Abstract
Purpose of Review
To provide an overview of the recent scientific literature about ramp lesions of the medial meniscus and to summarise the current evidence on their prevalence, classification, biomechanics, surgical techniques and clinical outcomes.
Recent Findings
Ramp lesions may be present in more than 1 patient undergoing ACL reconstruction out of 5 and almost half of the medial meniscal tears observed in this population. Due to the risk of persistent anterior and rotational laxity after ACL reconstruction, their repair has been advocated. There is no general agreement to date on whether and when ramp lesions should be treated surgically. Comparative studies have failed to show that the repair of stable lesions was superior in comparison to nonoperative approaches. A lower failure rate and secondary meniscectomy has been reported with a suture hook repair through the posteromedial portal in comparison with an all-inside technique. Furthermore, reconstructions of the anterolateral complex in association with ACL reconstruction may have a protective effect on ramp repair.
Summary
Ramp lesions of the medial meniscus in ACL-injured knees cannot be neglected anymore. Given their novelty, their clinical impact has not been fully assessed yet, but the evidence is growing that they need to be systematically identified and eventually repaired, for which they require advanced surgical knowledge. There is, to date, no consensus on whether and when ramp lesions should be treated surgically. Their subtypes, size and stability may influence the decision-making process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ramp lesions were first described in 1983 by Hamberg et al. [1] as “a peripheral vertical rupture in the posterior horn of the medial or lateral meniscus with an intact body”. The tear was initially identified through the Gillquist view with a 30- or 70-degree arthroscope, and repair was achieved by a mini-arthrotomy behind the posterior oblique ligament. In 1984, Lemaire et al. [2] described a similar entity: a posterior meniscocapsular tear of the medial compartment of the knee associated with an anterior cruciate ligament (ACL) rupture. These tears were then poorly investigated by the orthopaedic community until Ahn et al. [3, 4] brought the lesion back into the spotlight 20 years later, bringing new perspectives to the treatment of ACL injuries. Most of the scientific literature about ramp lesions of the medial meniscus thus concentrates over the last 5 years. In the context of this recent and rapid development, the purpose of this review is to summarise current knowledge on the prevalence, classification, biomechanics, surgical techniques and clinical outcomes of ramp lesions.
How Common are Ramp Lesions?
To properly interpret the existing literature on ramp lesions, it is important to consider the discrepancies in their definition and in their diagnostic approach as well as the year of publication of the respective study. Several terminologies have been used to describe these lesions such as meniscosynovial lesions, meniscocapsular separations, hidden lesions and ramp lesions. The authors recommend to use exclusively the term “ramp lesion” with the following definition: a traumatic tissue disruption between the posterior horn of the medial meniscus and its meniscoligamentous and capsular junction located in the so-called red-red zone according to zone 0 of the Warren classification [5].
With increasing knowledge and recognition of ramp lesions amongst orthopaedic surgeons over the last decade [6], the reported prevalence of these injuries in association with ACL injuries has significantly increased from 9% in 2010 [7] to an overall pooled prevalence of 21.9% (range: 9.0–41.7%) [8••]. The highest prevalence was reported by the most recent studies. To date, ramp lesions have thus to be recognised as a significant part of all medial meniscus tears in ACL-injured patients, representing up to 55% of them [9••, 10].
The observed prevalence may vary according to various patient and injury factors. For example, there is a moderate-to-strong evidence that ramp lesions are more likely to be observed in males, in patients under 30 years, presenting with complete ACL tears and in the presence of a concomitant lateral meniscal tear [8••]. The evidence that chronicity of the ACL injury (time from injury to surgery > 24 months) influences the rate of ramp lesions is lower [8••]. The definition of chronicity, however, varies between studies preventing from a proper comparison of results. Finally, several studies also mentioned that ramp lesions were also more likely to be observed in contact injuries [10,11,12] and in revision ACL reconstructions [12,13,14].
When assessed with magnetic resonance imaging (MRI), the highest reported prevalence of ramp lesions reached 42% [12] and 39.5% [15]. MRI sensitivity to detect ramp lesions varies from 48 to 86% and its specificity from 79 to 99% [16] with a pooled sensitivity of 65–71% and a pooled specificity of 92–94%, according to two recent systematic reviews [17, 18]. MRI currently remains less accurate to detect ramp lesions than to detect meniscal tears in general [19]. Their prevalence, as reported with this method, may thus be underestimated. To overcome these limitations, future studies may focus on better standardising influencing MRI factors such as magnet strength, knee position, MRI interpreter and diagnostic criteria [17].
Although less accurate than arthroscopy to diagnose ramp lesions, MRI can add important indirect information on the risk of a patient to present a ramp lesion. The medial meniscal slope has indeed recently been suggested as a risk factor for ramp lesions in knees with ACL injury [20]. Furthermore, posteromedial tibial plateau edema has been reported to be associated with ramp lesions [8••, 21, 22].
