Abstract
Purpose of Review
The most common complications warranting revision consideration in reverse shoulder arthroplasty (RSA) include instability and its associated causes: infection, periprosthetic fracture, and glenoid baseplate loosening. Management of complications can be challenging and the nuances of treatment are still being elucidated. The focus of this paper is to review the treatment of the failed RSA and discuss evidence-based recommendations for revision.
Recent Findings
The most common complications requiring revision RSA are instability and infection. The causes for instability can be subdivided into three main subcategories: loss of compression, loss of containment, and impingement. Loss of compression is further broken down into 6 subcategories revolving around abnormal prosthesis positioning, undersized prostheses, or intrinsic soft-tissue tension loss leading to instability. Periprosthetic infection can also lead to instability, yet the most appropriate management for infected RSA remains controversial.
Summary
Restoring stability by maximizing deltoid and soft tissue tension while avoiding impingement revolves around three basic methods: (1) lateralizing and/or upsizing the glenosphere to an inferior position on the glenoid, (2) use of a more constrained polyethylene insert, and (3) distalizing the humerus by increasing the polyethylene thickness and/or the thickness of the humeral tray. Management of periprosthetic joint infection can be performed in one-stage, two-stage, or “three-stage” procedures all showing good outcomes with two-stage procedures being the most commonly performed. However, persistent positive culture with propriobacterium acnes can occur in up to 25% of cases. In order to limit the associated morbidity from failed revision reverse shoulder arthroplasty, continued research on best management of associated complications is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis is a common degenerative condition of the glenohumeral joint with an estimated prevalence between 4 and 26% [1]. Reverse shoulder arthroplasty (RSA) is preferred in patients with osteoarthritis of the shoulder and significant rotator cuff arthropathy as it provides a greater fulcrum for the deltoid to assist in abduction than anatomic total shoulder arthroplasty [2,3,4, 5•, 6]. Documented complication rates range from 7 to 48% with a recent review citing an overall complication rate of 15% [7,8,9]. The most common complications leading to revision include instability or dislocation and its associated causes (Table 1), infection, periprosthetic fracture, and glenoid baseplate loosening [7, 10••, 11]. Management of complications can be challenging, and there is no consensus on the best treatment practices. The focus of this paper is to review the treatment of the failed RSA and discuss evidence-based recommendations for revision.
Biomechanics of Reverse Shoulder Arthroplasty
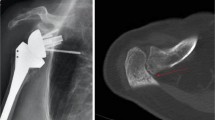
A clear understanding of the unique biomechanics of RSA is important for managing complications. From a biomechanical perspective, both the native shoulder and anatomic TSA work similarly to prevent instability through compressive forces of the rotator cuff equal to 200 N at 50° abduction [12]. After RSA, the arm is lengthened by approximately 2.5 cm (Fig. 1), which compensates for rotator cuff deficiency by increasing deltoid tension [2,3,4, 5•]. While vital for stability, overtensioning the deltoid defined as > 15 mm by Lädermann et al. should be avoided. Acromial and scapular stress fractures as well as deltoid degeneration can arise and are difficult to manage during revision RSA due to an inability to adequately tension the deltoid [4, 5•, 10••, 11, 13••, 14, 15].
Image adapted from Boileau et al. showing the medialization and lowering of the humerus in RSA compared with the native shoulder which increases deltoid tensioning and compressive forces across the prosthesis components [9]
Instability
Instability after RSA is the most common complication of RSA with reported incidence ranging from 0.5 to 9% [11, 16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. The initial evaluation for an unstable RSA is an appropriate history, physical examination, and radiographic analysis, including full-length humeral radiographs to evaluate for humeral shortening [13••]. Attempted closed versus open reduction and temporary immobilization for 6 weeks has been proposed for the management of first-time dislocations [31]. Teusink et al. reported a 62% success rate in 21 patients who underwent closed reduction for dislocation of RSA with comparable outcomes to operative revisions [31].
For recurrent instability, Abdelfattah et al. recently proposed a treatment-derived classification as seen in Table 1 [10••]. The classification system broke up the causes of instability into three main mechanisms of (1) loss of compression, (2) loss of containment, and (3) impingement. These three mechanisms are further subdivided, and the evidence behind each category will be described.
Loss of Compression
Loss of compressive forces to maintain stability of the joint can be due to (1) undersized implants, (2) loss of deltoid contour, (3) loss of humeral height, (4) subscapularis deficiency, (5) acromial/scapular fracture, and (6) deltoid dysfunction. This loss of compression can result in laxity of prosthetic components, which can lead to component gapping and subsequent instability or dislocation [31].
Loss of Compression: Undersized Implants
Increasing deltoid tension and compression across the shoulder predominantly revolves around three basic methods for increasing stability: (1) lateralizing and/or upsizing the glenosphere, (2) use of constrained polyethylene insert, and (3) distalizing the humerus by increasing the polyethylene thickness and/or the thickness of the humeral tray [13••, 14]. It is seldomly required to explant a well-fixed humeral stem or baseplate during initial revision even if implant position is not optimal, unless there is excessive humeral shortening or humeral medialization [13••, 33].
