Abstract
Purpose of Review
To characterize current concepts in capsular repair and hip instability, and examine findings from biomechanical and clinical studies on hip capsular management strategies as they pertain to hip stability, patient outcomes, and hip arthroscopy failure. Further, we discuss the clinical evaluation and treatment of capsular deficiency.
Recent Findings
There remains debate regarding the optimal capsular management strategies in hip arthroscopy, particularly concerning the necessity of routine capsular repair. A variety of capsulotomy techniques exist and may be used to access the hip joint. Additionally, a wide variety of techniques are employed to repair the hip capsule. Biomechanical evidence supports capsular closure restores hip joint stability to that of the intact, native state. Several clinical studies in both primary and revision hip arthroscopy settings have demonstrated improved pain and functional outcomes in patients who underwent capsular repair or capsular reconstruction. Studies have shown capsular repair may be especially important in patients with ligamentous laxity and hip dysplasia, and in competitive athletes.
Summary
Post-surgical hip instability secondary to capsular insufficiency is increasingly recognized as a cause of hip arthroscopy failure. Capsular closure restores native biomechanical stability to the hip joint, and several clinical studies report improved pain and functional outcomes following capsular repair or capsular reconstruction in both the primary and revision hip arthroscopy settings. There remains much to learn regarding capsular hip instability as it relates to optimal capsular management surgical technique, intra-operative capsular management decision-making, clinical diagnosis, and related advanced imaging findings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A substantial increase in the number of hip arthroscopy cases performed has been observed in recent years as the understanding of the pathology of femoroacetabular impingement syndrome (FAIS) has increased and surgical techniques to treat FAIS have improved [1]. As hip arthroscopy cases increase, so too has the prevalence of post-surgical hip instability following hip arthroscopy [2,3,4]. Post-surgical hip instability, presenting as hip microinstability, hip subluxation, or gross instability of the hip, occurs as a consequence of surgical alteration to the anatomy responsible for imparting biomechanical stability to the hip joint, including the hip capsule, labrum, articular congruity, suction seal, and musculature crossing the hip joint. Gross hip instability in the form of frank dislocation is rare; however, subtle instability, or microinstability, is becoming increasingly recognized as a source of pain and dysfunction in the hip preservation population [2, 3]. Microinstability of the hip occurs as extra-physiologic motion between the femoral head and acetabulum, resulting in hip pain with or without subjective complaints of hip joint instability. The abnormal, extra-physiologic micromotion of the hip joint leads to hip joint toggling and abnormal joint reaction forces which may lead to accelerated cartilage wear and degenerative changes. Further, the extra-physiologic hip motion stresses the labrum and hip capsule, which may result in labral degeneration and capsular attenuation [3].
Arthroscopic instrumentation of the central and peripheral hip compartments to address intra-articular pathology necessitates partial disruption of the capsuloligamentous anatomy surrounding the hip joint in the form of a capsulotomy. Several biomechanical studies have highlighted the effects capsulotomies and capsular defects have on hip joint stability [5,6,7,8], while additional biomechanical evidence shows that in the absence of frank capsular defects, hip capsular attenuation also significantly reduces the static stabilization provided by the iliofemoral ligament [9]. Moreover, multiple clinical studies have identified and reported on the presence of persistent capsular defects in the anterosuperior region of the hip capsule in symptomatic patients following arthroscopic treatment of FAIS [10,11,12]. Recently, O’Neill et al. described a grading system of capsular changes observed on magnetic resonance arthrography (MRA) in a cohort of patients with symptomatic post-surgical hip instability, which included normal capsular appearance and volume, increased capsular redundancy, focal capsular rents, and high-grade capsular irregularity, and gross capsular defect [11].
In an attempt to limit post-surgical capsular deficiency and maintain capsular integrity, routine capsular repair following arthroscopic hip surgery for FAIS has become increasingly common [13]. However, despite our increasing understanding of post-surgical hip instability, with one study reporting 35% of their hip arthroscopy revision patients manifesting hip instability [14], there remains debate on the importance of repairing the hip capsule and the clinical significance of a deficient hip capsule. The purpose of this review is to characterize current concepts in capsular repair and hip instability, specifically presenting findings from biomechanical experiments and clinical studies focused on hip capsular management strategies, patient outcomes, and the clinical workup and treatment of capsular deficiency.
Hip Capsule Anatomy and Function
Acetabular and femoral head bony congruity provides significant hip joint stability, which is further stabilized by a surrounding muscular envelope, fibrocartilaginous acetabular labrum, and joint capsule. The hip capsule is a fibrous capsuloligamentous complex composed of multiple distinct ligamentous thickenings, including the iliofemoral ligament, pubofemoral ligament, ischiofemoral ligament, and zona orbicularis. Of these ligaments, the iliofemoral ligament, also known as the Y ligament of Bigelow, is the strongest and most important soft tissue contributor to hip joint stability, providing restraint to anterior translation of the femoral head, hip extension, and external rotation [5]. Originating on the anterior inferior iliac spine and acetabular rim between 12:45 and 3:00 o’clock [15], the iliofemoral ligament provides static stabilization to an area of the hip joint devoid of bony coverage. Typically, the acetabulum is anteverted and laterally tilted approximately 15–20° and 45°, respectively, while the proximal femur is anteverted approximately 10–15°. This anatomic relationship between the femoral head and acetabulum situates the majority of femoral head coverage posteromedially and places an increased requirement on soft tissue stabilizers, including the iliofemoral ligament, in the anterolateral region of the hip joint.
