Abstract
The outcome of romiplostim for secondary failure of platelet recovery (SFPR) was investigated in children who had undergone hematopoietic stem cell transplantation (HSCT). Seven transfusion-dependent pediatric patients (median age 11 years), with platelet counts below 10 × 109/L, received four weekly doses of subcutaneous romiplostim to treat SFPR developed after HSCT. All patients, except one (patient 4), became platelet transfusion-independent in the second week from the beginning of treatment and no patient needed to discontinue drug treatment because of adverse events. Romiplostim could represent a beneficial first-line treatment, but further studies are required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endogenous thrombopoietin (e-TPO), also known as c-Mpl ligand, is a primary regulator of the proliferation and maturation of megakaryocytes as well as of platelet production. Having no storage form, e-TPO is made at a constant rate in the liver and is released from hepatocytes into circulation [1]. E-TPO was the last major hematopoietic growth factor to be identified, purified and cloned.
Romiplostim (Nplate®, Amgen) is used for platelet stimulation. This drug is obtained by synthesizing a fusion protein made by four little peptides consisting of 14 amino acids connected to an IgG Fc fragment. The mechanism of action of this drug consists of a linkage within the thrombopoietin receptor to start the kinase intracellular signaling pathways [2].
Romiplostim is approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for treating adult patients in whom first-line and other second-line therapies have failed or who have a contraindication to splenectomy. Previous reports have also documented the efficacy of romiplostim in several patients with thrombocytopenia associated with hepatitis C-related liver cirrhosis, low-risk myelodysplastic syndromes, aplastic anemia refractory to immune-suppressive therapy and in the management of patients with severe secondary failure of platelet recovery (SFPR) [3].
Little published data exist, however, on the use of thrombopoietin-receptor agonists in children. In this case series, we report the outcome of 7 pediatric patients who underwent hematopoietic stem cell transplantation (HSCT) and were treated with romiplostim for SFPR.
Case series
Seven pediatric patients admitted to IRCCS “Burlo Garofolo” Pediatric Hospital from 2011 to 2013 who underwent allogeneic Hematopoietic Stem Cell Transplantation (HSCT) and developed SFPR were successively treated with romiplostim.
Written informed consent was obtained from the patients’ parents before the romiplostim treatment, in accordance with established hospital policies.
The median age of patients was 11 years (with a range from 8 to 13 years). Four patients received a HSCT for acute leukemia, 2 for bone marrow failure and 1 for primary immunodeficiency. All patients received a myeloablative conditioning regimen. Three patients had human leukocyte antigen (HLA)-identical sibling donors, 3 patients had HLA-matched unrelated donors and one patient had a haploidentical donor. The median total nuclear cell dose (TNC) infused was 4. 9 (range 3–7, 9) × 108/kg. The stem cell source was the bone marrow (BM) in 5 cases, and granulocyte-colony-stimulating factor-mobilized peripheral blood stem cells (PBSC) were used in the remaining 2 cases. Six of 7 patients received tacrolimus (FK-506) alone or in combination with mycophenolate mofetil (MMF) as graft-versus-host disease (GVHD) prophylaxis. Only one patient received a combination of cyclosporine, MMF and prednisone as GVHD prophylaxis. All patients achieved a complete engraftment. The median day of recovery of polymorphonuclear neutrophils (PMN) and Reticulocyte Blood Count (RBC) was day +16.7 (range 11–20) and +21.2 (range 15–29), respectively. The successful engraftment of PMN was defined when absolute neutrophil count exceeded 0.5 × 109/L, and engraftment of RBC was defined when reticulocytes were >2 %.
GVHD was evaluated according to the Seattle standard criteria [4]. The incidence of GVHD was 57 % (4 patients had acute GVHD), but the severity of GVHD was very low: none of the patients developed grade 3–4 GVHD. All patients had post-transplant cytomegalovirus (CMV) reactivation, quantified in serum by polymerase-chain-reaction (PCR) testing. The median day of the first CMV detection was +31, 7 (range 15–44).
All patients had platelet engraftment, with a median day of PLT recovery at day +30 (range 11–63) following transplantation. All seven patients developed SFPR: in 4 SFPR followed a viral infection caused by one or more viruses, 2 had immune-related SFPR and one had SFPR due to a combination of viral infection and immune reaction. The 3 patients who developed immune-related SFPR were treated with prednisone and IVIG with or without rituximab.
Baseline characteristics of the patients, type of conditioning regiment, donor type, hematopoietic stem cell (HSC) source, engraftment, CMV reactivation, GVHD prophylaxis, grade of GVHD and SFPR first-line treatment are summarized in Table 1.
Inclusion criteria for the romiplostim stimulation (RS) were platelet transfusion-dependent development of SFPR, no response to first-line treatment of immune-related SFPR, absence of megakariocytes or hypomegakariocytosis in virus-related SFPR, bone marrow biopsy, no primary disease recurrence before RS and full donor chimerism.