Little is known about the pathogenesis of ramp lesions. One of the current main hypotheses is related to the fact that a disruption of the meniscotibial attachment at the posterior horn of the medial meniscus may occur at the moment of injury, when the medial femoral condyle subluxes posteriorly over the posterior border of the medial tibial plateau. This mechanism is comparable to Bankart lesions of the glenohumeral joint. By superimposing the bone bruise areas on the femur and the tibia on MRI to obtain a precise reproduction of the femoral position in relation to the tibia at the time of injury, some authors identified an anteroposterior displacement of up to 25 mm occurring at the time of the injury [23]. This significant displacement makes the disruption of the meniscotibial attachment and the occurrence of ramp lesions plausible.
To date, arthroscopic examination remains the diagnostic “gold standard” [8••]. Sonnery-Cottet et al. [24] have proposed a systematic arthroscopic exploration of the knee joint using a 30° scope. Under arthroscopy, ramp lesions can be suspected by anterior probing of the posterior horn of the medial meniscus which can reveal increased mobility of the medial meniscus [24]. Those lesions are underestimated through the standard anterolateral arthroscopic portal as they can be hidden under a membrane-like tissue which requires a minimal posteromedial debridement to discover the lesion [24]. The trans-condylar notch visualisation can be improved either by moving the foot in internal rotation or with a 70° arthroscope [16, 25]. Some lesions can only be visualised by the posteromedial approach [26] so that the additional posteromedial portal remains essential to allow a complete visualisation of the ramp as well as a full identification of the medio-lateral extension of the lesion. An additional direct posteromedial portal view does also allow for a dynamic stability testing of the ramp through several flexion-extension movements [26]. If possible, the posteromedial portal should be performed with trans-illumination to avoid iatrogenic injuries to the saphenous vein and nerve. This is currently the only way to ensure the identification of all ramp lesions, both in adult [16] and paediatric populations [27].
The Different Types of Ramp Lesions
Ramp lesions are as tears at the posterior meniscocapsular junction and/or tears of the posterior meniscotibial ligament [28] (Fig. 1). The posterior meniscocapsular junction of the medial meniscus is composed of the meniscocapsular ligament superiorly and the meniscotibial ligament inferiorly, both of which attach to the periphery of the posterior horn of the medial meniscus. Ramp lesions occur in the posterior aspect of the red-red zone of the medial meniscus, where the meniscocapsular and meniscotibial attachment merge into the posterior horn [28].
Magnetic resonance imaging (MRI) of a ramp lesion of the medial meniscus. Sagittal slice MRI of a typical sign of a ramp lesion in an ACL-deficient knee. In this right knee, a hyper T2-weighted signal can be observed at the meniscocapsular junction of the medial meniscus posterior horn. Abbreviations: MFC: medial femoral condyle; MTP: medial tibial plateau; ACL: anterior cruciate ligament; MCJ, meniscocapsular junction; MM: medial meniscus
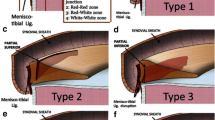
The first classification was described in 2016 by Thaunat et al. [29] and distinguishes 5 different types of ramp lesions (Fig. 2). Types 1 and 2 are stable lesions at probing of the posterior horn of the medial meniscus. The former corresponds to a lesion posterior to the meniscal attachment of the meniscotibial ligament. The latter is a partial superior lesion anterior to the meniscal attachment of the meniscotibial ligament that can only be diagnosed by a trans-notch approach. Type 3 is a partial inferior lesion, which cannot be identified with a trans-notch approach. It has low stability at probing. Types 4 and 5 are highly unstable at probing: type 4 being a complete lesion in front of the meniscal attachment of the meniscotibial ligament and type 5 is a double lesion with an associated meniscotibial ligament disruption. Out of 2156 primary or revision ACL reconstructions with 334 ramp lesions (15.5%), Thaunat et al. could further show that the subtypes of ramp lesions were distributed as follows: type 1, 47.9%; type 2, 4.8%; type 3, 11.4%; type 4, 28.7%; type 5, 7.2% [30••].