The iliofemoral ligament in the anterosuperior region of the hip capsule is particularly relevant to hip arthroscopy, as the most commonly used arthroscopic portals pierce the hip capsule in this region. The anterolateral portal pierces the hip capsule at approximately 1:00 o’clock, while the anterior portal pierces the hip capsule at approximately 3:00 o’clock [15]. Connecting these portals in line with the acetabular rim to create an interportal capsulotomy large enough to safely instrument the central and peripheral compartments of the hip joint results in nearly complete transection of the iliofemoral ligament, resulting in significant capsular disruption. Further, in order to provide increased visualization and instrument maneuverability, many surgeons prefer to extend the interportal capsulotomy distally, parallel to the axis of the femoral neck, reaching towards the zona orbicularis to create a T-type capsulotomy. Additionally, a periportal capsulotomy is another technique performed in a similar manner to the interportal capsulotomy without connecting the anterolateral and modified anterior portals [16, 17]. While variability in capsulotomy technique exists, the size and position of capsulotomies are strategically made to adequately visualize and surgically treat hip pathology. Pearls and pitfalls of these capsulotomy techniques are described in Table 1.
Ultimately, any capsulotomy disrupts the native capsular state and may result in diminished capsuloligamentous stabilization provided to the hip. In addition to demonstrating that capsulotomies increase the mobility of the hip joint relative to its native state, several biomechanical studies have also shown capsular repair restores normal hip joint biomechanical stability [6, 7, 18,19,20,21]. Therefore, the ideal arthroscopic capsular management strategy balances creating a capsulotomy large enough to adequately visualize and efficiently instrument the hip while retaining and restoring as much capsular integrity as possible.
Hip Capsule Management in Primary Hip Arthroscopy
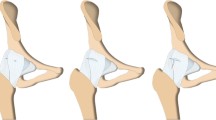
Considerable variation in surgical technique and decision-making exists regarding capsular management in the primary hip arthroscopy setting for FAIS. After comprehensively addressing pathology within the central and peripheral hip compartments, the hip capsule is either left unrepaired, partially repaired, or completely repaired. If the decision is made to close the capsule, the suture configuration employed is quite variable amongst surgeons, ranging from placement of a single simple stitch to utilizing several overlapping figure-of-eight sutures to provide a complete capsular closure [22]. Further, capsular repair tensioning ranges substantially from low-tension capsular apposition to capsular plication and inferior capsular shift [23, 24]. It is important to recognize the variability in surgical technique when comparing studies evaluating the biomechanical effects and clinical outcomes related to capsular repair [25]. An example of the senior author’s capsular closure technique is provided in Fig. 1.
Arthroscopic images of a right hip interportal capsulotomy which was repaired using a figure-of-eight suture technique. A Interportal capsulotomy prior to repair. B Three (#2) non-absorbable sutures placed in a figure-of-eight pattern, alternating between blue-striped and all-white suture to aid in identification. C Sutures tied and cut providing a complete capsular closure
There remains no clear consensus in the literature as to whether or not the hip capsule should be routinely repaired following arthroscopic hip procedures [26]; however, routine capsular closure has become increasingly popular in recent years [13, 27]. Studies have shown capsular repair is especially valuable in patients with generalized ligamentous laxity [28], hip dysplasia [29,30,31,32], and competitive athletes [33].
Several recent studies have compared outcomes between capsular management techniques. Economopoulos et al. prospectively randomized patients to three capsular management techniques: T-type capsulotomy with no repair, interportal with no repair, and interportal capsulotomy closed with three interrupted sutures. At 2-year follow-up, they found the capsular closure group had significantly superior outcomes than the unrepaired T-capsulotomy and interportal capsulotomy groups. Further, no patients in the repaired and unrepaired interportal capsulotomy groups underwent total hip arthroplasty (THA), while four patients in the unrepaired T-capsulotomy group underwent subsequent THA [34].
Recently, several comparative matched cohort studies have been published comparing patient-reported outcomes, complications, and rates of subsequent revision surgery and conversion to THA between capsular repair and unrepaired groups. Frank et al. studied patients who underwent hip arthroscopy for FAIS and found patients who underwent complete closure of T-type capsulotomy resulted in significantly superior HOS-SS outcome scores relative to patients who underwent partial closure (vertical limb only) of the T-type capsulotomy [35]. Bolia et al. evaluated patients with interportal capsulotomies and found patients who underwent capsular repair of an interportal capsulotomy had significantly higher HOS-ADL and mHHS scores than patients without capsular repair at mid-term follow-up. Further, they found the unrepaired group was 6.8 times more likely to undergo subsequent THA [36]. In another comparative study, Domb et al. demonstrated significantly improved outcome scores of both groups at 2- and 5-year follow-ups [37]. However, the unrepaired group had a higher rate of conversion to THA and their mHHS scores significantly declined between the 2- and 5-year follow-up time points. As part of their conclusions, they suggested routine capsular repair may improve the durability of capsular integrity following hip arthroscopy. Larson et al. performed a matched cohort study comparing hip arthroscopy outcomes between dysplastic patients and a control group without radiographic evidence of dysplasia and found labral repair and capsular plication were associated with improved clinical outcomes in the dysplastic group [30].