The median time from the start of the RS was at day +85 (range 62–104). All 7 patients received 4 weekly doses of subcutaneous romiplostim: 4 patients received romiplostim at a dosage of 5 μg/kg, one patient at a dosage of 3 μg/kg, and two patients received romiplostim at escalating dosages (from a minimum dose of 3 μg/kg to a maximum dose of 7 μg/kg).
Before romiplostim stimulation, all patients had platelet counts below 10 × 109/L (without platelet transfusion), and all patients were transfusion dependent. All patients, except one (patient 4), became platelet transfusion independent in the second week from the beginning of treatment. Their RS was discontinued after 4 weeks with a sustained platelet count of >60 × 109/L (range 63–124 × 109/L). After 8 weeks from the beginning of RS, all 6 patients who responded to treatment reached a platelet recovery count of more than 80 × 109/L (range 86–186 × 109/L) and at the last follow-up (minimum 303 days and maximum 790 days) reached a normal platelet count (range 263–384 ×109/L).
As an alternative approach to assessing platelet recovery after RS we have focused on a simple peripheral blood test as a sensitive and practical measurement of ongoing thrombopoiesis. The Sysmex XE-2100 blood cell counter has been developed to measure the fraction of newly released immature platelets containing high amounts of cytoplasmic RNA in the peripheral blood, analogous to measurement of reticulocytes in the erythroid lineage [5]. The immature platelet fraction (IPF) has been utilized to predict marrow recovery after hematopoietic transplantation and to assess the level of thrombopoiesis in patients with SFPR. IPF is expressed as a percentage, which represents the ratio of immature PLTs to the total number of PLTs ×100 (0.60–0.70).
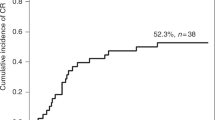
As reported in Fig. 1a all patients except one, after last RS, have a platelet growth counting above 50 × 109/L. As described by Dominietto et al. the platelet count below this cut-off is a negative predictor of transplant-related mortality (TRM) after HSCT [6]. Figure 1b shows a linear decrease of IPF and immature platelet growth after platelet count stabilization.
The post-transplant course of patient 4 was also complicated by systemic toxoplasmosis on day +31, which was treated with trimethoprim-sulfamethoxazole and subsequently needed a change to pyrimethamine and clindamycin therapy due to thrombocytopenia and a related bone marrow suppression.
On day +52 patient 4 developed a systemic hystoplasmosis associated with severe liver GVHD. She also had CMV, Epstein Barr Virus (EBV) and Adenovirus reactivation due to increasing immune suppression. On day +60 she developed deep neutropenia, anemia and transfusion refractory thrombocytopenia. Her bone marrow biopsy showed complete aplasia. This patient died on day +102 from multiorgan failure.
All patients tolerated romiplostim well, and no patient needed to discontinue drug treatment because of adverse events.
Discussion
Thrombocytopenia after allogeneic HSCT is a common complication, which can sometimes be really grievous. When thrombocytopenia is the result of poor platelet engraftment after myeloablative conditioning regimens used for HSCT, it is defined as a primary failure of platelet recovery (PFPR). PFPR depends on several factors, including HLA typing, stem cell source, stem cell doses infused, types and phases of disease, GVHD and infection complications [7]. Some patients present with secondary failure of platelet recovery (SFPR). SFPR has been defined, by the Seattle group, as a decline in platelet count to <20 × 109/L for 7 consecutive days, or the need for transfusion support after achieving a sustained platelet count of ≥50 × 109/L without transfusion support for 7 consecutive days after HSCT1. SFPR depends substantially on two factors: (1) a decline of platelet production in bone marrow due to a relapse of primary disease, allograft rejection, GVHD, pharmacological toxicity and virus infections, or (2) a rise of peripheral platelet destruction due to veno-occlusive disease (VOD), transplant-associated microangiopathy, immune-mediated mechanism, splenomegaly, impaired renal or liver function or platelet consumption due to bleeding. The incidence of SFPR in HSCT, reported in the literature, is estimated at around 20 % [7]. Several studies have suggested that poor platelet recovery following allo-HSCT is of adverse prognostic significance in transplant outcome [8–10]. Przepiorka et al. used a platelet count of 100 × 109/L as a criterion to define chronic GVHD as severe [8]. Those patients who have a platelet count below 100 × 109/L had increased overall mortality and increased treatment failure 18 months post transplant [8]. First et al. discussed thrombocytopenia after allogeneic HSCT in pediatric cohorts, observing that the patients with a platelet count below 100 × 109/L for 100 days post transplant have decreased survival and an increased incidence of severe acute and chronic GVHD [9]. Bolwell et al. demonstrated that the primary reason thrombocytopenic patients have a higher mortality risk was a striking incidence of treatment-related failure. The most common cause of treatment failure was chronic GVHD [10]. Infectious complications may also be associated with thrombocytopenia. The authors concluded that it is difficult to establish the precise etiology of thrombocytopenia in the post transplant period but, clearly, it is associated with other significant complications [10]. Therefore, measurement of e-TPO levels may be of diagnostic utility in discriminating between pathological processes in which thrombocytopenia has resulted primarily from bone marrow hypoplasia as opposed to peripheral platelet destruction. Measurement of e-TPO levels may serve as a surrogate marker for megakaryopoiesis. [11].
