Abstract
Purpose of Review
Fractures of the proximal humerus (PHF) and distal radius (DRF) are among the most common upper extremity fractures in the elderly. Recent randomized controlled trials support non-surgical treatment. Evidence behind the best non-surgical treatment strategy has been sparse and raises questions as to when and how to initiate exercises. The purpose of this systematic review and meta-analysis was to assess the benefits and harms of early mobilization versus late mobilization and supervised versus non-supervised exercises therapy after PHF and DRF.
Recent Findings
15 published and 5 unpublished trials were included. Early mobilization after PHF resulted in better function with a mean difference (MD) of 4.55 (95% CI 0.00–9.10) on the Constant Shoulder Score. However, the MD was not found to be clinically relevant. No clear evidence showed that early mobilization after PHF had a positive effect on range of motion or pain. Neither did it lead to more complications. Furthermore, no eligible evidence was found supporting early mobilization to be superior to late mobilization after DRF, or that supervised exercise therapy was superior to non-supervised exercise therapy after PHF and DRF. The quality of evidence on all outcomes was found to be low or very low.
Summary
Early mobilization after PHF may have a beneficial effect on function. Due to the lack of clear evidence, there is an urgent need for future studies to determine the effect of early mobilization and supervised exercise therapy after PHF and DRF.
Prospero ID number: CRD42020167656, date of registration 28.04.2020
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fractures of the proximal humerus (PHF) or distal radius (DRF) are among the most common fractures in the elderly population [1, 2]. The majority of patients suffering a PHF or DRF are aged 60 years or older, and the most representative patient is an elderly osteoporotic woman [2, 3]. Due to longer life expectancy in the elderly population, the incidence rates of these osteoporotic fractures are predicted to increase [2, 3]. In future, this increase will impose a substantial burden on healthcare systems and increase societal costs.
Both PHF and DRF can have a substantial impact on the patient´s physical function and independent living and are associated with higher morbidity and mortality [3, 4]. After sustaining a PHF or DRF, the main focus of treatment is to regain the best possible function of the shoulder or wrist. Recent evidence questions the benefit of surgical treatment compared with non-surgical treatment after PHF [5,6,7]. The same conclusion was reached in a recent review investigating the optimal treatment after DRF in which no clear benefit of surgical treatment was found in the elderly [8•]. Thus, the next important task is to address and optimize the non-surgical treatment strategy for these fractures.
The question of when to commence supervised exercise therapy is of high clinical importance. Sparse evidence suggests that there might be a preference for a short immobilization period after sustaining a PHF or DRF [9••].
Patients are usually referred to supervised rehabilitation after sustaining these types of fractures. However, supervised rehabilitation consumes considerable healthcare resources and raises the question as to what extent patients benefit from supervised exercise therapy [8, 10]. Bruder et al. have suggested that after PHF non-supervised exercises at home might be just as effective as exercises supervised by a therapist; however, this conclusion was based on scarse evidence [9••]. In 2015, the Cochrane review concluded that there is no evidence to determine the best possible non-surgical treatment after PHF [6].
Our aim therefore was to conduct a systematic review and meta-analysis to assess the benefits and harms of (1) early mobilization compared to late mobilization and of (2) supervised exercise therapy compared to non-supervised exercises after non-surgically treated PHF and DRF.
Materials and Methods
Study Design and Protocol
This is a systematic review with meta-analysis. Search strategy, trial selection, eligibility criteria, methodology assessment, data extraction, and analysis were performed in accordance with a predefined protocol (PROSPERO: CRD42020167656). Trial screening, selection of trials, data extraction, assessment of methodology, and quality of evidence were performed by two independent reviewers (H.K.Ø. and V.T.P.). Disagreements were resolved through a consensus process. The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) was used as a checklist throughout the reporting [11].
