotherapy, the patient received bone marrow transplantation using a total body irradiation (TBI)-containing regimen [12 Gy TBI, fractionated in six doses, 60 mg/kg (once daily i.v.) of etoposide, and 180 mg/m2 (90 mg/m2 once daily i.v. for 2 days) of melphalan] from an HLA-matched sibling donor. At the latest follow-up (over 9 years from diagnosis), the patient remained in CR.
Patient 2 was a 4-year-old female who presented with hepatomegaly and scattered petechiae on the surfaces of the extremities. The complete blood count revealed a WBC count of 209,000/µL with 93 % blasts, and a platelet count of 21,000/µL. Other than the bone marrow, no organ involvement was present at admission. Radiographic evaluation revealed no thoracic or abdominal masses. Bone marrow smears showed numerous blasts with FAB-L1, similar to for Patient 1. Flow cytometric immunophenotypic and genetic analyzes of the bone marrow were performed, and revealed that the blast cells were positive for λ sIg, CD10, CD19, CD22, and HLA-DR, and negative for CD34. RT-PCR detected MLL-AF9 gene fusion. The patient was treated according to the group D protocol of BFM-NHL95 [11]. She achieved CR after induction therapy, and the MLL-AF9 fusion gene transcript was negative by RT-PCR. Four months after the initial diagnosis, cord blood transplantation was performed using the same 12-Gy TBI-containing regimen as for patient 1, from an HLA 8/8 matched unrelated donor. At the latest follow-up (1 year from diagnosis), she was disease-free and without adverse events, including chronic graft-versus-host disease.
Patient 3 was a 4-month-old female who presented with purplish skin lesions on her back and hepatosplenomegaly. The complete blood count revealed a WBC count of 295,000/µL, with blasts. At admission, the patient had anemia (hemoglobin: 2.9 g/dL) and thrombocytopenia (platelets: 45,000/µL). Although bone marrow aspiration indicated ALL with FAB-L1 morphology, blast cell typing by flow cytometry revealed a mature B-ALL immunophenotype; the blast cells expressed λ sIg, CD10, CD19, CD20, and CD22. She had a normal karyotype by conventional G-banding. RT-PCR detected MLL-AF9 gene fusion, and MLL rearrangement was detected by FISH, whereas there was no evidence of MYC rearrangement. The patient received the Japanese Pediatric Leukemia/Lymphoma Study Group MLL03 treatment protocol [12], and complete remission was documented after the end of the induction phase upon bone marrow smears. At this time, no MLL-AF9 fusion gene transcripts were detected by RT-PCR. After two additional courses of chemotherapy, allogeneic SCT was performed using 5/6 HLA-matched unrelated cord blood with a busulfan-containing regimen comprising 9.6 mg/kg (0.6 mg/kg four times a day i.v. for 4 days) of busulfan, 60 mg/kg (once daily i.v.) of etoposide, and 120 mg/kg (60 mg/kg once a day i.v. for 2 days) of cyclophosphamide. At the latest follow-up (6 years from diagnosis), the patient remained disease-free.
Discussion
We here described three rare cases of mature B-ALL with non-L3 morphology and MLL-AF9 chimeric gene positive status. The immunophenotype data of our patients and previous cases are summarized in Table 1. As shown in this table, most leukemia cells with these features were negative for CD34 and TdT expression (0/9 and 1/8 positive cases, respectively). Iwamoto et al. demonstrated that mature B-ALL cells frequently show negative expressions of TdT and CD34 [13], whereas both of these immaturity markers, especially TdT, are often positive in MLL-rearranged B-lineage ALL [14]. On the other hand, although CD10, known to be a common ALL antigen, was positive in four of 10 cases, MLL-rearranged B-lineage ALL cases have been reported to usually lack significant CD10 expression [14, 15]. These findings may indicate a characteristic of mature B-ALL rather than childhood B-lineage ALL with MLL rearrangement. Additionally, Jansen et al. reported that MLL-AF9-positive patients have more mature immunoglobulin gene rearrangements than other MLL-rearranged subtypes [15]. Hence, further analysis is necessary to clarify the relation between MLL-AF9 gene fusion and B-lineage ALL.
Table 2 summarizes the clinical characteristic of pediatric patients with mature B-cell ALL with non-L3 and MLL-AF9 fusion. In the previous reports, the outcomes of ALL patients with a mature B immunophenotype, non-L3 morphology, and MLL-AF9 were poor, especially after relapse [6, 9, 10], and Pui et al. similarly reported that B-lineage ALL with MLL-AF9 gene fusion was associated with a poor prognosis [16]. Additionally, in one previous study, three of four patients (cases 1-4) who achieved molecular CR, as determined by RT-PCR for MLL-AF9 fusion transcripts, after induction therapy (shown in Table 1), relapsed after or during chemotherapy. Today, the detection of minimal residual disease after induction is a useful predictor of outcome and is used to guide the decision to perform a transplant after the first CR. Although it is necessary to consider the publication bias for past reports on such unique cases of ALL showing a poor prognosis, this finding may suggest that patients belonging to this rare subgroup of ALL require HSCT after the first CR regardless of the minimal residual disease levels.