Moreover, Makar et al. observed that high TPO levels were associated with a lack of a durable response to treatment with a TPO receptor agonist.
Only a few cases have been described in literature to date of patients successfully treated with romiplostim after having developed SFPR post allo-HSCT.
We found only 5 articles reporting on a total of 11 patients: only two cases are pediatric patients [2, 4, 12–14]. Of those 11 patients, 4 had a refractory immune thrombocytopenia, and 7 developed a SFPR in the course of acute or chronic GVHD with or without EBV or CMV reactivation. The rationale for using RS in immune thrombocytopenia (ITP) is based on this disorder’s pathophysiology. It is now recognized that ITP is a disorder of increased platelet destruction and inappropriately low platelet production. Kuter and Gernsheimer found megakaryocytes were present in increased numbers of ITP patients, but noted that these megakaryocytes often appeared immature and underwent accelerated apoptosis with a greatly diminished productivity of platelets [15]. Harker and colleagues [16] extensively studied 8 patients with human immunodeficiency virus (HIV) thrombocytopenia and have shown that there was no change after thrombopoietin (TPO) or thrombopoietin-mimetics therapy (TMT) in platelet survival, antiplatelet antibody or HIV viral load. These results suggested that TMT therapy reduced apoptosis of megakaryocyte progenitors and megakaryocytes and allowed the patients to produce platelets.
The rationale for using of RS in virus-related SFPR is a quite clear. Many researchers have demonstrated that CMV can incubate in the hemopoietic stem or ancestral cell to inhibit their generation and differentiation. This suggests that viruses can cause blood platelet reduction not only through the immunologic mechanism but also through the direct infection of megakaryoblast by inhibiting its proliferation [17]. Drugs for treatment or prophylaxis of the most common viral infections (CMV, adenovirus, HHV- 6, BKV) in patients undergoing a HSCT are known for their myelotoxicity, and thrombocytopenia is reported as a side effect in the data sheet of each antiviral drug. Pathophysiologic understanding of drops in platelet counts due to virus infection tells us that RS should be used as an adjuvant and targeted therapy by shortening the time for platelet recovery after obtaining viral clearance. The same considerations can also apply for SFPR developing during a GVHD. An increase of immunosuppressive therapy often results in an increased platelet count [18]. The use of RS for a GVHD-related SFPR should be reserved only for patients who cannot reach a spontaneous platelet recovery or whose recovery times are too long, when GVHD has improved.
In our cases all patients, except one (patient 4), have had an excellent response to RS without any complications.
Two of our patients with an immune form of SFPR were treated with RS after the failure of first-line therapy with prednisone and high-dose immunoglobulin (HDIG) and second-line treatment with rituximab. The third patient, who developed a mixed form, virus and immune-related SFPR, was treated only with cortisone and HDIG, but not with rituximab, because of a difficult management of of several viruses that reactivated.
These 3 patients had side effects from first-line treatment in the form of metabolic syndrome and virus reactivation. Moreover, the first two patients underwent rituximab treatment, and they showed infection complications related to use of rituximab.
Our case series includes a very small number of patients, and it is not easy to draw a valid conclusion on the efficacy and safety of the use of romiplostim in pediatric patients who develop a SFPR after allogeneic transplant. Most patients undergoing an allogeneic transplant are exposed to a variety of drugs, including those for prophylaxis (anti-infective, anti-rejection, organ-specific toxicity) and those for treatment of possible complications. Sometimes these drugs can cause more side effects or complications rather than significant benefits.
Thrombocytopenia post-HSCT is associated with a higher non-relapse mortality (NRM), and overall survival (OS) is significantly worse than that in patients with a normal platelet count. This is not due to the presence of thrombocytopenia per se, but due to the disease factors that have induced it; hence the more severe the triggering event, the more severe the derived thrombocytopenia. It would be interesting to see if a resolution of or at least an improvement in thrombocytopenia after RS is able to modify the OS and NRM in patients who undergo HSCT.
Conclusions
Since to date there are no recommendations about the use of thrombopoietin-receptor agonists in children1, our data may serve to add new information about the safety and efficacy of using romiplostim for the treatment of SFPR after HSCT in pediatric patients.