While several studies have shown improved subjective outcomes in patients who underwent capsular repair at the time of index surgery relative to those whose capsules were left unrepaired, other studies have shown no significant differences in outcome scores between repaired and unrepaired groups. Filan and Carton studied a large cohort of patients who underwent arthroscopic osseous resection and labral repair for FAIS and showed patients who underwent capsular repair did not report superior clinical outcomes compared to a group of patients with an unrepaired capsulotomy [38]. They found significantly superior scores in the unrepaired group in Short Form-36 and WOMAC scores, but not in UCLA activity scale and mHHS scores. However, they also found patients between the ages of 25 and 34 in the unrepaired group underwent revision hip arthroscopy at a significantly higher rate. Notably, an interportal capsulotomy was performed in all patients, and in patients who underwent capsular repair, a simple suture technique was utilized, ranging from 1 to 4 sutures. Therefore, there exists variability within the repair group as to the completeness and biomechanical strength of capsular closure provided in each case. In another study, Bech et al. prospectively randomized patients to capsular repair or no repair, and at final follow-up of 52 months, they found no significant differences in Copenhagen Hip and Groin Outcome Scores between groups [39]. They also performed an interportal capsulotomy in all patients, and in the repair group closed with 2–3 sutures. Similarly, Atzmon et al. demonstrated no significant differences in mHHS and HOS-ADL scores at minimum 2-year follow-up between groups [40].
Overall, there are several studies which support routine capsular repair following hip arthroscopy, and a few studies that challenge the necessity of routine capsular closure. The advantages of capsular repair include the potential for improved functional outcomes, diminished pain, and lower revision hip arthroscopy and conversion to THA rates. There are also potential disadvantages attributed to capsular closure. The technically challenging nature of the procedure may result in additional surgical time and anesthetic exposure and could result in iatrogenic labral or chondral damage. Additionally, over-tensioning the repair may result in hip joint stiffness. In the young hip preservation population, there remains much to learn regarding the various capsulotomy and capsular closure techniques and the effects they have on restoring stability, improving patient functionality, and alleviating hip pain.
Post-surgical Capsular Insufficiency—a Cause of Hip Arthroscopy Failure
Inadequate osteochondral resection resulting in residual femoroacetabular impingement is classically described as the most common cause of failure in hip arthroscopy for FAIS [14, 41, 42]. However, there are several other etiologies which may be solely responsible for, or contribute to, recalcitrant hip pain and functional disability following FAIS hip arthroscopy [43]. Appropriately diagnosing the correct pathology responsible in each specific case can be challenging, particularly in the absence of radiographic evidence suggestive of residual hip impingement. Common etiologies of persistent symptoms causing hip arthroscopy failure include post-surgical hip instability, labral pathology, capsular adhesions, chondral lesions, loose osteochondral bodies, and heterotopic ossification [14, 44, 45]. Capsular insufficiency as a cause of post-surgical hip instability is increasingly recognized as a cause of hip arthroscopy failure [45,46,47,48,49,50,51] (Fig. 2).
MRA imaging with contrast injected into the peripheral hip compartment clearly delineates the synovial surface of the hip capsule to provide valuable diagnostic information regarding the integrity and morphology of the surgically altered hip capsule [52] (Fig. 2). The region of primary interest when evaluating for hip capsular pathology in the revision setting is the anterosuperior hip capsule, approximately 12 to 3 o’clock, in the region of the previous capsulotomy (Fig. 3). Several studies have aimed to evaluate the clinical utility of magnetic resonance imaging assessment of capsular integrity.
In a small cohort of nine patients who underwent revision hip arthroscopy without radiographic evidence of residual osseous impingement on radiographs, McCormick et al. were the first to report on the incidence of post-surgical capsular defects observed on MRA and visualized arthroscopically in the revision hip arthroscopy setting. They found capsular irregularities were present in all patients, and seven (78%) patients had identifiable iliofemoral ligament defects on MRA [12]. In a cohort of 20 patients with evidence of a capsular defect on MRA and/or laxity of the hip observed on physical examination, Wylie et al. demonstrated significant clinical improvement following revision hip arthroscopy for capsular repair [53]. Furthermore, O’Neill et al. presented the subjective history, physical examination findings, and MRA findings in a cohort of patients diagnosed with post-surgical hip instability who made substantial improvements at mid-term follow-up in pain and function outcomes following isolated capsular repair at the time of revision surgery [11]. In their study, they described the use of oblique axial MRAs oriented in the plane parallel to the long axis of the femoral neck to assess for anterosuperior capsular insufficiency, and proposed the following classification system for capsular defects: 0—normal, 1—capsular redundancy, 2—focal capsular rent, and 3—gross extravasation of fluid from the capsule. Of note, all pre-revision MRAs displayed evidence of post-surgical capsular changes, with 31% showing capsular redundancy, 52% showing a focal capsular rent, and 17% showing gross extravasation of fluid from the capsule.
Indeed, the MRA studies outlined above are limited by the absence of a control group evaluating for capsular defects in an asymptomatic cohort; however, studying this is challenging given the risks attributed to the invasive nature of performing an MRA in an asymptomatic patient. In a relatively small cohort of both asymptomatic and symptomatic patients following hip arthroscopy, Kim et al. showed the presence of capsular defects on MRA was not statistically different between groups, and concluded capsular defects are not more common in symptomatic relative to asymptomatic patients. However, their study was likely underpowered to detect significant differences between groups in the various post-operative changes they observed for [54].