Arthroscopic classification of ramp lesions according to Thaunat et al. [29]. Five different types of ramp lesions are distinguished during arthroscopy: type 1: meniscocapsular lesions; type 2: partial superior lesions; type 3: partial inferior or hidden lesions; type 4: complete tears in the red-red zone; type 5: double tears. Abbreviations: MFC: medial femoral condyle; MTP: medial tibial plateau
This classification does neither consider the length of the lesion nor the stability of the capsuloligamentous complex during knee motion. Seil et al. [31] thus proposed an updated classification independent from the lesion being in the red-red zone or not. It includes the medio-lateral extension of the lesion as well as its stability. As such, complete lesions extend along the entire ramp and partial lesions are located either centrally or medially. In stable lesions, the capsuloligamentous complex adheres firmly to the posterior wall of the meniscus and does not move during movements of flexion and extension. Theoretically, these lesions have the potential to heal without surgical treatment. In unstable lesions, the capsuloligamentous complex is not adherent to the meniscus and may show a dehiscence or gap between the posterior wall of the medial meniscus and the capsuloligamentous complex of the ramp during knee flexion or extension movements.
Greif et al. [32••] modified Thaunat’s et al. [29] classification in MRI by detailing the lesion in the meniscocapsular complex according to the latest descriptions of DePhillipo’s cadaveric study [33] (Fig. 3). Cristiani et al. [15] recently reported the prevalence of each subtype of lesions in a retrospective study including 100 patients with ramp lesions out of 253 patients included: type 1: 13%; type 2: 4%; type 3A: 7%; type 3B: 7%; type 4A: 20%; type 4B: 43%; type 5: 6%. To date, the generalisation of results on MRI must, however, be done with caution as the method appears to have only moderate agreement with arthroscopic findings [34].
Magnetic resonance imaging (MRI) classification of ramp lesions according to Greif et al. [32••]. Five different types of ramp lesions are distinguished on MRI: type 1: meniscocapsular ligament tear; type 2: partial superior peripheral meniscal horn tear; type 3A: partial inferior peripheral posterior horn meniscal tear; type 3B: meniscotibial ligament tear; type 4A: complete peripheral posterior horn meniscal tear; type 4B: complete meniscojunction tear; type 5: peripheral posterior horn meniscal double tear. Abbreviations: MFC: medial femoral condyle; MTP: medial tibial plateau
Biomechanics
Several cadaver studies showed that ACL-associated ramp lesions lead to an increased anterior tibial translation, as well as in internal and external rotation of the knee compared to ACL deficiency alone [35,36,37]. These findings confirm that the posterior horn of the medial meniscus acts as a secondary restraint to anterior tibial translation and rotation in the ACL-deficient knee.
The biomechanical impact of ramp lesions was confirmed in clinical studies. Mouton et al. [38•] demonstrated in a series of 275 patients including 58 (21%) ramp lesions that patients with an isolated ramp lesion were more likely to have a grade III pivot shift compared to patients with isolated ACL injury and no ramp lesion. Thaunat et al. could further show a higher side-to-side laxity as well as a higher degree of pivot shift in complete lesions (subtypes 1, 4, 5) compared to partial lesions (subtypes 2 and 3) [30••]. Leaving ramp lesions unrepaired in the context of an ACL-deficient knee may therefore lead to persistent laxity after ACL reconstruction.
Surgical Techniques
There is no agreement on whether and when ramp lesions should be treated surgically [39]. The tear size may affect the stability of these lesions, and it has been suggested that small and stable tears may be managed with debridement alone [40]. Lesions localised in the red-red zone may have the potential to heal without surgical treatment unless the blood supply to the periphery of the meniscus is interrupted, preventing proper natural healing of the lesion [4].
Meniscal ramp lesions can be repaired using an all-inside technique or a posteromedial portal approach. In all-inside techniques [41, 42], the device is advanced to the ramp lesion through the anteromedial portal. With a trans-condylar notch view, the first implant is inserted under the meniscus and obliquely into the joint capsule. The second implant is inserted into the peripheral edge of the meniscus. The pre-tied self-sliding knot is tensioned to achieve secure fixation.
The most frequent technique to repair ramp lesions is a direct suture repair by using a curved hook with a curvature between 25° [24] and 90° [43••], depending on the used approach. If a single posteromedial approach with a trans-condylar notch arthroscopic view is used (Fig. 4), the tear is debrided with a motorised cutter through the posteromedial portal. Then the suture hook is passed through the meniscal peripheral rim tissue from superior to inferior and to the posterior horn of the meniscus from inferior to superior. The suture is advanced and retrieved with a grasper, and a knot is tied with an arthroscopic pusher. Sutures are placed every 5 mm along the length of the tear. Usually, we recommend a strong monofilament absorbable suture (e.g. PDS 1). If a double posteromedial (PM) approach is used (Fig. 5), the posteromedial viewing portal is placed proximal and posterior to the knee joint and the working portal more distal and anterior. For proper portal placement, the use of an orientation needle is strongly recommended while the arthroscope helps visualising the posteromedial capsular structures through an intercondylar notch view. The use of a cannula is generally not required. A double PM approach provides a full visualisation of the ramp, reaching from its medial attachment at the medial collateral ligament to the centre of the knee (Fig. 6). This direct view does also allow to get additional information on the stability of the ramp lesion through flexion-extension movements as well as the tension of the posteromedial capsule [43••] (Fig. 7).