Search Strategy and Trial Selection
Electronic databases were systematically searched for primary trials (Supplementary material). Searches were conducted in MEDLINE (OvidSP), Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), CINAHL, Physiotherapy Evidence Database (PEDro), Scopus, ClinicalTrials.gov, and WHO International Clinical Trials Registry Platform (WHO ICTRP). Other sources involved the hand searching of reference lists of systematic reviews or guidelines. The search was limited to randomized controlled trials, non-randomized trials, and prospective observational studies. Searches were undertaken on September 20 and December 2, 2019 and repeated on June 11, 2020. Results were loaded into EndNote (version X9.2; Clarivate Analytics) software for deduplication. Full, database-specific search strategies are available in the online appendix. The identified trials were uploaded in systematic review management software (Covidence, Aus) and screened at title/abstract level. Eligible trials were then full-text screened for final inclusion. Reference lists from the full-text trials were also screened for supplementary relevant trials.
Eligibility Criteria
Eligibility criteria were defined according to the Population, Intervention, Comparison, and Outcome (PICO) method [12]. The population consisted of adults ≥ 18 years with a verified PHF or DRF due to recent trauma and referred to non-operative treatment. The interventions and comparisons were defined as (1) early mobilization (≤ within 2 weeks after time of fracture) compared to late mobilization or (2) supervised exercise therapy compared to non-supervised exercises. The included outcome measures were function, pain, and health-related quality of life (HRQoL). Function was defined as either function assessed by patient-reported outcome measures (PROMs), performance-based function including range of motion (ROM) and strength measures, or by questionnaires comprising both of these subjective and objective measurements.
Methodological Assessment
The included trials were evaluated using the Cochrane Risk of Bias tool (RoB 2.0) [13]. The risk of bias was rated as low, unclear, or high. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to rate the body of evidence for each outcome as either very low, low, moderate, or high [14].
Data extraction and statistical analysis
The following trial information was extracted: author, year, country, trial design, number of participants, population characteristics, description of intervention/control, outcome measures, and time to follow-up. Effect estimates were extracted as reported in the trials. In three cases, the corresponding authors were contacted for extended data details. If a minimum of two outcome measures were found eligible for comparison, a meta-analysis was undertaken and values were presented as either mean difference (MD) or standard mean difference (SMD) with 95% CIs, using the random-effect model. The I2 value was calculated and if higher than 50%, the heterogeneity was considered substantial and not eligible for meta-analysis. In cases where meta-analysis could not be undertaken, narrative synthesis was performed based on the conclusions reported in the trials. Statistics were performed using Stata 16 (TX, USA) and R 3.6.2 (R Foundation, Vienna).
Results
Search and Selection of Trials
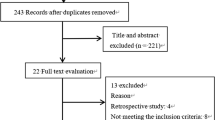
In total, 1924 trials were found eligible for screening. After screening and full-text reading, 15 RCTs (943 participants) were included. Furthermore, 1 abstract (98 participants) and 5 trials (736 participants) registered in ClinicalTrials.gov and WHO ICTRP were identified. A summary of the search results and trial selection process is provided in a PRISMA flow diagram (Fig. 1).
Trial Characteristics
The mean age of the participants in the included trials ranged from 52.5 to 77.3 years. The follow-up times ranged from 2 weeks to 2 years. Specific details on the outcome measures used can be found in Tables 1, 2, and 3.
Benefits and harms of Early Mobilization after Proximal Humerus Fracture
Function
Functional scores were reported in three trials. [15,16,17,18]. One of the trials was outlined in two different papers [15, 16]. In addition, two unpublished trials (ClinicalTrials.gov) provided preliminary results [19, 20]. The outcome measures used are outlined in Tables 1 and 2. Data extracted from two of the trials [17, 20] allowed meta-analysis and an overall MD of 4.55 (95% CI 0.00–9.10) on the Constant Shoulder Score (CS) at 6-month follow-up in favor of early mobilization was found (Fig. 2). This finding was supported by two other trials concluding that early mobilization within 2 weeks after sustaining a PHF resulted in better function when compared to late mobilization [16, 18].