The influence of capsular closure on the long-term biomechanical integrity of the hip capsule remains uncertain. Weber et al. found “…in a subset of symptomatic patients after hip arthroscopy for FAI, the majority (92.5%) of the repaired hip capsules remained closed at greater than 1 year of follow-up. The hip capsule adjacent to the capsulotomy and subsequent repair is thickened compared with the same location on the contralateral, nonoperative hip.” Additionally, Strickland et al. performed a randomized controlled trial in which patients with FAIS who underwent bilateral hip arthroscopy with an interportal capsulotomy were assigned capsular repair of one hip while the contralateral hip was left unrepaired [55]. MRI (without arthrogram) evaluation for capsular defects found all hip capsules healed at 24 weeks post-operatively with no significant differences between groups in capsular thickness in the area of capsulotomy. Notably, patients with hip dysplasia and hyperlaxity were excluded from the study protocol as the surgeon always performed capsular repair in these patients. Certainly, the results of this study question the need to routinely repair the hip capsule in all cases. However, it is important to note the limitations of this study which only assessed for capsular thickness and capsular continuity and did not assess for capsular laxity or biomechanical strength of the repaired and unrepaired capsules.
Overall, several studies have identified capsular defects in patients with a history of hip arthroscopy, and multiple studies have demonstrated significantly improved patient outcomes with capsular closure aimed to restore hip stability at revision hip arthroscopy.
Clinical Recognition of Capsular Insufficiency
The clinical recognition and diagnosis of capsular insufficiency are continually evolving as our understanding of this relatively new pathology expands. Several key elements of a patient’s history, physical examination, and imaging should be used in conjunction to accurately diagnose capsular insufficiency. Subjectively, patients typically complain of persistent hip pain following hip arthroscopy, which tends to gradually worsen as hip instability progresses. Patients may also complain of a feeling of the hip giving way, particularly in positions of hip extension and external rotation. Occasionally patients may describe a subjective feeling of increased hip instability relative to their contralateral, native hip. Historical details of previous arthroscopic hip surgery may also provide valuable information, including specifics regarding acetabuloplasty, femoral osteochondroplasty, labral management, additional procedures performed, capsulotomy type, capsulotomy size, capsular closure strategy, and the number of previous surgeries.
Patient characteristics, including hip dysplasia and generalized ligamentous laxity, place patients at a higher risk of developing instability following hip arthroscopy. Additionally, multiple studies have shown a greater percentage of females are diagnosed with post-surgical hip instability, which may be a result of increased ligamentous laxity and thinner hip capsules in females [10, 12, 56]. Failure to adhere to a prescribed physical therapy regimen resulting in excessive early hip motion could compromise labral or capsular repair, leading to surgical failure [43].
There are several physical examination maneuvers aimed at evaluating hip stability (Table 2). A clinically based axial distraction test and an intra-operative axial stress exam under anesthesia (Fig. 4) are the preferred physical examination maneuvers of the senior author to assess for post-surgical capsular instability. The clinically based axial distraction test is performed with the patient positioned supine on the examination table with the affected hip flexed to 45°, the knee flexed to 90°, and the contralateral leg relaxed in a neutral position. The examiner places their knee against the patient’s ischium to stabilize the pelvis while applying an axial force through the hip with the examiner’s hand placed on the proximal leg. Pain, apprehension, objective hip toggling, and asymmetry compared to the contralateral hip are the four major components assessed while axial distraction is applied. Pain is subjectively reported by the patient; apprehension is assessed by observing for anxiety or resistance to distraction; toggling is felt by the examiner as hip joint subluxation; and asymmetry is assessed by repeating the examination on the contralateral hip joint and comparing the axial distractibility between both hips. In the cohort of 31 patients studied by O’Neill et al. who were all easily distractable with gentle traction under anesthesia, 24 (77%) had at least 1 positive finding of pain, apprehension, or toggle on axial distraction testing [11].
Fluoroscopic images of an intra-operative axial stress exam under anesthesia in a patient undergoing right-sided revision hip arthroscopy. A Non-operative, asymptomatic left hip showing no joint space widening at 100 lbs of axial traction. B Symptomatic right hip showing significant joint space widening at 100 lbs of axial traction
Additional examination maneuvers evaluating for hip stability and capsular integrity include the abduction-extension-external rotation test, dial test, anterior apprehension (hyperextension, external rotation) test, and the prone external rotation (instability) test. Hoppe et al. studied the diagnostic value of the abduction-hyperextension-external rotation test, prone instability test, and the hyperextension-external rotation test and found when each of these exams produced positive findings; the combined positive predictive value was 95% [57]. Beighton criteria should be obtained to gain an understanding of generalized ligamentous laxity. Further, in patients undergoing revision surgery, capsular laxity can be assessed with gentle manual traction in anesthetized patients evaluating for ease of distractibility. The distractibility of the contralateral lower extremity can also be assessed to provide comparison to the native hip joint and capsule in patients with no history of contralateral hip surgery.
In addition to the physical exam, MRA evidence of capsular redundancy, focal capsular rent, or gross capsular defects with fluid extravasation should be used, supporting the clinical diagnosis of post-surgical capsular insufficiency. Overall, clinical recognition of capsular insufficiency can be challenging, and more research is needed in this domain.