For an unstable RSA with less than 15 mm of humeral shortening, Chae et al. suggested that the primary problem was undersized implants and recommended the use of thicker polyethylene liners, a metallic spacer, or placement of a larger glenosphere [13••, 33]. Clinical outcomes with use of this cutoff are promising. Boileau et al. found that 11 of 16 patients undergoing revision RSA for instability showed evidence of humeral shortening prior to revision [33]. In their study, 3 patients of the 11 with evidence of humeral shortening had humeral shortening < 15 mm. These patients underwent revision with a metallic spacer and a thicker polyethylene liner resulting in “restoration of contralateral humeral length and a stable shoulder” though no specific outcome measures for this sub-cohort of patients were included [33]. Cheung et al. also demonstrated good patient satisfaction and moderate to good post-operative ASES scores in 5 patients who underwent revision RSA with polyethylene exchange [11].
Another metric used to assess implant size in Chae et al.’s approach to instability was humeral medialization [13••, 17, 33]. A biomechanic study examining 6 RSAs implanted in fresh frozen cadaveric upper extremities showed a direct correlation between increased force needed for anterior dislocation and increased glenoid lateral offset from 0 to + 15 mm [34]. For unstable RSAs with less than 15 mm of humeral medialization, Chae et al. hypothesized that undersized implants were the root cause of instability, and recommended the use of a larger glenosphere with or without additional lateralization [13••, 33]. Both implant stability and clinical outcome scores have shown improvement with a combination of upsized glenospheres with or without glenoid lateralization and adding a constrained liner.
With regard to liner constraint, most systems use semi-constrained liners but high mobility (lower constraint) and constrained liners are options which vary the socket depth as seen in Fig. 2. Some systems will alternatively elevate the lip angle from 60 to 65° in their constrained liners [35]. Constrained liners have a deeper concavity and higher peripheral rim theorized to provide more intrinsic stability to the construct at the cost of higher articular contact stresses and decreased range of motion [36]. Biomechanical models have supported this stability claim with use of a more constrained humeral socket showing increased force to dislocate of 140.8 N (88.0%), 109.6 N (66.3%), and 70.5 N (35.9%) for superior, posterior, and anterior loading, respectively [37]. However, cadaveric studies comparing standard-, low-, and high- (retentive) polyethylene liners show no difference in joint load during active abduction with small but significant differences in passive external rotation between low- and high-constraint inserts (57° ± 26° and 51° ± 27° respectively) [38]. Small cohort clinical studies have shown good improvement in patient reported outcome scores and range of motion without recurrent instability following revision RSA with constrained liners [39]. Constrained inserts should be used judiciously in the management of patients during revision for instability given their negative effect on shoulder range of motion [13••].
Image adapted from Abdulla et al. Socket depth variations in high mobility, semi-constrained, and constrained liners [2]
Loss of Compression: Loss of Humeral Height or Deltoid Contour
Significant loss of humeral height (> 15 mm), excessive medialization of the humerus (> 15 mm), and loss of deltoid contour from proximal bone absorption of the tuberosities present additional challenges to revision that may warrant full revision of either the humeral or glenoid components with possible use of bone allograft to restore the deltoid wrapping effect [13••, 17, 40, 41]. In Boileau et al.’s analysis of 11 of 16 patients undergoing revision RSA for instability with evidence of humeral shortening, 8 patients had shortening > 15 mm and subsequently underwent removal of prosthesis with re-implantation of a longer stem with good functional improvement [17].
The use of prosthesis-proximal humerus bone allograft (Fig. 3) has also been shown to both relieve pain and improve function in patients with significant humeral height or proximal bone resorption of the tuberosities [40, 42, 43]. Chacon et al. evaluated the use of prosthesis-proximal humeral bone allograft in 25 patients with a mean humeral height loss of 53.6 mm who underwent revision RSA with minimum 2-year follow-up [40]. They found significant improvement in average clinical outcomes and good incorporation of the allograft in the diaphyseal region in 76% of patients [40].
Images adapted from Chacon et al. A Sawbones model of the prepared proximal humeral allograft (a). Immediate post-operative (b) and last available radiographs (c) demonstrating incorporation at the allograft-bone junction in both the metaphyseal region and the diaphyseal region (arrow) [40]
Management of excessive medialization (> 15 mm) can be similarly approached by use of a larger glenosphere with lateral offset. If implants alone are unable to achieve adequate lateralization, removal of the baseplate and placement of bone graft under the glenoid baseplate can further lateralize the glenoid construct [13••]. Despite computer-simulated models showing substantially increased muscle tension with 10-mm bone lateralizing glenoid bone graft, research of its use is limited and warrants further investigation [41].
Loss of Compression: Subscapularis Deficiency
The role of the subscapularis tendon in providing stability for RSA has been heavily debated. Biomechanical studies have demonstrated improved stability of the joint with subscapularis repair while clinical studies are mixed with some showing no significant difference in complications, dislocation, range of motion, strength and patient reported outcome scores in patients undergoing subscapularis repair vs tenotomy [21, 26, 44, 45]. While these studies show low rates of instability regardless of repair, other clinical studies display a different picture. Trappey et al. reported that patients with an irreparable subscapularis tendon had a 12% rate of instability compared with less than 1% in patients with a repairable subscapularis tendon [46]. The relative risk of post-operative dislocation has been reported to be 1.90 [95% CI 1.61–2.23] after RSA in patients with an irreparable subscapularis tendon compared with those with a repaired tendon [25]. Most recently, Cheung et al. showed that successful subscapularis repair was independently associated with a decreased odds of post-operative instability 2 years after RSA [11]. Continued analysis of the role of the subscapularis tendon is required given the significant controversy that remains in the literature.