ROM was reported in two trials [17, 18]. In addition, two unpublished trials reported ROM figures on which a meta-analysis could be conducted [19, 20]. No eligible evidence of a difference between the groups was found at 6-month follow-up, either in flexion with a MD equal to 2.18° (95% CI −4.83° to 9.19°) or in abduction with a MD equal to 3.75° (95% CI −5.76° to 13.27°) (Figs. 3 and 4). The same conclusion was reached in a third trial that found no eligible evidence of a difference in ROM between groups [18]. A fourth trial reported a higher active and passive abduction and anterior elevation in favor of the early mobilization group [17] (Table 1).
Pain
Pain scores were reported in two published trials [17, 18] and the two previously mentioned unpublished trials [19, 20]. Meta-analysis based on data from the two unpublished trials [19, 20] showed no evidence suggestive of between-group difference in pain 6 months after the fracture, with an MD equal to 0.20 (95% CI −0.36 to 0.76) (Fig. 5). In contrast, two other trials concluded individually that early mobilization resulted in a decrease of pain in favor of the early mobilization group [17, 18] (Table 1).
Health-Related Quality of Life
The Short Form-36 (SF-36) was used to measure HRQoL in one trial. The trial reported a positive effect in the role limitation and pain domains in favor of early mobilization. This positive effect was not, however, found between groups in any of the other domains [16].
Complications
One trial identified a single case (1/32) of frozen shoulder in the late mobilization group [17] while another trial found one case (1/39 and 1/41) of reflex dystrophy in both groups [18].
Benefits and Harms of Early Mobilization after Distal Radius Fracture
Function
Functional scores were reported in five trials. The outcomes used are described in Tables 1 and 2. Two trials reported an improvement of function in favor of early mobilization after DRF [21, 22]. This finding was not, however, in agreement with the results reported in three other trials, which concluded that early mobilization after DRF did not lead to improvement of function [23,24,25]. Recovery of domestic abilities was assessed in one trial that reported a higher number of patients in the early mobilization group regained their abilities within 5 weeks [21].
ROM was reported in two trials which stated that early mobilization improved movement of the wrist [22, 25]. Grip strength was assessed in three trials [21, 22, 25]. One trial reported grip strength being higher in the early mobilization group [22]. However, no increase in grip strength was reported in the other two trials [21, 25].
Pain
Pain scores were reported in two trials. The trials did not find eligible evidence of a difference between groups at any of the time points [21, 25].
Health-Related Quality of Life
HRQoL was not assessed in any of the trials investigating early versus late mobilization after DRF.
Complications
One trial identified three cases (3/54) of treatment failure in the early mobilization group, defined as problems leading to abandonment of given treatment or operation of malunited fracture [25]. Another trial reported four cases of reflex dystrophy, but they did not state in which groups [24].
Benefits and harms of supervised exercise therapy after proximal humerus fracture
Function
ROM was assessed in two trials. One trial measured movements comprising hand on back and hand on neck [26], and one trial measured active and passive elevation [27]. Both trials concluded that supervised exercise therapy did not result in a better ROM at any time point. Muscle strength was also assessed in the two trials. One trial measured isometric muscle strength by horizontal and vertical push [27], and the other trial measured shoulder and grip strength [26]. No eligible evidence of a difference between groups at any time point was detected.
Pain
Pain was assessed in two trials [26, 27]. One trial rated pain as insignificant, moderate, or severe and the other trial used a modified Borg scale (from 0 to 8). The trials did not show a higher degree of pain relief in the supervised exercise therapy group [26, 27].
Health-Related Quality of Life
HRQoL was not assessed in any of the trials investigating supervised exercise therapy after PHF.
Complications
One trial reported three cases of frozen shoulder. Two cases (2/20) were identified in the supervised group and one (1/22) case in the non-supervised group. In addition, one (1/22) patient in the non-supervised group had an unexplained pain over a longer period [26].
Benefits and Harms of Supervised Exercise Therapy after Distal Radius Fracture
Function
Patient-Rated Wrist Evaluation (PRWE) was used in two trials and the results were inconsistent. One trial found that supervised exercise therapy improved function [28••]. The second trial, however, reported a greater improvement in the non-supervised group [29], and a third trial did not find any eligible evidence of a between-group difference [30].
