Abstract
Purpose of Review
Heart failure is a significant and growing cause of disease in the developed world with substantial cost and morbidity associated with it. This review focuses on methamphetamine-associated cardiomyopathy (MACM) as an increasingly recognized cause of heart failure.
Recent Findings
MACM is increasingly recognized worldwide. Prevalence of MACM in heart failure hospital admissions has been increasing in the western USA. Risk factors for development of MACM include male sex, hypertension, and alcohol use disorder. MACM is associated with severe left ventricular dysfunction, greater heart failure symptom burden, and younger age of presentation than non-ischemic controls. Improvement of ejection fraction has been seen with guideline-directed therapy and methamphetamine cessation. EF improvement is correlated with lack of evidence of chronicity on transthoracic echocardiography and extent of fibrosis on endomyocardial biopsy. Limited studies show white and Māori patients are most often affected, though race/ethnicity has not been shown to be an independent risk factor in the development of MACM.
Summary
MACM represents a subset of heart failure that affects predominantly young men, and tends to present with severely reduced ejection fraction and significant symptom burden. There is evidence that ejection fraction can be improved in patients without extensive cardiac fibrosis or evidence of cardiomyopathy chronicity on imaging in the setting of abstinence and medical therapy. In this context, methamphetamine use is a modifiable risk factor in the development of heart failure that would likely benefit from early intervention, though larger longitudinal studies are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methamphetamine (MA) is a widely accepted cause of cardiomyopathy (CM). This phenomenon was first reported in the late 1970s [1], and reports from around the world have since emerged documenting its presence [2,3,4,5].
MA is a sympathomimetic amine that can be smoked, inhaled, ingested, or injected and increases intrasynaptic levels of monoamines (serotonin, norepinephrine, and dopamine) [6]. This creates euphoria and stimulant effects leading to potential abuse of the drug. Cardiovascular effects have been well-documented and include tachycardia, hypertension, direct myocardial toxicity, pulmonary arterial hypertension, and neurotransmitter depletion. These phenomena can then lead to cardiovascular complications such as malignant hypertension, aortic dissection, coronary vasospasm and myocardial infarction, cardiomyopathies, and arrhythmias. Of the cardiomyopathies, 3 patterns have been identified. Dilated cardiomyopathy (DCM) is thought to be due to direct cardiotoxic effects of MA, hypertrophic CM is thought to be due to profound hypertension, and stress CM (Takotsubo or reverse Takotsubo pattern) which is thought to be due to the acute effect of catecholaminergic overload [6]. This review seeks to synthesize recent data and begin to explore racial/ethnic differences and social vulnerabilities specific to this type of CM.
The Burden of Heart Failure
The burden of disease of heart failure (HF) remains substantial worldwide. In the United States of America (USA), HF affects approximately 6.5 million adults, or 2.42% of the population, and is projected to increase to 8 million by 2030 [7]. This projected increase is driven by the increased incidence and prevalence with age [7], an aging population, and modest increases in survival rates [8]. In the UK, the prevalence has remained fairly stable over the past 10 years at 1.6% of the population, but similarly, the absolute number of HF patients has increased with the increase in population [9]. Overall lifetime risk for developing HF in the USA between the ages of 45–95 is estimated at 20–45%, and mortality rates remain high at approximately 50% at 5 years [7].
HF continues to have a significant cost burden for the USA health care system, totaling an estimated $30.7 billion in 2012 in total medical costs [10]. With such a substantial burden of disease and systemic cost, there is significant research and regulatory energy in addressing this. Much of the discussion centers around modifiable risk factors such as hypertension, diabetes, tobacco use, and obesity [11,12,13] and improving adherence to guideline-based therapies [14]. Less focus has been directed toward primordial prevention of heart failure and care of vulnerable patients, specifically those with substance use disorders.
Methamphetamine Use Globally
Substance use disorders are a global malady with generally a higher prevalence in developed nations, but certainly affect all regions of the world [15]. It is estimated that 5.5% of the global population uses drugs recreationally, and use of amphetamine-type stimulants (ATS), including MA, remains substantial with an estimated global prevalence of 0.6% [16]. Within the USA, MA began in and is still most readily available in the western costal region and Hawai’i, but is becoming more prevalent in areas where it was previously less so, such as the Midwest and the Northeast. The majority of MA is produced in Mexico and trafficked across the Southwestern border, resulting in a particularly high availability in southwestern regions [17].