Loss of Compression: Acromial/Scapular Fractures and Periprosthetic Fractures
Compressive forces are vital for maintaining joint stability. Unfortunately, excessive tensioning of the deltoid can lead to acromial and scapular stress fractures which have a prevalence of 3.1–10% in patients undergoing RSA [5•]. The management of these fractures is still debated. There have been variable results following ORIF of acromial and scapular stress fractures, including high rates of malunion or non-union, and decreased functional outcomes [5•]. In a survey of 54 orthopedic surgeons, Hamid et al. found that 75% of surgeons treat acromion fractures conservatively, 22% with ORIF, and 3% with revision RSA [47]. Non-operative management of acromial and scapular spine fractures consists of shoulder immobilization with activity modification and cessation of physical therapy for at least 6 weeks [5•]. Union rates for non-operative management of acromion and scapular fractures range from 50 to 57% with good improvement in clinical outcomes compared with baseline [5•,32, 48, 49]. To determine those that may benefit from operative management, Crosby et al. developed a classification system for the management of scapular fractures that algorithmically decides treatment based on fracture location (Fig. 4) [15].
Classification of acromial fractures from Levy et al. Type I—small fractures of the anterior acromion; type II—fractures through the anterior acromion just posterior to AC joint; type III—fractures of the posterior acromion or scapular spine [52]
Non-operative treatment is recommended for type I fractures of the anterior acromion [15]. Type II fractures are generally associated with varying severities of AC joint arthrosis which is thought to contribute to the stress fracture due to the inability of the stiff AC joint to dissipate forces across the acromion [15]. Therefore, with stable type II fractures, Crosby et al. recommend management with AC joint resection [15].
Unstable type II and all type III fractures involve more of the deltoid origin which increases risks for malunion or non-union and worsening deltoid function. For this reason, ORIF is more heavily considered with improved clinical outcomes following rigid plate fixation compared with tension band fixation [5•, 50, 51]. Camarada et al. and Rouleau et al. demonstrated fracture union and good clinical outcomes with the use of a mesh plate and perpendicular 90/90 small fragment locking plate configurations for operative treatment of displaced scapular spine fractures [53, 54]. Despite these promising results, this classification system has been shown to have mixed inter-observer reliability [5•, 52, 55]. Non-operative management should still be considered for all fracture types as there is limited evidence to show a clinically significant decreased time to union or improved clinical outcomes compared with non-operative treatment [5•].
Loss of Compression: Deltoid Dysfunction
Deltoid dysfunction can be described as a notable laxity or clinical instability with no other obvious cause, and a relative oversizing of implants [10••]. Deltoid dysfunction can arise from axillary nerve palsies, cervical radiculopathy, or poor muscle quality from fatty infiltration, rupture, or atrophy [10••]. These cases are often seen after multiple revisions and are challenging to manage with the highest recurrent rates of instability [10••]. Management techniques remain limited as this classification subcategory currently remains more of a diagnosis of exclusion.
Loss of Containment
Loss of containment is the failure of the prosthetic articulation which allows for arm elevation. It is predominantly caused by eccentric inferior or posterior polyethylene wear that can be result of prosthesis design, progressive alteration in deltoid tensioning, or chronic impingement with the scapular border [10••, 56,57,58]. The mainstay of treatment for loss of containment is polyethylene exchange with standard or retentive liners. The design of RSA is thought to produce a greater amount of polyethylene wear as compared with anatomic shoulder arthroplasty due to the larger surface area of contact. The definition of significant wear is still under investigation [56]. Computational models by Terrier et al. predicted an average polyethylene wear of 44.6 mm3 in 1 year which was four times the anatomic model wear of 8.4 mm3 annually [59]. Clinical studies have not yet supported this difference. In a retrieval analysis of 7 RSAs undergoing revision at a mean of 1.9 years following implantation, Day et al. found an average wear of 2.1 mm (range 0.1–4.7 mm) which was less than suggested by computational modeling [56].
Impingement
Impingement is caused by an obstruction of the prosthetic articulation due to tuberosity malunion from fracture, heterotopic ossification, prosthetic malalignment, or large body habitus [10••]. These physical obstructions can cause the prosthetic articulation to be levered out of place. The management of this condition depends on adequate exposure to remove impinging soft tissue and bone while simultaneously upsizing the glenosphere to provide a larger arc of motion with or without use of a more constrained polyethylene liner [10••]. It is important to expose the inferior glenoid which can have scar tissue or heterotopic bone causing instability and impingement [13••, 14].