Treatment
Capsular repair and capsular reconstruction are the two primary definitive arthroscopic treatment options for capsular insufficiency. It is also important to note that an open periacetabular osteotomy should be considered as a potential treatment for post-surgical hip instability [58]. As with most orthopaedic conditions, non-operative measures are first attempted prior to surgical intervention following recognition of post-surgical hip instability. In cases of recalcitrant hip pain and dysfunction, there are several described capsular repair and capsular reconstruction techniques [22, 59, 60]. A direct capsular repair can be considered in cases with sufficient capsular tissue in both the proximal and distal capsular limbs. If the tissue of the proximal capsule is insufficient or of inadequate quality to perform a direct repair, then techniques involving acetabular suture anchors may be utilized. Furthermore, in cases with large capsular defects, poor capsular tissue quality, or substantial capsular laxity, patient’s may benefit from an augmented capsular closure or capsular reconstruction using one of the many previously published techniques [60,61,62,63,64].
Multiple studies have shown capsular management aimed at restoring hip stability at the time of revision arthroscopic hip surgery improves outcomes. Larson et al. demonstrated capsular plication was predictive of significant improvements in mHHS outcome scores, and they discussed the importance of restoring capsular integrity in the revision setting [65]. Newman et al. found capsular plication at the time of revision hip arthroscopy to be significantly associated with clinical improvement [23]. The case series by Wylie et al. demonstrated significant improvements in mHHS, HOS-ADL, and HOS-SS outcome scores [53]. In O’Neill et al.’s cohort of patients who underwent isolated capsular repair for capsular hip instability, mean improvement in HOS-SS exceeded both the minimally clinically important difference and substantial clinical benefit values previously defined in the literature, while also observing excellent post-operative mHHS and HOS-ADL scores [11]. Finally, Fagotti et al. is one of few currently published studies to demonstrate the effect of capsular reconstruction on patient outcomes [66]. They compared outcomes between groups who underwent capsular reconstruction with two different allografts, iliotibial band versus dermal allograft. They found the failure rate (22%) between groups was the same, while the outcome scores favored the iliotibial band group. However, interpretation of the differences in post-operative outcome scores is limited by a relatively small cohort size, concomitant pathology and procedures performed, and greater mean pre-operative outcome scores in the iliotibial band group. Overall, there remains much to learn regarding patient outcomes following arthroscopic hip capsular reconstruction surgery.
Conclusions
Post-surgical hip instability secondary to capsular insufficiency is increasingly recognized as a cause of hip arthroscopy failure. Capsular closure restores native biomechanical hip joint stability, and several clinical studies report improved pain and functional outcomes following capsular repair or capsular reconstruction in both the primary and revision hip arthroscopy settings. There remains much to learn regarding post-surgical hip instability as it relates to optimal capsular management techniques, intra-operative capsular management decision-making, clinical diagnosis, and related advanced imaging findings.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bonazza NA, Homcha B, Liu G, Leslie DL, Dhawan A. Surgical trends in arthroscopic hip surgery using a large national database. Arthrosc - J Arthrosc Relat Surg. 2018;34(6):1825–30.
Canham CD, Domb BG, Giordano BD. Atraumatic hip instability. JBJS Rev. 2016;4(5). https://doi.org/10.2106/JBJS.RVW.15.00045.
Safran MR. Microinstability of the hip—gaining acceptance. J Am Acad Orthop Surg. 2019;27(1):12–22.
Yeung M, Simunovic N, Belzile E, Philippon MJ, Ayeni OR. Systematic review gross instability after hip arthroscopy: An analysis of case reports evaluating surgical and patient factors. Arthrosc: J Arthrosc Relat Surg. 2016;32(6):1196–204. https://doi.org/10.1016/j.arthro.2016.01.011.
Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: An in vitro biplane fluoroscopy study. Ame J Sports Med. 2011;39(1_suppl):85S–91S. https://doi.org/10.1177/0363546511412161.
Baha P, Burkhart TA, Getgood A, Degen RM. Complete capsular repair restores native kinematics after interportal and T-capsulotomy. Am J Sports Med. 2019;47(6):1451–8. https://doi.org/10.1177/0363546519832868.
Abrams GD, Hart MA, Takami K, Bayne CO, Kelly BT, Espinoza Orías AA, Nho SJ. Biomechanical evaluation of capsulotomy, capsulectomy, and capsular repair on hip rotation. Arthrosc – J Arthrosc Relat Surg. 2015;31(8):1511–7. https://doi.org/10.1016/j.arthro.2015.02.031.
Han S, Alexander JW, Thomas VS, Choi J, Harris JD, Doherty DB, Jeffers JRT, Noble PC. Does capsular laxity lead to microinstability of the native hip? Am J Sports Med. 2018;46(6):1315–23. https://doi.org/10.1177/0363546518755717.
Johannsen AM, Behn AW, Shibata K, Ejnisman L, Thio T, Safran MR. The role of anterior capsular laxity in hip microinstability: a novel biomechanical model. Am J Sports Med. 2019;47(5):1151–8. https://doi.org/10.1177/0363546519827955.
Weber AE, Kuhns BD, Cvetanovich GL, Lewis PB, Mather RC, Salata MJ, Nho SJ. Does the hip capsule remain closed after hip arthroscopy with routine capsular closure for femoroacetabular impingement? A magnetic resonance imaging analysis in symptomatic postoperative patients. Arthrosc - J Arthrosc Relat Surg. 2017;33(1):108–15. https://doi.org/10.1016/j.arthro.2016.07.022.