ROM was reported in four trials [28, 29, 31, 32] and in one trial abstract [33]. Only one of the trials found no eligible evidence of a difference in ROM between the two groups [29], whereas the remaining trials concluded that supervised exercise therapy after DRF leads to an increased ROM [28, 31,32,33].
Grip strength was reported in four trials and the results were inconsistent [28, 29, 31, 32]. Two trials found increased grip strength in the supervised group [28, 32], the third trial found no eligible evidence of a difference between the groups at any time point [31], and the fourth trial found grip strength to be greater in the non-supervised group [29].
Pain
VAS was used to measure pain in two trials [28, 31]. One trial reported a lower degree of pain in the supervised group [28••], but another trial found no eligible evidence of a clear difference between the groups at any time point [31].
Health-Related Quality of Life
HRQoL was assessed in one trial using SF-36. The trial concluded that supervised exercise therapy after DRF did not increase HRQoL [31].
Quality of Evidence
Risk of Bias
Randomization was applied in all 15 trials. However, only six trials provided sufficient details leading to low risk of bias (Table 4). Blinding of patients in these study settings was not possible, and therefore the risk of bias was rated high in this domain in all trials. Blinding of the outcome assessors was rated as unclear in seven of the trials. Based on adherence of the methods described in the trials, selective reporting of outcomes was not found to be an overall problem, and in most cases the risk of bias was rated as low. However, only a few trials had prior protocol registrations or published protocols, and thus the assessment of selective reporting of outcomes was not comprehensive.
GRADE assessment
As a result of incomparable outcome measures, difference in time to follow-up, and high I2 values, meta-analysis could only be undertaken on a limited number of outcomes. Thus, GRADE assessment was based on the meta-analysis and on the substance of the narrative synthesis. As a result of risk of bias, imprecision, and inconsistency of the trial results, the quality of evidence on all outcomes was found to be low or very low (Tables 5, 6, 7 and 8).
Discussion
This systematic review and meta-analysis suggests that early mobilization within 2 weeks of fracture may result in better function after PHF. However, these findings are based on low quality evidence. Furthermore, the overall MD (4.55) on the CS is not considered to be a clinically important difference, which has been reported to be between 5.4 and 11.6 [34]. Moreover, no evidence showed early mobilization after PHF has a clear positive effect on ROM or pain. Neither did it lead to more complications. No eligible evidence was found supporting early mobilization to be superior to late mobilization after DRF in terms of improved wrist function, grip strength, HRQoL, or reduced pain. Finally, no clear evidence showed a clear benefit of supervised exercise therapy compared with non-supervised home exercises on function of the upper limb, HRQoL, or reduced pain after PHF or DRF.
These results confirm the conclusions of previous systematic reviews that have reported a lack of clear evidence to support the decision on when to commence exercise therapy and to what extent it should be supervised [6, 35].
Limitations
The low number of included trials is a limitation of the present study, and changes to the inclusion criteria may have resulted in a larger study sample. Several trials did not include PROMs as an outcome measure, and therefore most of the measurements were clinician assessed and do not necessarily reflect the patients’ own perception of function. Several trials did not provide sufficient information on the inclusion and exclusion criteria. Furthermore, sample size calculation was only reported in 6 out of the 15 trials [16, 17, 25, 28, 29, 31] and only 4 trials referred to a minimal clinical important difference for their main outcome [16, 17, 28, 31]. This adds additional uncertainty to the reported results.
Implications
The consequences of immobilizing elderly people who have sustained a PHF or DRF for longer than necessary is an important consideration. A longer period of immobilization may lead to physical inactivity, with an increased risk of compromising general health [36]. The present study identified three unpublished RCTs that had investigated the effects of early mobilization after PHF, leading us to believe that more evidence will be available in the future [19, 20, 37].
Rehabilitation after upper limb fractures must be viewed from a broader and more complex perspective than that investigated in this systematic review. Therefore, other independent risk factors for poor function after these type of fractures, such as social deprivation, low self-efficacy, and fear of movement, must be acknowledged [38, 39].