All-inside suture hook through a posteromedial approach with a trans-condylar notch arthroscopic control. Superior (A) and posterior (B) views of an anatomical section of the upper end of the tibia. Illustration of how the arthroscope is situated through the notch, between the posterior cruciate ligament and the medial femoral condyle. The suture hook is inserted through a posteromedial approach. Abbreviations: MM: medial meniscus; LM: lateral meniscus; PCL: posterior cruciate ligament; ACL: anterior cruciate ligament
All-inside suture hook through a 2-portal posteromedial approach. Superior (A) and posterior (B) views of an anatomical section of the upper end of the tibia. Placement of the arthroscope through a first posteromedial portal and the suture hook through a second posteromedial approach. Abbreviations: MM: medial meniscus; LM: lateral meniscus; PCL: posterior cruciate ligament; ACL: anterior cruciate ligament
All-inside suture hook through a 2-portal posteromedial approach – arthroscopic views. This is a right knee at 90° knee flexion. (A) The entry of the posteromedial viewing portal is identified through a trans-notch articular view with the help of a needle and trans-illumination to protect from saphenous vessel injury. (B) Direct visualisation of the posteromedial corner and a ramp lesion. The camera is in the posteromedial viewing portal. (C) The lesion is debrided with a shaver through the second posteromedial portal located approximately 3 to 4 cm posteriorly and distally to the viewing portal. (D). Suture of a ramp lesion with a 90° curved hook and a PDS 1 wire. (E) After suture retrieval, a sliding knot is made using a knot pusher. (F) After section of the suture, the knot can be visualised. Abbreviations: PMC: posteromedial capsule; MM: medial meniscus; MFC: Medial femoral condyle; MCJ: meniscocapsular junction
Posteromedial view before and after repair of a ramp lesion and during flexion and extension. This is a right knee. The camera is in the posteromedial viewing portal. Before repair, the ramp lesion is observed at 90° (A) and 20° (B) knee flexion. A cleft between the posterior wall of the medial meniscus and the ramp tissue can be identified in both positions. After repair, posteromedial view at 90° (C) and 20°(D) knee flexion. The black star indicates adequate tensioning of the posterior capsule by the repair (B and D). Abbreviations: MFC: medial femoral condyle; MM: medial meniscus; MCJ: meniscocapsular junction; PMC: posteromedial capsule
Outcomes
As clinical research on the impact of ramp lesions is relatively new, there is a relative paucity of evidence regarding outcome studies on the clinical impact of ramp lesions and their treatment. A recent systematic review found that only 7 to 11 studies reported the outcome of ramp lesions [16, 18, 39, 44, 45]. Current studies mainly consist of case series or studies with a poor level of evidence, with a lack of homogeneity between them. Likewise, postoperative follow-up periods were highly variable, reaching from 6 to 47 months.
Few authors left the ramp lesions unrepaired or treated them with debridement or trephination only. Albayrak et al. [46] compared 33 ACL-injured patients with a stable ramp lesion left untreated and 33 ACL-injured patients without associated ramp lesions. At a minimum 3-year follow-up, there were no differences in Lachman, pivot-shift test, Lysholm score, IKDC score and complication rate. The only difference was a longer time to return to sport for the patients with a meniscal ramp lesion. Some authors advocated that the repair of stable lesions should be foreseen in athletes [44]. Yang et al. [47] compared the debridement (n = 31) to anterior all-inside suture using a hybrid repair device (n = 37) in ramp lesions which were inferior to 2 cm. They showed a postoperative improvement in both groups for the Lysholm and the IKDC scores but no significant difference between the two groups at 24 months. This is in agreement with Liu et al. [40] who compared in a randomised controlled trial abrasion and trephination (n = 33) to the all-inside suture hook repair (n = 40) in ramp lesions which were inferior to 1.5 cm. No significant differences could be found between groups in terms of subjective IKDC and Lysholm scores, clinical laxity, nor for the MRI healing status of ramp lesion. The later finding differed with the study by Hatayama et al. [48] which compared the healing status of ramp lesions left untreated or repaired using an all-inside technique through the posteromedial portal and found significantly higher healing rates in the repaired group compared with the non-repaired group. To date, comparative studies have thus failed to show that the repair of stable ramp lesions is superior compared to conservative approaches.
It currently remains unsure whether ramp repair should be recommended in both stable and unstable ramp lesions. Thaunat et al. [49] examined 132 patients at a minimum follow-up of 24 months and differentiated between limited tears (n = 81) and extended tears (n = 51). The limited tears required posterior meniscal suture repair alone, and for the extended tears, reparation was completed with suture anchors or outside-in sutures. No significant difference could be found in terms of revision surgery between the limited and extended groups.