O’Neill DC, Mortensen AJ, Cannamela PC, Aoki SK. Clinical and radiographic presentation of capsular iatrogenic hip instability after previous hip arthroscopy. Am J Sports Med. 2020;48(12):2927–32.
McCormick F, Slikker W, Harris JD, et al. Evidence of capsular defect following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):902–5. https://doi.org/10.1007/s00167-013-2591-z.
Ekhtiari S, de Sa D, Haldane CE, et al. Hip arthroscopic capsulotomy techniques and capsular management strategies: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2017;25(1):9–23. https://doi.org/10.1007/s00167-016-4411-8.
Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007;35(11):1918–21.
Telleria JJM, Lindsey DP, Giori NJ, Safran MR. An anatomic arthroscopic description of the hip capsular ligaments for the hip arthroscopist. Arthroscopy - Journal of Arthroscopic and Related Surgery. 2011;27(5):628–36. https://doi.org/10.1016/j.arthro.2011.01.007.
Monroe EJ, Chambers CC, Zhang AL. Periportal capsulotomy: a technique for limited violation of the hip capsule during arthroscopy for femoroacetabular impingement. Arthrosc Tech. 2019;8(2). https://doi.org/10.1016/j.eats.2018.10.015.
Chambers CC, Monroe EJ, Flores SE, Borak KR, Zhang AL. Periportal capsulotomy: Technique and outcomes for a limited capsulotomy during hip arthroscopy. Arthrosc: J arthrosc & relat surg : Official publi Arthrosc Assoc North Ame Int Arthrosc Assoc. 2019;35(4). https://doi.org/10.1016/j.arthro.2018.10.142.
Wuerz TH, Song SH, Grzybowski JS, Martin HD, Mather III RC, Salata MJ, Espinoza Orías AA, Nho SJ Capsulotomy size affects hip joint kinematic stability. In: Arthroscopy - Journal of Arthroscopic and Related Surgery. Vol 32. W.B. Saunders; 2016:1571-1580
Philippon MJ, Trindade CAC, Goldsmith MT, Rasmussen MT, Saroki AJ, Løken S, LaPrade RF. Biomechanical assessment of hip capsular repair and reconstruction procedures using a 6 degrees of freedom robotic system. Am J Sports Med. 2017;45(8):1745–54. https://doi.org/10.1177/0363546517697956.
Khair MM, Grzybowski JS, Kuhns BD, Wuerz TH, Shewman E, Nho SJ. The effect of capsulotomy and capsular repair on hip distraction: a cadaveric investigation. Arthrosc – J Arthrosc Relat Surg. 2017;33(3):559–65. https://doi.org/10.1016/j.arthro.2016.09.019.
Jackson TJ, Peterson AB, Akeda M, Estess A, McGarry MH, Adamson GJ, Lee TQ. Biomechanical effects of capsular shift in the treatment of hip microinstability. Am J Sports Med. 2016;44(3):689–95. https://doi.org/10.1177/0363546515620391.
Aoki SK, Karns MR, Hananouchi T, Todd DC. Hip arthroscopy capsular closure: the figure of eight technique. Arthrosc Tech. 2017;6(2):e505–9. https://doi.org/10.1016/j.eats.2016.10.021.
Newman JT, Briggs KK, McNamara SC, Philippon MJ. Revision hip arthroscopy: a matched-cohort study comparing revision to primary arthroscopy patients. Am J Sports Med. 2016;44(10):2499–504. https://doi.org/10.1177/0363546516650888.
Camp CL, Reardon PJ, Levy BA, Krych AJ. A simple technique for capsular repair after hip arthroscopy. Arthrosc Tech. 2015;4(6):e737–40. https://doi.org/10.1016/j.eats.2015.07.020.
Chahla J, Mikula JD, Schon JM, Dean CS, Dahl KD, Menge TJ, Soares E, Turnbull TL, LaPrade RF, Philippon MJ. Hip capsular closure: a biomechanical analysis of failure torque. Am J Sports Med. 2017;45(2):434–9. https://doi.org/10.1177/0363546516666353.
Ortiz-Declet V, Mu B, Chen AW, Litrenta J, Perets I, Yuen LC, Domb BG. Should the capsule be repaired or plicated after hip arthroscopy for labral tears associated with femoroacetabular impingement or instability? A systematic review. Arthrosc – J Arthrosc Relat Surg. 2018;34(1):303–18.
Riff AJ, Kunze KN, Movassaghi K, et al. Systematic review of hip arthroscopy for femoroacetabular impingement: the importance of labral repair and capsular closure. Arthrosc - J Arthrosc Relat Surg. 2019;35(2):646–656.e3.
Larson CM, Stone RM, Grossi EF, Giveans MR, Cornelsen GD. Ehlers-Danlos syndrome: arthroscopic management for extreme soft-tissue hip instability. Arthrosc - J Arthrosc Relat Surg. 2015;31(12):2287–94. https://doi.org/10.1016/j.arthro.2015.06.005.
Krych AJ, Sousa PL, King AH, Engasser WM, Levy BA. Intra-articular diagnostic injection exhibits poor predictive value for outcome after hip arthroscopy. Arthrosc - J Arthrosc Relat Surg. 2016;32(8):1592–600. https://doi.org/10.1016/j.arthro.2016.02.005.
Larson CM, Ross JR, Stone RM, Samuelson KM, Schelling EF, Giveans MR, Bedi A. Arthroscopic management of dysplastic hip deformities: predictors of success and failures with comparison to an arthroscopic FAI cohort. Am J Sports Med. 2016;44(2):447–53.