Conclusion
There is an urgent need for high-quality randomized controlled trials to substantiate the current evidence regarding the optimal time to initiate mobilization and the need for supervision after PHF and DRF.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691–7. https://doi.org/10.1016/j.injury.2006.04.130.
Launonen AP, Lepola V, Saranko A, Flinkkila T, Laitinen M, Mattila VM. Epidemiology of proximal humerus fractures. Arch Osteoporos. 2015;10:209. https://doi.org/10.1007/s11657-015-0209-4.
Rundgren J, Bojan A, Mellstrand Navarro C, Enocson A. Epidemiology, classification, treatment and mortality of distal radius fractures in adults: an observational study of 23,394 fractures from the national Swedish fracture register. BMC Musculoskelet Disord. 2020;21(1):88. https://doi.org/10.1186/s12891-020-3097-8.
Lander ST, Mahmood B, Maceroli MA, Byrd J, Elfar JC, Ketz JP, et al. Mortality rates of humerus fractures in the elderly: does surgical treatment matter? J Orthop Trauma. 2019;33(7):361–5. https://doi.org/10.1097/bot.0000000000001449.
• Launonen AP, Sumrein BO, Reito A, Lepola V, Paloneva J, Jonsson KB, et al. Operative versus non-operative treatment for 2-part proximal humerus fracture: a multicenter randomized controlled trial. PLoS Med. 2019;16(7):e1002855. https://doi.org/10.1371/journal.pmed.1002855This recent trial found no benefits of surgical treatment with locking plate compared to non-surgical treatment after 2-part proximal humerus fractures in the elderly. They suggest non-surgical treatment on the majority of the patients with this kind of fracture, which is in line with the conclusion from a Cochrane Review from 2015 by Handoll et al.
Handoll HH, Brorson S. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2015;(11):Cd000434. https://doi.org/10.1002/14651858.CD000434.pub4.
Rangan A, Handoll H, Brealey S, Jefferson L, Keding A, Martin BC, et al. Surgical vs nonsurgical treatment of adults with displaced fractures of the proximal humerus: the PROFHER randomized clinical trial. Jama. 2015;313(10):1037–47. https://doi.org/10.1001/jama.2015.1629.
• Mellstrand Navarro C, Brolund A, Ekholm C, Heintz E, Hoxha Ekstrom E, Josefsson PO, et al. Treatment of radius or ulna fractures in the elderly: a systematic review covering effectiveness, safety, economic aspects and current practice. PLoS One. 2019;14(3):e0214362. https://doi.org/10.1371/journal.pone.0214362This review does not find that surgical treatment after moderately displaced distal radius fracture in the elderly is superior to non-surgical treatment. Furthermore, surgical treatment was found to increase the risk of major complications.
•• Bruder AM, Shields N, Dodd KJ, Taylor NF. Prescribed exercise programs may not be effective in reducing impairments and improving activity during upper limb fracture rehabilitation: a systematic review. Aust J Phys. 2017;63(4):205–20. https://doi.org/10.1016/j.jphys.2017.08.009This review suggests that current prescribed exercise regimes do not lead to an improvement of activity following distal radius fracture or proximal humerus fracture. However, patients with these fractures may benefit from early exercises and shorter immobilization.
Handoll H, Brealey S, Rangan A, Keding A, Corbacho B, Jefferson L, et al. The ProFHER (PROximal Fracture of the Humerus: Evaluation by Randomisation) trial – a pragmatic multicentre randomised controlled trial evaluating the clinical effectiveness and cost-effectiveness of surgical compared with non-surgical treatment for proximal fracture of the humerus in adults. Health Technol Assess. 2015;19(24):1–280. https://doi.org/10.3310/hta19240.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj. 2015;350:g7647. https://doi.org/10.1136/bmj.g7647.
Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16. https://doi.org/10.1186/1472-6947-7-16.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928. https://doi.org/10.1136/bmj.d5928.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. https://doi.org/10.1016/j.jclinepi.2010.04.026.