Globally, MA is found most commonly in the USA data based on drug seizures, but is also prevalent in China, Thailand, and Mexico. Southeast and East Asian markets are continuing to grow with a shift toward inexpensive MA pills, a form of the drug that would presumably lower the barrier for use [16]. Prevalence of use is also high in Oceania, with last-year use having declined slightly in Australia in recent years but remains substantial at 1.1%. In New Zealand, the estimated prevalence is stable at 1% of the population, though waste water measurements and qualitative input suggests it is on the rise. Southeast Asia is also an area of rising concern, as MA is indicated as the main drug of concern in treatment, and prevalence of past-year use in countries of that region ranges from 0.5–1.1% [16]. Because MA use is a global issue with apparent increasing population usage, increased understanding of methamphetamine-associated cardiomyopathy (MACM) and its management is necessary.
Trends and Risk Factors in MACM
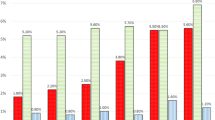
Concordant with the rise in prevalence of MA use is an increase in the prevalence of MACM. Comprehensive prevalence data remains lacking for many regions, though in the southwestern USA, a region reported to have a “high” and increasing availability of MA [17], prevalence of MACM has almost tripled from 1.8% of heart failure admissions in 2009 to 5.6% in 2014 [18•]. Another study of veterans in this same region reported an increase from 3.4 to 6.7% of MACM in heart failure admissions [19]. Other studies have found rates of 19.0–38.0% of MA use in patients admitted with HF exacerbations, albeit HF etiology was uncertain [20, 21].
Cohort data about MA use and MACM are emerging. These studies largely consist of young (ages 30–60) white men in the western USA. This likely reflects patterns of use, though male gender has been identified as an independent risk factor for development of systolic dysfunction in MACM with an OR of up to 3.1 (CI 2.1–4.6) [21, 22•]. Other risk factors for development of MACM are also emerging. For example, hypertension and alcohol use disorder were also identified as independent risk factors for severe systolic dysfunction among MA users [22•]. Biomarker data are emerging. In a work by Richards et al., screens of MA-positive emergency room patients with symptoms of heart failure showed higher age and smoking history to be associated with severely reduced EF and a higher brain natriuretic peptide (BNP) [23].
MACM Symptoms, Systolic Dysfunction, and Severity
Although clinical and research studies are linking MA with CM, the course of left ventricular (LV) dysfunction is not currently well-understood. Recent studies have begun to shed light on this relationship. Several groups note that MACM patients tend to have a severely reduced left ventricular ejection fraction (EF), either compared with non-ischemic cardiomyopathy (NICM) or MA-positive controls [19, 22•, 23, 24•]. These patients also have elevated BNP [19, 23, 24•, 25, 26••], and LV dilation [22, 24•, 25, 26••].
Significant symptom burden is also associated with MACM. A high percentage of MACM patients have New York Heart Association (NYHA) classification III/IV on admission [25, 26••]. Other work indicates improvement in symptom burden in those persons who abstained from MA with worsening heart failure among individuals who continued MA use [18•, 26••].
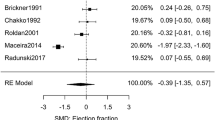
Cardiomyopathy trajectory data are mixed. Kueh et al. evaluated a group of MACM patients with severely reduced EF on presentation (22.0 ± 8.0%) and found modest improvement in systolic function on median follow-up of 17.7 months (IQR 7.4–35.8), but overall EF remained severely impaired [24, 25]; results were not stratified by ongoing MA use. By contrast, other research demonstrates EF improvement, especially with the cessation of MA use [18•, 26••]. Indeed, Schurer et al. demonstrated an EF improvement to 43.0 ± 13.0% from a baseline of 19.0 ± 6.0% in abstinent MACM patients, compared with no significant difference in the group with continued MA use. Table 1 summarizes available EF data and trajectory where available.
Factors predicting LVEF recovery are forthcoming. Voskoboinik et al. determined predictors of early LVEF recovery to include “lack of transthoracic echocardiographic (TTE) evidence of chronicity,” such as a smaller left ventricular and left atrial cavities, and reverse Takotsubo pattern, as well as shorter duration of MA use [27•], while Schurer et al. found only cardiac fibrosis was independently negatively associated with LVEF at follow-up [26••].