Scapular notching from prosthetic malalignment can also cause direct mechanical impingement of the humeral bone or humeral prosthesis of the inferior scapular neck. Increasing the neck shaft angle places the polyethylene cup in a more horizontal position and has been shown to increase notching-related impingement. A recent systematic review comprising over 2000 RSAs showed the rate of scapular notching was 2.83% for those with a 135° prosthesis and 16.8% for those with a 155° prosthesis though no differences in dislocation rate were noted [60]. Use of a lateral off-set and inferior positioning of the glenosphere have also been recommended and shown to decrease the incidence of scapular notching [12, 14]. Holcomb et al. recommended adding a 15° inferior tilt to the glenoid rim to limit notching and maximize compressive forces; however, more recent data has shown no clinical benefit of inferior tilt in reducing scapular notching [13••, 61,62,63].
Though rare, it has been reported that excessive retroversion of the humeral component can lead to abutment of the prosthetic humerus against the anterior glenoid neck with internal rotation [10••]. Care should be taken if revision of humeral components is necessary to restore 10–20° of humeral retroversion which is comparable with the anatomic shoulder and has been found in cadaveric studies to cause the least inferior impingement [64].
Periprosthetic Joint Infection
Periprosthetic joint infection (PJI) has a relatively low incidence ranging from 0.80 to 1.46% though is a significant burden on the medical system with median hospitalization cost of $20,007.87 in 2011 [65]. Diagnosis of infection can be challenging given the indolent nature of common infecting organisms and lower efficacy of commonly used diagnostic markers [66]. A standard work-up should include a complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein level (CRP), and aspiration with cultures. ESR and CRP have a reported sensitivity of 16–32% and 42–63% respectively in the diagnosis of shoulder PJI [66, 67].
The consensus diagnostic criteria (Tables 2 and 3) for PJI were established by the Musculoskeletal Infection Society (MSIS) and remain the most widely used criteria for diagnosing infection [66, 68••, 69]. Recently, synovial IL-6 has also been used to assist in diagnosis of PJI with a reported sensitivity of 87% and specificity of 90% when compared with alternative PJI diagnostic criteria [70,71,72,73,74]. After diagnostic confirmation, treatment with one-stage, two-stage, or “three-stage” revision is still being debated [70, 71].
Both one- and two-stage procedures have been proposed for the management of infected joint arthroplasty. One-stage management includes complete removal of components, radical excision of infected tissue with irrigation and debridement, and re-implantation of components with antibiotic-impregnated cement for humeral fixation [70, 71]. Two-stage management includes complete implant removal and placement of an antibiotic spacer with a period of IV antibiotics. The duration of IV antibiotics has varied from 10 days to 3 months depending on patient response [70, 71]. A two-stage protocol for the management of infected joint arthroplasty is standard of care for hip and knee PJI and generally considered the most accepted treatment algorithm for management of shoulder PJI [68••, 74].
Despite two-stage management being widely performed, a recent systematic review by George et al. of 6 one-stage revision studies (n = 75) and 13 two-stage revision studies (n = 142) found no significant difference in eradication rates of 94.7% and 90.8%, respectively. They also found significantly better clinical outcome scores after one-stage revision (mean Constant score = 51) compared with two-stage revision (mean Constant score = 44). One limitation acknowledged by its authors is that the study did not account for differences in patient selection, and that the vast majority of studies failed to describe the indications for their treatment [75•].
A three-stage protocol has also been described. Following explanation of implants, placement of an antibiotic spacer and a 6-week course of antibiotics, Zhang et al. described an intermediate stage open biopsy, to confirm eradication of infection, prior to the second stage of a two-stage revision for management of shoulder PJI [74]. In their study, 22% of patients had evidence of persistent infection following an open biopsy, which were treated with subsequent rounds of I&D prior to definitive shoulder arthroplasty. Propionibacterium acnes was the most common bacteria found in 75% of patients with persistently positive cultures. Utilizing this three-stage process, all 18 patients included showed no signs of recurrent infection at 24 months with an average ASES score of 71 [74]. The management of prosthetic joint infections is still controversial with varying methods outlined in the literature. Surgeons must take multiple factors into account when determining treatment protocols including patient comorbidities, the risk of missing subclinical indolent infections, longer hospital stays with more aggressive treatment, and the risk of multiple operative interventions.
Periprosthetic Humerus Fracture
Periprosthetic humerus fractures can occur intraoperatively and post-operatively. Post-operative fractures have been reported to have a prevalence of 1.0–3.0% [76•]. A classification of periprosthetic humeral fractures has recently been proposed that takes into account the type of prosthesis, location of fracture, type of glenoid resurfacing, quality of the rotator cuff, height of the tuberosities, and whether the prosthesis was stable or loose [76•, 77]. Figure 5 shows the portion of this classification system focused on periprosthetic fractures of the RSA prosthesis. Similar to the Vancouver classification for periprosthetic fractures of the hip, fractures of the proximal stem are more stable and can be treated conservatively while fractures within the stem metaphysis or diaphysis are more likely unstable and should be treated operatively [76•, 78]. Unstable fractures are addressed with revision to a longer stem with or without ORIF for further stability of the fracture [76•, 79].