A recent review of HSCT in pediatric ALL patients did not support allogeneic SCT when MLL-rearranged ALL is the sole adverse risk factor, and described that the presence of MLL-positive cells along with other risk factors (age, WBC, prednisone response, and other cytogenetic abnormalities) could be used to define very high-risk groups, for which allogeneic SCT may be recommended [17]. From this review, our cases do not necessarily satisfy the transplant indications, and late effects, such as growth failure, endocrine abnormalities, and secondary cancer caused by the HSCT, are major problem for pediatric patients. Accumulation of cases with similar features and further biological studies of ALL will help clarify the clinical significance of this unique phenotype in childhood ALL.
References
Bene MC, Castoldi G, Knapp W, Ludwig WD, Matutes E, Orfao A, et al. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL). Leukemia. 1995;9:1783–6.
Rubnitz JE, Behm FG, Downing JR. 11q23 rearrangements in acute leukemia. Leukemia. 1996;10:74–82.
Brunning RD, Borowitz M, Matutes E, Head D, Flandrin G, Swerdlow SH, et al. Precursor B lymphoblastic leukaemia/lymphoblastic lymphoma. Pathology and Genetics of Tumors of Hematopoietic an Lymphoid tissues. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization Classification of Tumours. Lyon: IARC Press; 2001. p. 111–4.
Frater JL, Batanianet JR, O’Connor DM, Grosso LE. Lymphoblastic leukemia with mature B-cell phenotype in infancy. J Pediatr Hematol Oncol. 2004;26:672–7.
Lee GR, Foerster J, Lukens J, Paraskevas I, Greer JP, Rodgers GM, editors. Wintrobe’s Clinical Hematology. 10th ed. Baltimore: Williams & Wilkins; 1999. p. 2246.
Tsao L, Draoua HY, Osunkwo I, Nandula SV, Murty VV, Mansukhani M, et al. Mature B-cell acute lymphoblastic leukaemia with t(9;11) translocation: a distinct subset of B-cell acute lymphoblastic leukemia. Mod Pathol. 2004;17:832–9.
Meyer C, Schneider B, Jakob S, Strehl S, Attarbaschi A, Schnittger S, et al. The MLL recombinome of acute leukemias. Leukemia. 2006;20:777–84.
Yokoyama A. Molecular mechanisms of MLL-associated leukemia. Int J Hematol. 2015;101:352–61.
Blin N, Mëchinaud F, Talmant P, Garand R, Boutard P, Dastugue N, et al. Mature B-cell lymphoblastic leukemia with MLL rearrangement: an uncommon and distinct subset of childhood acute leukemia. Leukemia. 2008;22:1056–9.
Lorenzana AN, et al. Immunoglobulin gene rearrangements in acute lymphoblastic leukemia with the 9;11 translocation. Genes Chromosom Cancer. 1991;3:74–7.
Woessmann W, Seidemann K, Mann G, Zimmermann M, Burkhardt B, Oschlies I, et al. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: a report of the BFM group study NHL-BFM95. Blood. 2005;105:948–58.
Koh K, Tomizawa D, Moriya Saito A, Watanabe T, Miyamura T, Hirayama M, et al. Early use of allogeneic hematopoietic stem cell transplantation for infants with MLL gene-rearrangement-positive acute lymphoblastic leukemia. Leukemia. 2015;29:290–6.
Iwamoto S, Deguchi T, Ohta H, Kiyokawa N, Tsurusawa M, Yamada T, et al. Flow cytometric analysis of de novo acute lymphoblastic leukemia in childhood: report from the Japanese Pediatric Leukemia/Lymphoma Study Group. Int J Hematol. 2011;94:185–92.
Attarbaschi A, Mann G, König M, Steiner M, Strehl S, Schreiberhuber A, et al. Mixed lineage leukemia-rearranged childhood pro-B and CD10-negative pre-B acute lymphoblastic leukemia constitute a distinct clinical entity. Clin Cancer Res. 2006;12:2988–94.
Jansen MW, Corral L, van der Velden VH, Panzer-Grümayer R, Schrappe M, Schrauder A, et al. Immunobiological diversity in infant acute lymphoblastic leukemia is related to the occurrence and type of MLL gene rearrangement. Leukemia. 2007;21:633–41.
Pui CH, Gaynon PS, Boyett JM, Chessells JM, Baruchel A, Kamps W, et al. Outcome of treatment in childhood acute lymphoblastic leukemia with rearrangements of the 11q23 chromosomal region. Lancet. 2002;359:1909–15.
Oliansky DM, Camitta B, Gaynon P, Nieder ML, Parsons SK, Pulsipher MA, et al. Role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of pediatric acute lymphoblastic leukemia: update of the 2005 evidence-based review. Biol Blood Marrow Transplant. 2012;18:505–22.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
About this article
Cite this article
Sarashina, T., Iwabuchi, H., Miyagawa, N. et al. Hematopoietic stem cell transplantation for pediatric mature B-cell acute lymphoblastic leukemia with non-L3 morphology and MLL-AF9 gene fusion: three case reports and review of the literature. Int J Hematol 104, 139–143 (2016). https://doi.org/10.1007/s12185-016-1971-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-016-1971-9