Regardless of the size of the tear or the type of repair, there is a general agreement that the outcome scores (i.e. subjective IKDC score, Lyshom and Tegner scores) show significant improvements after ACL reconstruction and ramp repair [44]. Two studies [50, 51•] have, however, suggested a lower failure rate and secondary meniscectomy with a suture hook repair through the posteromedial portal in comparison with an all-inside technique. Additional anterolateral ligament (ALL) reconstruction had significantly better meniscal repair survivorship compared with all other subgroups [51•]. This is confirmed by a retrospective study [13] which reported an overall rate of secondary meniscectomies of 10.8% at a mean follow-up of 45.6 months. Patients with ACL reconstruction combined with ALL reconstruction had a two-fold lower risk of subsequent medial meniscectomy than patients with ACL reconstruction alone. ALL reconstruction may thus have a protective effect on ramp lesion repair or at least on the medial meniscus. However, in terms of knee laxity, it has been shown that repairing a meniscal lesion was more effective to decrease a high-grade pivot shift than performing a lateral extra-articular tenodesis [52]. The latter should thus not prevent from a thorough diagnosis and repair of all meniscal tears.
Conclusions
Ramp lesions of the medial meniscus may be present in more than 1 patient undergoing ACL reconstruction out of 5, representing up to half of all medial meniscal tears observed in this population. Biomechanical studies have shown that they increase knee laxity, but that their repair may restore laxity values. Their clinical impact is currently under investigation. Their diagnosis and management is difficult and requires advanced surgical skills. Given the novelty of their recognition as well as the remaining diversity in definitions, there is no consensus on whether and when they should be treated surgically. Many factors, such as their subtype, size, stability and repair technique, may indeed influence the decision-making process.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hamberg P, Gillquist J, Lysholm J. Suture of new and old peripheral meniscus tears. J Bone Joint Surg Am. 1983;65(2):193–7. https://doi.org/10.2106/00004623-198365020-00007.
Lemaire M, Combelles F, Miremad C, Van Vooren P. Postero-internal menisco-capsular disinsertions associated with chronic instabilities of the knee caused by rupture of the anterior cruciate ligament. Rev Chir Orthop Reparatrice Appar Mot. 1984;70(8):613–22.
Ahn JH, Kim SH, Yoo JC, Wang JH. All-inside suture technique using two posteromedial portals in a medial meniscus posterior horn tear. Arthroscopy. 2004;20(1):101–8. https://doi.org/10.1016/j.arthro.2003.11.008.
Ahn JH, Wang JH, Yoo JC. Arthroscopic all-inside suture repair of medial meniscus lesion in anterior cruciate ligament–deficient knees: results of second-look arthroscopies in 39 cases. Arthroscopy. 2004;20(9):936–45. https://doi.org/10.1016/j.arthro.2004.06.038.
Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med. 1982;10(2):90–5. https://doi.org/10.1177/036354658201000205.
DePhillipo NN, Engebretsen L, LaPrade RF. Current trends among US surgeons in the identification, treatment, and time of repair for medial meniscal ramp lesions at the time of ACL surgery. Orthop J Sports Med. 2019;7(2):2325967119827267. https://doi.org/10.1177/2325967119827267.
Bollen SR. Posteromedial meniscocapsular injury associated with rupture of the anterior cruciate ligament: a previously unrecognised association. J Bone Joint Surg Br. 2010;92(2):222–3. https://doi.org/10.1302/0301-620x.92b2.22974.
•• Kunze KN, Wright-Chisem J, Polce EM, DePhillipo NN, LaPrade RF, Chahla J. Risk factors for ramp lesions of the medial meniscus: a systematic review and meta-analysis. Am J Sports Med. 2021;49(13):3749–57. https://doi.org/10.1177/0363546520986817. (A systematic review on risk factors for ramp lesions in ACL-injured patients.)
•• Hatayama K, Terauchi M, Saito K, Aoki J, Nonaka S, Higuchi H. Magnetic resonance imaging diagnosis of medial meniscal ramp lesions in patients with anterior cruciate ligament injuries. Arthroscopy. 2018;34(5):1631–7. https://doi.org/10.1016/j.arthro.2017.12.022. (Includes 12 studies with 8410 patients and presents the overall pooled prevalence of ramp lesions is 21.9% (range, 9.0–41.7%) as well as the different risk factors analysed in the literature.)
Magosch A, Mouton C, Nührenbörger C, Seil R. Medial meniscus ramp and lateral meniscus posterior root lesions are present in more than a third of primary and revision ACL reconstructions. Knee Surg Sports Traumatol Arthrosc. 2021;29(9):3059–67. https://doi.org/10.1007/s00167-020-06352-3.