Kirchhoff et al. did a validation of this algorithmic approach with 8 patients undergoing revision RSA (4 ORIF, 2 ORIF with augmentation, and 2 long stem humeral component implantation) all with good radiographic and clinical outcomes at 3 and 12 months post-op [77]. Overall union rate after surgery for periprosthetic humerus fractures is high, with one series reporting 97% union rate in 36 patients treated either with revision arthroplasty or ORIF. In the ORIF group (n = 17), fractures healed at an average of 6.8 months [80].
Glenoid Baseplate Loosening
With the initial RSA devices, inadequate fixation coupled with long lever arms led to failure rates between 11.7 and 40% [14, 23, 81]. The transition to the use of locking screws in the glenoid baseplate has significantly improved these outcomes with subsequent analysis using large diameter locking screws and an inferior tilt to the glenoid baseplate showing a 0.5% baseplate failure rate [14].
Scapular notching has also been theorized to play a potential role in baseplate loosening though more recent studies have disputed this role [14, 63, 82,83,84,85]. Using multiple regression analysis to analyze factors associated with aseptic glenoid baseplate in 202 primary or revision RSAs, Bitzer et al. found only use of peripheral non-locking screws and bone graft for glenoid bone defects were associated with baseplate failure while scapular notching, glenosphere center-of-rotation offset, patient age, and sex were not [82]. Though the rates of recurrent loosening after RSA with bone graft are mixed, ranging from 0 to 13.6%, it does remain an option for significant glenoid loss leading to < 50% baseplate coverage, a cut-off past which micromotion significantly increases [82, 86,87,88,89].
Conclusion
Reverse shoulder arthroplasty is a commonly performed procedure though complications of instability, infection, periprosthetic fracture, and glenoid baseplate loosening can necessitate revision. Adequate clinical and radiographic analysis of the patient as well as understanding of implant design, shoulder anatomy, shoulder biomechanics, and procedural approach is needed to help manage these challenging complications. Though there have been multiple studies suggesting treatment strategies to address each of these causes of failure, there is still no consensus on a standard treatment protocol. Surgeons managing these complications should have a good understanding of treatment options and outcomes to appropriately counsel their patients.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Thomas M, Bidwai A, Rangan A, Rees JL, Brownson P, Tennent D, et al. Glenohumeral osteoarthritis. Should Elb. 2016;8(3):203–14.
Berliner JL, Regalado-Magdos A, Ma CB, Feeley BT. Biomechanics of reverse total shoulder arthroplasty. J Shoulder Elb Surg. 2015;24:150–60.
Gagey O, Hue E. Mechanics of the deltoid muscle. A new approach. Clin Orthop. 2000;375:250–7.
Lädermann A, Williams MD, Melis B, Hoffmeyer P, Walch G. Objective evaluation of lengthening in reverse shoulder arthroplasty. J Shoulder Elb Surg. 2009;18:588–95.
• Mayne IP, Bell SN, Wright W, Coghlan JA. Acromial and scapular spine fractures after reverse total shoulder arthroplasty. Should Elb. 2016;8(2):90–100 Systematic review discussing the diagnosis, etiology and management of acromial and scapular spine fractures after reverse shoulder arthroplasty. They confidently conclude that there is no substantial evidence showing a clinically significant decrease in time to union or improved clinical outcomes when comparing ORIF with non-operative treatment.
Schwartz DG, Kang SH, Lynch TS, Edwards S, Nuber G, Zhang LQ, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elb Surg. 2013;22(3):357–64.
Barco R, Savvidou OD, Sperling JW, Sanchez-Sotelo J, Cofield RH. Complications in reverse shoulder arthroplasty. EFORT Open Rev. 2017;1(3):72–80.
Groh GI, Groh GM. Complications rates, reoperation rates, and the learning curve in reverse shoulder arthroplasty. J Shoulder Elb Surg. 2014;23:388–94.
Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-balland-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87:1476–86.
•• Abdelfattah A, Otto RJ, Simon P, Christmas KN, Tanner G, LaMartina J 2nd, et al. Classification of instability after reverse shoulder arthroplasty guides surgical management and outcomes. J Shoulder Elb Surg. 2018;27(4):e107–18 Reports on a treatment-based classification system for causes of instability broken up into three main mechanisms of (1) loss of compression, (2) loss of Containment, and (3) impingement.
Cheung EV, Sarkissian EJ, Sox-Harris A, Comer GC, Saleh JR, Diaz R, et al. Instability after reverse total shoulder arthroplasty. J Shoulder Elb Surg. 2018;27(11):1946–52.
Gutiérrez S, Keller TS, Levy JC, Lee WE, Luo ZP. Hierarchy of stability factors in reverse shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):670–6.
•• Chae J, Siljander M, Wiater JM. Instability in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2018;26(17):587–96 Reports on current recommendations for management of shoulder instability providing an algorithmic approach to the treatment of instability following reverse total shoulder arthroplasty.
Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439–49.
Crosby LA, Hamilton A, Twiss T. Scapula fractures after reverse total shoulder arthroplasty: classification and treatment. Clin Orthop Relat Res. 2011;469:2544.
Bohsali KI, Wirth MA, Rockwood CA. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88:2279–92.
Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elb Surg. 2005;15:527–40.
Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25:129–33.
Bufquin T, Hersan A, Hubert L, Massin P. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly:a prospective review of 43 cases with a short-term follow-up. J Bone Joint Surg (Br). 2007;89:516–20.
Chalmers PN, Rahman Z, Romeo AA, Nicholson GP. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elb Surg. 2014;23:737–44.
Clark JC, Ritchie J, Song FS, Kissenberth MJ, Tolan SJ, Hard ND, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elb Surg. 2012;21:36–41.
Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90:1244–51.
Delloye C, Joris D, Colette A, Eudier A, Dubuc JE. Mechanical complications of total shoulder inverted prosthesis [French]. Rev Chir Orthop Reparatrice Appar Mot. 2002;88:410–4.
De Wilde L, Mombert M, Van Petegem P, Verdonk R. Revision of shoulder replacement with a reversed shoulder prosthesis (Delta III): report of five cases. Acta Orthop Belg. 2001;67:348–53.
Edwards TB, Williams MD, Labriola JE, Elkousy HA, Gartsman GM, O’Connor DP. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elb Surg. 2009;18:892–6.
Friedman RJ, Flurin PH, Wright TW, Zuckerman JD, Roche CP. Comparison of reverse total shoulder arthroplasty outcomes with and without subscapularis repair. J Shoulder Elb Surg. 2017;26:662–8.
Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92:2544–56.
Seebauer L, Walter W, Keyl W. Reverse total shoulder arthroplasty for the treatment of defect arthropathy. Oper Orthop Traumatol. 2005;17:1–24.
Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicenter study of 80 shoulders. J Bone Joint Surg (Br). 2004;86:388–95.
Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89:1476–85.
Teusink MJ, Pappou IP, Schwartz DG, Cottrell BJ, Frankle MA. Results of closed management of acute dislocation after reverse shoulder arthroplasty. J Shoulder Elb Surg. 2015;24:621–7.
Teusink MJ, Otto RJ, Cottrell BJ, Frankle MA. What is the effect of postoperative scapular fracture on outcomes of reverse shoulder arthroplasty? J Shoulder Elb Surg. 2014;23:782–90.
Boileau P, Melis B, Duperron D, Moineau G, Rumian AP, Han Y. Revision surgery of reverse shoulder arthroplasty. J Shoulder Elb Surg. 2013;22:1359–70.
Henninger HB, Barg A, Anderson AE, Bachus KN, Burks RT, Tashjian RZ. Effect of lateral offset center of rotation in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elb Surg. 2012;21:1128–35.
Carpenter S, Pinkas D, Newton MD, Kurdziel MD, Baker KC, Wiater JM. Wear rates of retentive versus nonretentive reverse total shoulder arthroplasty liners in an in vitro wear simulation. J Shoulder Elb Surg. 2015;24(9):1372–9.
Gutiérrez S, Luo ZP, Levy J, Frankle MA. Arc of motion and socket depth in reverse shoulder implants. Clin Biomech (Bristol, Avon). 2009;24(6):473–9.
Clouthier AL, Hetzler MA, Fedorak G, Bryant JT, Deluzio KJ, Bicknell RT. Factors affecting the stability of reverse shoulder arthroplasty: a biomechanical study. J Shoulder Elb Surg. 2013;22:439–44.
Abdulla I, Langohr DG, Giles JW, Johnson JA, Athwal GS. The effect of humeral polyethylene insert constraint on reverse shoulder arthroplasty biomechanics. Should Elb. 2018;10(1):25–31.
Thangarajah T, Higgs D, Bayley JI, Lambert SM. Constrained fixed-fulcrum reverse shoulder arthroplasty improves functional outcome in epileptic patients with recurrent shoulder instability. World J Orthop. 2016;7(7):434.
Chacon A, Virani N, Shannon R, Levy JC, Pupello D, Frankle M. Revision arthroplasty with use of a reverse shoulder prosthesis-allograft composite. J Bone Joint Surg Am. 2009;91:119–27.
Roche CP, Diep P, Hamilton M, Crosby LA, Flurin PH, Wright TW, et al. Impact of inferior glenoid tilt, humeral retroversion, bone grafting, and design parameters on muscle length and deltoid wrapping in reverse shoulder arthroplasty. Bull Hosp Jt Dis. 2013;71:284–93.
Kassab M, Dumaine V, Babinet A, Ouaknine M, Tomeno B, Anract P. Twenty nine shoulder reconstructions after resection of the proximal humerus for neoplasm with mean 7-year follow-up. Rev Chir Orthop Reparatrice Appar Mot. 2005;91:15–23.
Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg Am. 2007;89:292–300.
Oh JH, Shin SJ, McGarry MH, Scott JH, Heckmann N, Lee TQ. Biomechanical effects of humeral neck-shaft angle and subscapularis integrity in reverse total shoulder arthroplasty. J Shoulder Elb Surg. 2014;23:1091–8.
Vourazeris JD, Wright TW, Struk AM, King JJ, Farmer KW. Primary reverse total shoulder arthroplasty outcomes in patients with subscapularis repair versus tenotomy. J Shoulder Elb Surg. 2017;26:450–7.