Hodgson SA, Mawson SJ, Saxton JM, Stanley D. Rehabilitation of two-part fractures of the neck of the humerus (two-year follow-up). J Shoulder Elb Surg. 2007;16(2):143–5. https://doi.org/10.1016/j.jse.2006.06.003.
Hodgson SA, Mawson SJ, Stanley D. Rehabilitation after two-part fractures of the neck of the humerus. J Bone Joint Surg (Br). 2003;85(3):419–22. https://doi.org/10.1302/0301-620x.85b3.13458.
Lefevre-Colau MM, Babinet A, Fayad F, Fermanian J, Anract P, Roren A, et al. Immediate mobilization compared with conventional immobilization for the impacted nonoperatively treated proximal humeral fracture. A randomized controlled trial. J Bone Joint Surg Am. 2007;89(12):2582–90. https://doi.org/10.2106/jbjs.F.01419.
Kristiansen B, Angermann P, Larsen TK. Functional results following fractures of the proximal humerus. A controlled clinical study comparing two periods of immobilization. Arch Orthop Trauma Surg. 1989;108(6):339–41.
Early vs Delayed Physical Therapy (Exercises) for Non-Operatively-Treated Proximal Humerus Fractures: A Prospective Randomized Trial [database on the Internet]. National Library of Medicine (US). 2000 Feb 29, (cited July 2. 2020), Available at: https://clinicaltrials.gov/ct2/show/NCT00438633. Accessed 30 May 2020.
Conservative Treatment of Proximal Humeral Fractures – Immobilization for 1 Week Compared to Three Weeks: Prospective Randomized Study [database on the Internet]. National Library of Medicine (US). 2000 Feb 29 , (cited July 2, 2020), Available from: https://clinicaltrials.gov/ct2/show/study/NCT03217344. Accessed 30 May 2020.
Davis TR, Buchanan JM. A controlled prospective study of early mobilization of minimally displaced fractures of the distal radial metaphysis. Injury. 1987;18(4):283–5. https://doi.org/10.1016/0020-1383(87)90015-5.
Dias JJ, Wray CC, Jones JM, Gregg PJ. The value of early mobilisation in the treatment of Colles' fractures. J Bone Joint Surg (Br). 1987;69(3):463–7.
Stoffelen D, Broos P. Minimally displaced distal radius fractures: do they need plaster treatment? J Trauma. 1998;44(3):503–5. https://doi.org/10.1097/00005373-199803000-00014.
Jensen MR, Andersen KH, Jensen CH. Management of undisplaced or minimally displaced Colles' fracture: one or three weeks of immobilisation. J Orthop Sci. 1997;2(6):424–7.
Christersson A, Larsson S, Sandén B. Clinical outcome after plaster cast fixation for 10 days versus 1 month in reduced distal radius fractures: a prospective randomized study. Scand J Surg. 2018;107(1):82–90. https://doi.org/10.1177/1457496917731184.
Lungberg BJ, Svenungson-Hartwig E, Wikmark R. Independent exercises versus physiotherapy in nondisplaced proximal humeral fractures. Scand J Rehabil Med. 1979;11(3):133–6.
Bertoft ES, Lundh I, Ringqvist I. Physiotherapy after fracture of the proximal end of the humerus. Comparison between two methods. Scand J Rehabil Med. 1984;16(1):11–6.
•• Gutierrez-Espinoza H, Rubio-Oyarzun D, Olguin-Huerta C, Gutierrez-Monclus R, Pinto-Concha S, Gana-Hervias G. Supervised physical therapy vs home exercise program for patients with distal radius fracture: a single-blind randomized clinical study. J Hand Ther. 2017;30(3):242–52. https://doi.org/10.1016/j.jht.2017.02.001This trial is the most recent trial comparing a supervised versus a non-supervised exercise program after distal radius fracture. They reported that a supervised exercise program is more effective for improving function compared to a non-supervised program. This was found on the PRWE score at 6 weeks and 6 months of follow-up.