Our data obtained from children suggest that in selected cases of very complex patients who develop life-threatening complications such as SFPR, romiplostim could be a beneficial first-line treatment, with less risk than standard treatments. Further and larger studies are needed to better define the efficacy and safety of the use of romiplostim in children.
In conclusion, as described before, e-TPO levels in patients with SFPR after allo-HSTC need to be better investigated.
References
Imbach P, Crowther M. Thrombopoietin-receptor agonists for primary immune thrombocytopenia. N Engl J Med. 2011;365:734–41.
Poon LM, Di Stasi A, Popat U, Champlin RE, Ciurea SO. Romiplostim for delayed platelet recovery and secondary thrombocytopenia following allogeneic stem cell transplantation. Am J Blood Res. 2013;3(3):260–4.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8.
Beck JC, Burke MJ, Tolar J. Response of refractory immune thrombocytopenia after bone marrow transplantation to romiplostim. Pediatr Blood Cancer. 2010;54:490–1.
Bat T, Seth M, Steinberg SM, Childs R, Calvo KR, Barrett AJ, et al. Active thrombopoiesis associated with worse severity and activity of chronic graft-versus-host disease. Bone Marrow Transplant. 2013;48(12):1569–73.
Dominietto A, Raiola AM, van Lint MT, Lamparelli T, Gualandi F, Berisso G, et al. Factors influencing haematological recovery after allogeneic haemopoietic stem cell transplants: graft-versus-host disease, donor type, cytomegalovirus infections and cell dose. Br J Haematol. 2001;112:219–27.
Bruno B, Gooley T, Sullivan KM, Sullivan KM, Davis C, Bensinger WI, Storb R, et al. Secondary failure of platelet recovery after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:154–62.
Przepiorka D, Anderlini P, Saliba R, Cleary K, Mehra R, Khouri I, et al. Chronic graft-versus-host disease after allogeneic blood stem cell transplantation. Blood. 2001;98:1695–700.
First LR, Smith BR, Lipton J, Nathan DG, Parkman R, Rappeport JM. Isolated thrombocytopenia after allogeneic bone marrow transplantation: existence of transient and chronic thrombocytopenic syndromes. Blood. 1985;65:368–74.
Bolwell B, Pohlman B, Sobecks R, Andresen S, Brown S, Rybicki L, et al. Prognostic importance of the platelet count 100 days post allogeneic bone marrow transplant. Bone Marrow Transplant. 2004;33:419–23.
Makar RSL, Zhukov OS, Sahud MA, Kuter DJ. Thrombopoietin levels in patients with disorders of platelet production: diagnostic potential and utility in predicting response to TPO receptor agonists. Am J Hematol. 2013;88(12):1041–4.
Buchbinder D, Hsieh L, Krance R, Nugent DJ. Successful treatment of post-transplant thrombocytopenia with romiplostim in a pediatric patient with X-linked chronic granulomatous disease. Pediatr Transplantation. 2014;18(7):E252–4.
Calmettes C, Vigouroux S, Tabrizi R, Milpied N. Romiplostim (AMG531, Nplate) for secondary failure of platelet recovery after allo-SCT. Bone Marrow Transplant. 2011;46:1587–9.
Bollag RJ, Sterett M, Reding MT, Key NS, Cohn CS, Ustun C. Response of complex immune-mediated thrombocytopenia to romiplostim in the setting of allogeneic stem cell transplantation for chronic myelogenous leukemia. Eur J Haematol. 2012;89:361–4.
Kuter DJ, Gernsheimer TB. Thrombopoietin and platelet production in chronic immune thrombocytopenia. Hematol Oncol Clin North Am. 2009;23(6):1193–211.
Harker LA, Carter RA, Marzec UM, Cherry JK, Gunthel CJ, Lennox JL, et al. Correction of thrombocytopenia and ineffective platelet production in patients infected with human immunodeficiency virus (HIV) by PEG-rHuMGDF therapy [abstract]. Blood. 1998;92:707a.
Xiao Y, Lin W, Liu Q, Jin R, Fei H. Direct infection of colony forming unit-megakaryocytes by human cytomegalovirus contributes the pathogenesis of idiopathic thrombocytopenic purpura. J Huazhong Univ Sci Technol. 2006;26(5):555–7.
First LR, Smith BR, Lipton J, Nathan DG, Parkman R, Rappeport JM. Isolated thrombocytopenia after allogeneic bone marrow transplantation: existence of transient and chronic thrombocytopenic syndromes. Blood. 1985;65(2):368–74.
Acknowledgments
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Maximova, N., Zanon, D., Rovere, F. et al. Romiplostim for secondary thrombocytopenia following allogeneic stem cell transplantation in children. Int J Hematol 102, 626–632 (2015). https://doi.org/10.1007/s12185-015-1821-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-015-1821-1