Seil R, Mouton C, Coquay J, Hoffmann A, Nührenbörger C, Pape D, et al. Ramp lesions associated with ACL injuries are more likely to be present in contact injuries and complete ACL tears. Knee Surg Sports Traumatol Arthrosc. 2018;26(4):1080–5. https://doi.org/10.1007/s00167-017-4598-3.
Balazs GC, Greditzer HGT, Wang D, Marom N, Potter HG, Marx RG, et al. Ramp lesions of the medial meniscus in patients undergoing primary and revision ACL reconstruction: prevalence and risk factors. Orthop J Sports Med. 2019;7(5):2325967119843509. https://doi.org/10.1177/2325967119843509.
Sonnery-Cottet B, Praz C, Rosenstiel N, Blakeney WG, Ouanezar H, Kandhari V, et al. Epidemiological evaluation of meniscal ramp lesions in 3214 anterior cruciate ligament-injured knees from the SANTI study group database: a risk factor analysis and study of secondary meniscectomy rates following 769 ramp repairs. Am J Sports Med. 2018;46(13):3189–97. https://doi.org/10.1177/0363546518800717.
Kim SH, Seo HJ, Seo DW, Kim KI, Lee SH. Analysis of risk factors for ramp lesions associated with anterior cruciate ligament injury. Am J Sports Med. 2020;48(7):1673–81. https://doi.org/10.1177/0363546520918207.
Cristiani R, van de Bunt F, Kvist J, Stålman A. High prevalence of meniscal ramp lesions in anterior cruciate ligament injuries. Knee Surg Sports Traumatol Arthrosc. 2023;31(1):316–24. https://doi.org/10.1007/s00167-022-07135-8.
Bumberger A, Koller U, Hofbauer M, Tiefenboeck TM, Hajdu S, Windhager R, et al. Ramp lesions are frequently missed in ACL-deficient knees and should be repaired in case of instability. Knee Surg Sports Traumatol Arthrosc. 2020;28(3):840–54. https://doi.org/10.1007/s00167-019-05521-3.
Koo B, Lee SH, Yun SJ, Song JG. Diagnostic performance of magnetic resonance imaging for detecting meniscal ramp lesions in patients with anterior cruciate ligament tears: a systematic review and meta-analysis. Am J Sports Med. 2020;48(8):2051–9. https://doi.org/10.1177/0363546519880528.
Moreira J, Almeida M, Lunet N, Gutierres M. Ramp lesions: a systematic review of MRI diagnostic accuracy and treatment efficacy. J Exp Orthop. 2020;7(1):71. https://doi.org/10.1186/s40634-020-00287-x.
Wang W, Li Z, Peng HM, Bian YY, Li Y, Qian WW, et al. Accuracy of MRI diagnosis of meniscal tears of the knee: a meta-analysis and systematic review. J Knee Surg. 2021;34(2):121–9. https://doi.org/10.1055/s-0039-1694056.
Jiang J, Liu Z, Wang X, Xia Y, Wu M. Increased posterior tibial slope and meniscal slope could be risk factors for meniscal injuries: a systematic review. Arthroscopy. 2022;38(7):2331–41. https://doi.org/10.1016/j.arthro.2022.01.013.
Beel W, Mouton C, Tradati D, Nührenbörger C, Seil R. Ramp lesions are six times more likely to be observed in the presence of a posterior medial tibial bone bruise in ACL-injured patients. Knee Surg Sports Traumatol Arthrosc. 2022;30(1):184–91. https://doi.org/10.1007/s00167-021-06520-z.
Willinger L, Balendra G, Pai V, Lee J, Mitchell A, Jones M, et al. Medial meniscal ramp lesions in ACL-injured elite athletes are strongly associated with medial collateral ligament injuries and medial tibial bone bruising on MRI. Knee Surg Sports Traumatol Arthrosc. 2022;30(5):1502–10. https://doi.org/10.1007/s00167-021-06671-z.
Owusu-Akyaw KA, Kim SY, Spritzer CE, Collins AT, Englander ZA, Utturkar GM, et al. Determination of the Position of the knee at the time of an anterior cruciate ligament rupture for male versus female patients by an analysis of bone bruises. Am J Sports Med. 2018;46(7):1559–65. https://doi.org/10.1177/0363546518764681.
Sonnery-Cottet B, Conteduca J, Thaunat M, Gunepin FX, Seil R. Hidden lesions of the posterior horn of the medial meniscus: a systematic arthroscopic exploration of the concealed portion of the knee. Am J Sports Med. 2014;42(4):921–6. https://doi.org/10.1177/0363546514522394.