Chandrasekaran S, Darwish N, Martin TJ, Suarez-Ahedo C, Lodhia P, Domb BG. Arthroscopic capsular plication and labral seal restoration in borderline hip dysplasia: 2-year clinical outcomes in 55 cases. Arthrosc - J Arthrosc Relat Surg. 2017;33(7):1332–40.
Domb BG, Chaharbakhshi EO, Perets I, Yuen LC, Walsh JP, Ashberg L. Hip arthroscopic surgery with labral preservation and capsular plication in patients with borderline hip dysplasia: minimum 5-year patient-reported outcomes. Am J Sports Med. 2018;46(2):305–13.
Hassebrock JD, Makovicka JL, Chhabra A, Anastasi MB, Menzer HM, Wilcox JG, Economopoulos KJ. Hip arthroscopy in the high-level athlete: does capsular closure make a difference? Am J Sports Med. 2020;48(10):2465–70.
Economopoulos KJ, Chhabra A, Kweon C. Prospective randomized comparison of capsular management techniques during hip arthroscopy. Am J Sports Med. 2020;48(2):395–402.
Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing t-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634–42.
Bolia IK, Fagotti L, Briggs KK, Philippon MJ. Midterm outcomes following repair of capsulotomy versus nonrepair in patients undergoing hip arthroscopy for femoroacetabular impingement with labral repair. Arthrosc - J Arthrosc Relat Surg. 2019;35(6):1828–34.
Domb BG, Chaharbakhshi EO, Perets I, Walsh JP, Yuen LC, Ashberg LJ. Patient-reported outcomes of capsular repair versus capsulotomy in patients undergoing hip arthroscopy: minimum 5-year follow-up—a matched comparison study. Arthrosc - J Arthrosc Relat Surg. 2018;34(3):853–863.e1.
Filan D, Carton P. Routine interportal capsular repair does not lead to superior clinical outcome following arthroscopic femoroacetabular impingement correction with labral repair. Arthrosc - J Arthrosc Relat Surg. 2020;36(5):1323–34.
Bech NH, Sierevelt IN, de Waard S, Joling BSH, Kerkhoffs GMMJ, Haverkamp D. Capsular closure versus unrepaired interportal capsulotomy after hip arthroscopy in patients with femoroacetabular impingement, results of a patient-blinded randomised controlled trial. HIP Int. Published online April 12. 2021:112070002110057.
Atzmon R, Sharfman ZT, Haviv B, Frankl M, Rotem G, Amar E, Drexler M, Rath E. Does capsular closure influence patient-reported outcomes in hip arthroscopy for femoroacetabular impingement and labral tear? J Hip Preserv Surg. 2019;6(3):199–206.
Kyin C, Maldonado DR, Go CC, Shapira J, Lall AC, Domb BG. Mid- to long-term outcomes of hip arthroscopy: a systematic review. Arthrosc - J Arthrosc Relat Surg. 2021;37(3):1011–25. https://doi.org/10.1016/j.arthro.2020.10.001.
Ross JR, Larson CM, Adeoyo O, Kelly BT, Bedi A. Residual deformity is the most common reason for revision hip arthroscopy: a three-dimensional CT study. Clin Orthop Relat Res. 2015;473(4):1388–95. https://doi.org/10.1007/s11999-014-4069-9.
Makhni EC, Ramkumar PN, Cvetanovich G, Nho SJ. Approach to the patient with failed hip arthroscopy for labral tears and femoroacetabular impingement. J Ame Acad Orthop Surg. 2020;28(13):538–45.
Cvetanovich GL, Harris JD, Erickson BJ, Bach BR, Bush-Joseph CA, Nho SJ. Revision hip arthroscopy: A systematic review of diagnoses, operative findings, and outcomes. Arthrosc - J Arthrosc Relat Surg. 2015;31(7):1382–90. https://doi.org/10.1016/j.arthro.2014.12.027.
Ross JR, Clohisy JC, Bedi A, Zaltz I. Why does hip arthroscopy fail? Indications and PEARLS for revision success. Sports Med Arthrosc Rev. 2021;29(1):44–51.
Bedi A, Zbeda RM, Bueno VF, Downie B, Dolan M, Kelly BT. The incidence of heterotopic ossification after hip arthroscopy. Am J Sports Med. 2012;40(4):854–63. https://doi.org/10.1177/0363546511434285.
Bedi A, Kelly BT, Khanduja V. Arthroscopic hip preservation surgery. Bone Joint J. 2013;95-B(1):10–9. https://doi.org/10.1302/0301-620X.95B1.29608.
Beckmann JT, Wylie JD, Potter MQ, Maak TG, Greene TH, Aoki SK. Effect of naproxen prophylaxis on heterotopic ossification following hip arthroscopy: A double-blind randomized placebo-controlled trial. J Bone Joint Surg (Am Vol). 2014;97(24):2032–7. https://doi.org/10.2106/JBJS.N.01156.
Beckmann JT, Wylie JD, Kapron AL, Hanson JA, Maak TG, Aoki SK. The effect of NSAID prophylaxis and operative variables on heterotopic ossification after hip arthroscopy. Am J Sports Med. 2014;42(6):1359–64. https://doi.org/10.1177/0363546514526361.