Trappey GJ 4th, O’Connor DP, Edwards TB. What are the instability and infection rates after reverse shoulder arthroplasty? Clin Orthop Relat Res. 2011;469:2505–11.
Hamid N, Connor PM, Fleischli JF, D’Alessandro DF. Acromial fracture after reverse shoulder arthroplasty. Am J Orthop. 2011;40:E125–9.
Hattrup SJ. The influence of postoperative acromial and scapular spine fractures on the results of reverse shoulder arthroplasty. Orthopedics. 2010;33.
Lópiz Y, Rodríguez-González A, García-Fernández C, Marco F. Scapula insufficiency fractures after reverse total shoulder arthroplasty in rotator cuff arthropathy: what is their functional impact? Rev Esp Cir Ortop Traumatol. 2015;59(5):318–25.
Wahlquist TC, Hunt AF, Braman JP. Acromial base fractures after reverse total shoulder arthroplasty: report of five cases. J Shoulder Elb Surg. 2011;20:1178–83.
Walch G, Mottier F, Wall B, Boileau P, Molé D, Favard L. Acromial insufficiency in reverse shoulder arthroplasties. J Shoulder Elb Surg. 2009;18:495–502.
Levy JC, Anderson C, Samson A. Classification of postoperative acromial fractures following reverse shoulder arthroplasty. J Bone Joint Surg Am. . https://doi.org/10.1007/s12178-020-09602-6.
Camarada L, Phadnis J, Clitherow HD, et al. Mesh plates for scapula fixation. Tech Should Surg. 2015;16:79–84.
Rouleau DM, Gaudelli C. Successful treatment of fractures of the base of the acromion after reverse shoulder arthroplasty: case report and review of the literature. Int J Shoulder Surg. 2013;7:149–52.
Otto RJ, Virani NA, Levy JC, Nigro PT, Cuff DJ, Frankle MA. Scapular fractures after reverse shoulder arthroplasty: evaluation of risk factors and the reliability of a proposed classification. J Shoulder Elb Surg. 2013;22:1514–21.
Day JS, MacDonald DW, Olsen M, Getz C, Williams GR, Kurtz SM. Polyethylene wear in retrieved reverse total shoulder components. J Shoulder Elb Surg. 2012;21:667–74.
Kohan EM, Chalmers PN, Salazar D, Keener JD, Yamaguchi K, Chamberlain AM. Dislocation following reverse total shoulder arthroplasty. J Shoulder Elb Surg. 2017;26:1238–45.
Nam D, Kepler CK, Nho SJ, Craig EV, Warren RF, Wright TM. Observations on retrieved humeral polyethylene components from reverse total shoulder arthroplasty. J Shoulder Elb Surg. 2010;19(7):1003–12.
Terrier A, Merlini F, Pioletti DP, Farron A. Comparison of polyethylene wear in anatomical and reversed shoulder prostheses. J Bone Joint Surg (Br). 2009;91:977–82.
Erickson BJ, Frank RM, Harris JD, Mall N, Romeo AA. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elb Surg. 2015;24:988–93.
Edwards TB, Trappey GJ, Riley C, O’Connor DP, Elkousy HA, Gartsman GM. Inferior tilt of the glenoid component does not decrease scapular notching in reverse shoulder arthroplasty: results of a prospective randomized study. J Shoulder Elb Surg. 2012;21:641–6.
Holcomb JO, Cuff D, Petersen SA, Pupello DR, Frankle MA. Revision reverse shoulder arthroplasty for glenoid baseplate failure after primary reverse shoulder arthroplasty. J Shoulder Elb Surg. 2009;18(5):717–23.
Kempton LB, Balasubramaniam M, Ankerson E, Wiater JM. A radiographic analysis of the effects of prosthesis design on scapular notching following reverse total shoulder arthroplasty. J Shoulder Elb Surg. 2011;20:571–6.
Berhouet J, Garaud P, Favard L. Evaluation of the role of glenosphere design and humeral component retroversion in avoiding scapular notching during reverse shoulder arthroplasty. J Shoulder Elb Surg. 2014;23(2):151–8.
Padegimas EM, Maltenfort M, Ramsey ML, Williams GR, Parvizi J, Namdari S. Periprosthetic shoulder infection in the United States: incidence and economic burden. J Shoulder Elb Surg. 2015;24(5):741–6.
Frangiamore SJ, Saleh A, Kovac MF, Grosso MJ, Zhang X, Bauer TW, et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. JBJS Am. 2015;97:63–70.
Piper KE, Fernandez-Sampedro M, Steckelberg KE, Mandrekar JN, Karau MJ, Steckelberg JM, et al. C-reactive protein, erythrocyte sedimentation rate and orthopedic implant infection. PLoS One. 2010;5(2):e9358.
•• Fink B, Sevelda F. Periprosthetic joint infection of shoulder arthroplasties: diagnostic and treatment options. Biomed Res Int. 2017;2017:4582756 Presents an overview of diagnostic and therapeutic options for periprosthetic joint infection of shoulder athroplasties including treatment of early infection as well one-stage and two-stage procedures for treatment of late infections.
Parvizi J, Gehrke T. Definition of periprosthetic joint infection. J Arthroplast. 2014;29(7):1331.