Bedi A, Dines J, Dines DM, Kelly BT, O’Brien SJ, Altchek DW, et al. Use of the 70° arthroscope for improved visualization with common arthroscopic procedures. Arthroscopy. 2010;26(12):1684–96. https://doi.org/10.1016/j.arthro.2010.04.070.
Peltier A, Lording TD, Lustig S, Servien E, Maubisson L, Neyret P. Posteromedial meniscal tears may be missed during anterior cruciate ligament reconstruction. Arthroscopy. 2015;31(4):691–8. https://doi.org/10.1016/j.arthro.2014.12.003.
Malatray M, Raux S, Peltier A, Pfirrmann C, Seil R, Chotel F. Ramp lesions in ACL deficient knees in children and adolescent population: a high prevalence confirmed in intercondylar and posteromedial exploration. Knee Surg Sports Traumatol Arthrosc. 2018;26(4):1074–9. https://doi.org/10.1007/s00167-017-4471-4.
DePhillipo NN, Cinque ME, Chahla J, Geeslin AG, Engebretsen L, LaPrade RF. Incidence and detection of meniscal ramp lesions on magnetic resonance imaging in patients with anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45(10):2233–7. https://doi.org/10.1177/0363546517704426.
Thaunat M, Fayard JM, Guimaraes TM, Jan N, Murphy CG, Sonnery-Cottet B. Classification and surgical repair of ramp lesions of the medial meniscus. Arthrosc Tech. 2016;5(4):e871–5. https://doi.org/10.1016/j.eats.2016.04.009.
•• Thaunat M, Ingale P, Penet A, Kacem S, Haidar I, Bauwens PH, et al. Ramp lesion subtypes: prevalence, imaging, and arthroscopic findings in 2156 anterior cruciate ligament reconstructions. Am J Sports Med. 2021;49(7):1813–21. https://doi.org/10.1177/03635465211006103. (Shows the prevalence of the different subtypes of ramp lesions according to the arthroscopic classification.)
Seil R, Hoffmann A, Scheffler S, Theisen D, Mouton C, Pape D. Ramp lesions: tips and tricks in diagnostics and therapy. Orthopade. 2017;46(10):846–54. https://doi.org/10.1007/s00132-017-3461-z.
•• Greif DN, Baraga MG, Rizzo MG, Mohile NV, Silva FD, Fox T, et al. MRI appearance of the different meniscal ramp lesion types, with clinical and arthroscopic correlation. Skeletal Radiol. 2020;49(5):677–89. https://doi.org/10.1007/s00256-020-03381-4. (First MRI classification of ramp lesions)
DePhillipo NN, Moatshe G, Chahla J, Aman ZS, Storaci HW, Morris ER, et al. Quantitative and qualitative assessment of the posterior medial meniscus anatomy: defining meniscal ramp lesions. Am J Sports Med. 2019;47(2):372–8. https://doi.org/10.1177/0363546518814258.
Chagas-Neto FAD, Alencar LS, Aquino HLA, Taneja AK, Magalhães JFG, Sousa Filho PGT, et al. Is there a good agreement between MRI readers for Thaunat’s classification in arthroscopically-proven meniscal ramp lesions? Knee. 2021;28:371–82. https://doi.org/10.1016/j.knee.2020.12.029.
DePhillipo NN, Moatshe G, Brady A, Chahla J, Aman ZS, Dornan GJ, et al. Effect of meniscocapsular and meniscotibial lesions in ACL-deficient and ACL-reconstructed knees: a biomechanical study. Am J Sports Med. 2018;46(10):2422–31. https://doi.org/10.1177/0363546518774315.
Stephen JM, Halewood C, Kittl C, Bollen SR, Williams A, Amis AA. Posteromedial meniscocapsular lesions increase tibiofemoral joint laxity with anterior cruciate ligament deficiency, and their repair reduces laxity. Am J Sports Med. 2016;44(2):400–8. https://doi.org/10.1177/0363546515617454.
Ahn JH, Bae TS, Kang KS, Kang SY, Lee SH. Longitudinal tear of the medial meniscus posterior horn in the anterior cruciate ligament-deficient knee significantly influences anterior stability. Am J Sports Med. 2011;39(10):2187–93. https://doi.org/10.1177/0363546511416597.
• Mouton C, Magosch A, Pape D, Hoffmann A, Nührenbörger C, Seil R. Ramp lesions of the medial meniscus are associated with a higher grade of dynamic rotatory laxity in ACL-injured patients in comparison to patients with an isolated injury. Knee Surg Sports Traumatol Arthrosc. 2020;28(4):1023–8. https://doi.org/10.1007/s00167-019-05579-z. (Shows in vivo the impact of the ramp lesion on dynamic rotational laxity as expressed by the pivot shift test.)
Acosta J, Ravaei S, Brown SM, Mulcahey MK. Examining techniques for treatment of medial meniscal ramp lesions during anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2020;36(11):2921–33. https://doi.org/10.1016/j.arthro.2020.05.041.