Krischak G, Krasteva A, Pandorf-Frediani S, Dehner C, Schneider F, Gebhard F, et al. Effect of a home exercise program in rehabilitation of non operatively treated wrist fractures a prospectively randomized study. Physikalische Medizin Rehabilitationsmedizin Kurortmedizin. 2009;19(4):185–92. https://doi.org/10.1055/s-0029-1225336.
Christensen OM, Kunov A, Hansen FF, Christiansen TC, Krasheninnikoff M. Occupational therapy and Colles' fractures. Int Orthop. 2001;25(1):43–5.
Wakefield AE, McQueen MM. The role of physiotherapy and clinical predictors of outcome after fracture of the distal radius. J Bone Joint Surg Br Vol. 2000;82(7):972–6.
Watt CF, Taylor NF, Baskus K. Do Colles' fracture patients benefit from routine referral to physiotherapy following cast removal? Arch Orthop Trauma Surg. 2000;120(7-8):413–5. https://doi.org/10.1007/pl00013772.
Bache S. Two different approaches to the physiotherapeutic management of patients with distal radial fractures. Physiotherapy. 2000;86(7):383.
Dabija DI, Jain NB. Minimal clinically important difference of shoulder outcome measures and diagnoses: a systematic review. Am J Phys Med Rehabil. 2019;98(8):671–6. https://doi.org/10.1097/phm.0000000000001169.
Bruder AM, Taylor NF, Dodd KJ, Shields N. Physiotherapy intervention practice patterns used in rehabilitation after distal radial fracture. Physiotherapy. 2013;99(3):233–40. https://doi.org/10.1016/j.physio.2012.09.003.
Cunningham C, O’Sullivan R, Caserotti P, Tully MA. Consequences of physical inactivity in older adults: a systematic review of reviews and meta-analyses. Scand J Med Sci Sports. 2020;30(5):816–27. https://doi.org/10.1111/sms.13616.
Non-operative Treatment in Sweden of Proximal Humeral Fractures, a Randomised Multicenter Trial. [database on the Internet]. National Library of Medicine (US). 2000 Feb 29 (cited July 2 2020), Available at: https://clinicaltrials.gov/ct2/show/NCT03786679. Accessed 30 May 2020.
Johnson NA, Dias JJ. The effect of social deprivation on fragility fracture of the distal radius. Injury. 2019;50(6):1232–6. https://doi.org/10.1016/j.injury.2019.04.025.
Jayakumar P, Teunis T, Williams M, Lamb SE, Ring D, Gwilym S. Factors associated with the magnitude of limitations during recovery from a fracture of the proximal humerus: predictors of limitations after proximal humerus fracture. Bone Joint J. 2019;101-b(6):715–23. https://doi.org/10.1302/0301-620x.101b6.Bjj-2018-0857.R1.
Acknowledgments
We wish to thank Jaana Isojärvi for her assistance with the literature search. Furthermore, we wish to thank Carlos Torrens for sharing his unpublished data.
Author information
Authors and Affiliations
Contributions
Conception and design of the work: H.K.Ø., I.M., A.P.L., V.M.M., and V.T.P. Data collection, selection, evaluation, and analysis: H.K.Ø. and V.T.P. Drafting the article: H.K.Ø. Guidance and critical revision of the manuscript: H.K.Ø., I.M., A.P.L., M.T.V., V.M.M., and V.T.P.
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subject performed by any of the authors.
Conflict of Interest
H.K.Ø., I.M.,, A.P.L., M.T.V., V.M.M., and V.T.P. declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 57 kb)
Rights and permissions
About this article
Cite this article
Østergaard, H.K., Mechlenburg, I., Launonen, A.P. et al. The Benefits and Harms of Early Mobilization and Supervised Exercise Therapy after Non-surgically Treated Proximal Humerus or Distal Radius fracture: A systematic Review and Meta-analysis. Curr Rev Musculoskelet Med 14, 107–129 (2021). https://doi.org/10.1007/s12178-021-09697-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12178-021-09697-5