Anderson LA, Kapron AL, Aoki SK, Peters CL. Coxa profunda: Is the deep acetabulum overcovered? In: Clinical Orthopaedics and Related Research. Vol 470. Springer New York LLC; 2012:3375-3382. https://doi.org/10.1007/s11999-012-2509-y
Acuña AJ, Samuel LT, Roth A, Emara AK, Kamath AF. How capsular management strategies impact outcomes: a systematic review and meta-analysis of comparative studies. J Orthop. 2020;19:237–43. https://doi.org/10.1016/j.jor.2020.02.002.
Magerkurth O, Jacobson JA, Morag Y, Caoili E, Fessell D, Sekiya JK. Capsular laxity of the hip: findings at magnetic resonance arthrography. Arthrosc - J Arthrosc Relat Surg. 2013;29(10):1615–22. https://doi.org/10.1016/j.arthro.2013.07.261.
Wylie JD, Beckmann JT, Maak TG, Aoki SK. Arthroscopic capsular repair for symptomatic hip instability after previous hip arthroscopic surgery. Am J Sports Med. 2016;44(1):39–45.
Kim CHO, Dietrich TJ, Zingg PO, Dora C, Pfirrmann CWA, Sutter R. Arthroscopic hip surgery: frequency of postoperative MR arthrographic findings in asymptomatic and symptomatic patients. Radiology. 2017;283(3):779–88.
Strickland CD, Kraeutler MJ, Brick MJ, Garabekyan T, Woon JTK, Chadayammuri V, Mei-Dan O. MRI evaluation of repaired versus unrepaired interportal capsulotomy in simultaneous bilateral hip arthroscopy: a double-blind, randomized controlled trial. J Bone Joint Surg (Am Vol). 2018;100(2):91–8. https://doi.org/10.2106/JBJS.17.00365.
Packer JD, Foster MJ, Riley GM, Stewart R, Shibata KR, Richardson ML, Boutin RD, Safran MR. Capsular thinning on magnetic resonance arthrography is associated with intra-operative hip joint laxity in women. J Hip Preserv Surg. 2020;7(2):298–304. https://doi.org/10.1093/jhps/hnaa018.
Hoppe DJ, Truntzer JN, Shapiro LM, Abrams GD, Safran MR. Diagnostic accuracy of 3 physical examination tests in the assessment of hip microinstability. Orthop J Sports Med. 2017;5(11). https://doi.org/10.1177/2325967117740121.
Brusalis CM, Peck J, Wilkin GP, et al. Periacetabular osteotomy as a salvage procedure. J Bone Joint Surg. 2020;102(Suppl 2). https://doi.org/10.2106/JBJS.20.00087.
Levy DM, Grzybowski J, Salata MJ, Mather RC, Aoki SK, Nho SJ. Capsular plication for treatment of iatrogenic hip instability. Arthrosc Tech. 2015;4(6):e625–30. https://doi.org/10.1016/j.eats.2015.07.007.
Featherall J, Tomasevich KM, O’Neill DC, Mortensen AJ, Aoki SK. Arthroscopic Hip Capsule Reconstruction for Anterior Hip Capsule Insufficiency in the Revision Setting. Arthrosc Tech. 2021;10(5):e1339–44.
Trindade CAC, Sawyer GA, Fukui K, Briggs KK, Philippon MJ. Arthroscopic capsule reconstruction in the hip using iliotibial band allograft. Arthrosc Tech. 2015;4(1):e71–4. https://doi.org/10.1016/j.eats.2014.11.008.
Kurz AZ, Memon M, Williams D, Ayeni OR. Anterior capsule reconstruction of the native hip: a technique guide. Arthrosc Tech. 2019;8(10):e1247–53. https://doi.org/10.1016/j.eats.2019.06.014.
Mei-Dan O, Garabekyan T, McConkey M, Pascual-Garrido C. Arthroscopic anterior capsular reconstruction of the hip for recurrent instability. Arthrosc Tech. 2015;4(6):e711–5.
Larson CM, Williams BT, Bessa F, McGaver RS, Suppauksorn S, Faucett S, Chahla J. Revision hip capsular repair and augmentation with a bioinductive implant after a post-arthroscopy hip subluxation event. Arthrosc Tech. 2020;9(4):e453–8.
Larson CM, Giveans MR, Samuelson KM, Stone RM, Bedi A. Arthroscopic hip revision surgery for residual femoroacetabular impingement (FAI): surgical outcomes compared with a matched cohort after primary arthroscopic fai correction. Am J Sports Med. 2014;42(8):1785–90. https://doi.org/10.1177/0363546514534181.
Fagotti L, Soares E, Bolia IK, Briggs KK, Philippon MJ. Early outcomes after arthroscopic hip capsular reconstruction using iliotibial band allograft versus dermal allograft. Arthrosc - J Arthrosc Relat Surg. 2019;35(3):778–86. https://doi.org/10.1016/j.arthro.2018.10.110.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Alexander Mortensen, Allan Metz, and Devin Froerer declare that they have no conflicts of interest.
Stephen Aoki is a paid education consultant for Stryker Medical, outside of the submitted work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on HIP/FAI
Rights and permissions
About this article
Cite this article
Mortensen, A.J., Metz, A.K., Froerer, D.L. et al. Hip Capsular Deficiency—A Cause of Post-Surgical Instability in the Revision Setting Following Hip Arthroscopy for Femoroacetabular Impingement. Curr Rev Musculoskelet Med 14, 351–360 (2021). https://doi.org/10.1007/s12178-021-09732-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12178-021-09732-5