Liu X, Zhang H, Feng H, Hong L, Wang XS, Song GY. Is it necessary to repair stable ramp lesions of the medial meniscus during anterior cruciate ligament reconstruction? A prospective randomized controlled trial. Am J Sports Med. 2017;45(5):1004–11. https://doi.org/10.1177/0363546516682493.
Li WP, Chen Z, Song B, Yang R, Tan W. The fast-fix repair technique for ramp lesion of the medial meniscus. Knee Surg Relat Res. 2015;27(1):56–60. https://doi.org/10.5792/ksrr.2015.27.1.56.
Chen Z, Li WP, Yang R, Song B, Jiang C, Hou JY, et al. Meniscal ramp lesion repair using the fast-fix technique: evaluating healing and patient outcomes with second-look arthroscopy. J Knee Surg. 2018;31(8):710–5. https://doi.org/10.1055/s-0037-1606378.
•• Siboni R, Pioger C, Jacquet C, Mouton C, Seil J, Toanen C, et al. Meniscal ramp repair: a 2-portal posteromedial approach. Arthrosc Tech. 2022;11(7):e1163–9. https://doi.org/10.1016/j.eats.2022.02.026. (Describes a new surgical approach with a 2 portal posteromedial approach.)
D’Ambrosi R, Meena A, Raj A, Giorgino R, Ursino N, Mangiavini L, et al. Good results after treatment of RAMP lesions in association with ACL reconstruction: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2023;31(1):358–71. https://doi.org/10.1007/s00167-022-07067-3.
Alessio-Mazzola M, Lovisolo S, Capello AG, Zanirato A, Chiarlone F, Formica M, et al. Management of ramp lesions of the knee: a systematic review of the literature. Musculoskelet Surg. 2020;104(2):125–33. https://doi.org/10.1007/s12306-019-00624-z.
Albayrak K, Buyukkuscu MO, Kurk MB, Kaya O, Kulduk A, Misir A. Leaving the stable ramp lesion unrepaired does not negatively affect clinical and functional outcomes as well as return to sports rates after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2021;29(11):3773–81. https://doi.org/10.1007/s00167-020-06402-w.
Yang J, Guan K, Wang JZ. Clinical study on the arthroscopic refreshing treatment of anterior cruciate ligament injury combined with stable medial meniscus ramp injury. J Musculoskelet Neuronal Interact. 2017;17(2):108–13.
Hatayama K, Terauchi M, Saito K, Takase R, Higuchi H. Healing status of meniscal ramp lesion affects anterior knee stability after ACL reconstruction. Orthop J Sports Med. 2020;8(5):2325967120917674. https://doi.org/10.1177/2325967120917674.
Thaunat M, Jan N, Fayard JM, Kajetanek C, Murphy CG, Pupim B, et al. Repair of meniscal ramp lesions through a posteromedial portal during anterior cruciate ligament reconstruction: outcome study with a minimum 2-year follow-up. Arthroscopy. 2016;32(11):2269–77. https://doi.org/10.1016/j.arthro.2016.02.026.
Gousopoulos L, Hopper GP, Saithna A, Grob C, Levy Y, Haidar I, et al. Suture hook versus all-inside repair for longitudinal tears of the posterior horn of the medial meniscus concomitant to anterior cruciate ligament reconstruction: a matched-pair analysis from the SANTI study group. Am J Sports Med. 2022;50(9):2357–66. https://doi.org/10.1177/03635465221100973.
• Thaunat M, Foissey C, Ingale P, Haidar I, Bauwens PH, Penet A, et al. Survival and risk factor analysis of arthroscopic ramp lesion repair during anterior cruciate ligament reconstruction. Am J Sports Med. 2022;50(3):637–44. https://doi.org/10.1177/03635465211068524. (Shows that secondary arthroscopy occurred more frequently with all inside suture implant vs suture hook through a posteromedial portal.)
Jacquet C, Pioger C, Seil R, Khakha R, Parratte S, Steltzlen C, et al. Incidence and risk factors for residual high-grade pivot shift after ACL Reconstruction with or without a lateral extra-articular tenodesis. Orthop J Sports Med. 2021;9(5):23259671211003590. https://doi.org/10.1177/23259671211003590.
Author information
Authors and Affiliations
Contributions
RSi, CP, CM and CJ equally participated in the drafting of the manuscript. All authors had a critical revision of the manuscript. Each author has given final approval of the version to be published and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Siboni, R., Pioger, C., Jacquet, C. et al. Ramp Lesions of the Medial Meniscus. Curr Rev Musculoskelet Med 16, 173–181 (2023). https://doi.org/10.1007/s12178-023-09834-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12178-023-09834-2