Beekman P, Katusic D, Berghs B, Karelse A, de Wilde I. One-stage revision for patients with a chronically infected reverse total shoulder replacement. J Bone Joint Surg (Br). 2010;92(B):817–22.
Klatte t, Junghans K, Al-Khateeb h, et al. Single-stage revision for peri- prosthetic shoulder infection: outcomes and results. Bone Joint J. 2013;95-B:391–5.
Ortmaier R, Resch H, Hitzl W, et al. Treatment strategies for infection after reverse shoulder arthroplasty. Eur J Orthop Surg Traumatol. 2014;24:723–31.
Strickland JP, Sperling JW, Cofield RH. The results of two-stage re- implantation for infected shoulder replacement. J Bone Joint Surg (Br). 2008;90-B:460–5.
Zhang AL, Feeley BT, Schwartz BS, Chung TT, Ma CB. Management of deep postoperative shoulder infections: is there a role for open biopsy during staged treatment? J Shoulder Elb Surg. 2015;24(1):e15–20.
• George DA, Volpin A, Scarponi S, Haddad FS, Romanò CL. Does exchange arthroplasty of an infected shoulder prosthesis provide better eradication rate and better functional outcome, compared to a permanent spacer or resection arthroplasty? a systematic review. BMC Musculoskelet Disord. 2016;17(1):52 Systematic review comparing eradication rates and functional outcomes scores of single- or two-stage exchange arthroplasty, use of permanent spacer, or resection arthroplasty for management periprosthetic shoulder infection.
• Gebrelul A, Green A, Schacherer T, Khazzam M. Periprosthetic humerus fractures: classification, management, and review of the literature. Ann Joint. 2018;3(6) Reports on the Kirchhoff classification system for periprosthetic shoulder fractures and provides a treatment algorithm for the best predictable functional outcome using this classification system.
Kirchhoff C, Beirer M, Brunner U, Buchholz A, Biberthaler P, Crönlein M. Validation of a new classification for periprosthetic shoulder fractures. Int Orthop. 2018;1:1–7.
Marsland D, Mears SC. A review of periprosthetic femoral fractures associated with total hip arthroplasty. Geriatr Orthop Surg Rehabil. 2012;3(3):107–20.
Kirchhoff C, Kirchhoff S, Biberthaler P. Classification of periprosthetic shoulder fractures. Unfallchirurg. 2016;119:264–72.
Andersen JR, Williams CD, Cain R, Mighell M, Frankle M. Surgically treated humeral shaft fractures following shoulder arthroplasty. J Bone Joint Surg Am. 2013;95:9–18.
Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency: a minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697–705.
Bitzer A, Rojas J, Patten IS, Joseph J, McFarland EG. Incidence and risk factors for aseptic baseplate loosening of reverse total shoulder arthroplasty. J Shoulder Elb Surg. 2018;27(12):2145–52.
Cazeneuve JF, Cristofari DJ. The reverse shoulder prosthesis in the treatment of fractures of the proximal humerus in the elderly. J Bone Joint Surg. British Volume. 2010;92(4):535–539.
Feeley BT, Zhang AL, Barry JJ, Shin E, Ho J, Tabaraee E, et al. Decreased scapular notching with lateralization and inferior baseplate placement in reverse shoulder arthroplasty with high humeral inclination. Int J Shoulder Surg. 2014;8:65–71.
Lévigne C, Boileau P, Favard L, Garaud P, Molé D, Sirveaux F, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elb Surg. 2008;17(6):925–35.
Formaini NT, Everding NG, Levy JC, Santoni BG, Nayak AN, Wilson C, et al. The effect of glenoid bone loss on reverse shoulder arthroplasty baseplate fixation. J Shoulder Elb Surg. 2015;24(11):e312–9.
Jones RB, Wright TW, Zuckerman JD. Reverse total shoulder arthroplasty with structural bone grafting of large glenoid defects. J Shoulder Elb Surg. 2016;25:1425–32.
Lorenzetti AJ, Streit JJ, Cabezas AF, Christmas KN, LaMartina JI, Simon P, et al. Bone graft augmentation for severe glenoid bone loss in primary reverse total shoulder arthroplasty: outcomes and evaluation of host bone contact by 2D-3D image registration. JB JS Open Access. 2017;2(3):e0015.
Wagner E, Houdek MT, Griffith T, Elhassan BT, Sanchez-Sotelo J, Sperling JW, et al. Glenoid bone-grafting in revision to a reverse total shoulder arthroplasty. JBJS. 2015;97(20):1653–60.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Alexander R Markes and Edward Cheung declare that they have no conflict of interest. C.Benjamin Ma reports research support from Zimmer, Histogenics, Samumed and Consultancy fees from Stryker, Zimmer, Conmed, Valeris outside of the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Reverse Shoulder Arthroplasty
Rights and permissions
About this article
Cite this article
Markes, A.R., Cheung, E. & Ma, C.B. Failed Reverse Shoulder Arthroplasty and Recommendations for Revision. Curr Rev Musculoskelet Med 13, 1–10 (2020). https://doi.org/10.1007/s12178-020-09602-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12178-020-09602-6