Although the most commonly described pattern of MACM is dilated CM [22•, 24, 25, 26••, 27•], one group also noted a reverse Takotsubo (RT) pattern in a minority of MACM patients. The latter consists of apical preservation with basal-mid segment hypokinesis. While the entire cohort was predominantly male, the RT phenotype in this study was observed primarily among females (83%). This group was followed for early recovery of LVEF, and within a 6-week follow-up period, all patients with the RT phenotype experienced LVEF recovery (compared with 32% overall). This phenotype is still poorly understood, but in addition to having elevated cardiac biomarkers (troponin and creatine kinase) and dynamic ECG changes, these patients tended to have a shorter duration of MA use [27•].
In summary, MACM appears to present with a predominantly dilated pattern of CM, frequently with severe systolic dysfunction. In addition, cessation of MA appears to result in improvement in LVEF, albeit less frequently in normalization. Predictors of improvement in LVEF on follow-up include lack of evidence of chronicity on initial TTE and lack of fibrosis on biopsy. Data are emerging regarding mortality statistics associated with MACM.
Histopathology and Radiology
Animal studies have previously demonstrated histopathologic changes such as cardiac myocytolysis, eosinophilic degeneration, intracellular vacuolization, altered calcium homeostasis, and contraction band necrosis and fibrosis in the myocardia of animals exposed to chronic MA, with gradual recovery of these changes with use cessation of MA [28,29,30,31,32]. Human analyses are limited, but post-mortem data largely corroborate changes noted in animal studies, while additionally showing increased coronary atherosclerosis [31]. Cardiovascular pathology is noted in up to 68.0% of autopsy cases for MA-associated death, including myocardial fiber hypertrophy, perivascular fibrosis, and acute myocardial infarction [33].
Endomyocardial biopsy (EMB) offers insights into histological changes in living patients. Schurer et al. analyzed a cohort of patients with MACM and EF < 40% utilizing EMB in patients. Histological findings included markers of inflammation, fibrosis, and myocyte damage. Severe inflammation was equally distributed over the duration of MA use, but fibrosis demonstrated a relationship with duration of MA use—more severe fibrosis being was associated with longer duration of MA exposure. Compared with control dilated cardiomyopathy (DCM) patients, MACM patients showed more severe inflammation [26••]. The presence of inflammation as opposed to fibrosis early on in MA use invokes a plausible mechanism for the recovery of LV function. Presence of fibrosis on EMB was shown to be a predictor of poor LVEF outcome [26••].
While EMB offers insights into the mechanism and possible prognostication of impaired LV function in MACM, it is an invasive procedure with potential risks. Cardiac magnetic resonance imaging (CMR) is an alternative non-invasive modality to assess the presence and extent of fibrosis. Thus far, studies using CMR are few, though reports show potential promise in regard to the absence of myocardial scar with improved chance of LVEF recovery in MACM in patients who abstain from MA use [34, 35]. While further studies are needed, though these case reports support the observation of a relationship between presence of fibrosis and sustained systolic dysfunction using a more available and non-invasive modality.
Women in MACM
To date, most studies suggest a male predominance of MACM [18•, 21, 22•, 24•, 25, 26••, 27•]. This could be in part due to patterns of use in the USA—though globally, MA use is felt to be fairly evenly distributed between men and women [16]. For example, one study found male gender to be an independent predictor of developing MACM with an OR of 3.791 (2.508–5.730) [22•]. Data on MA-associated pulmonary arterial hypertension (PAH) are mixed. Some studies report no significant association with gender [36], while others report female sex as a strong independent predictor of development of MA-associated PAH [22•].
Race and Ethnicity in MACM
Although there are significant differences in the prevalence and incidence of heart failure by race and ethnicity, data regarding the influence of race and ethnicity in MACM are relatively lacking. Epidemiologic data for HF in general is primarily limited to high-income countries, and in the USA, blacks have the highest incidence of new heart failure, are affected at younger ages [37], and have higher HF readmission rates [38]. These differences are largely driven by differences in cardiovascular risk factors such as hypertension, diabetes, and obesity, as well as socioeconomic factors compared with other groups [39]. Hispanic/LatinX Americans have the second highest incidence of HF [40]. Racial differences in mortality data are mixed, though overall HF-related mortality is higher in blacks [12, 41].
Given these documented racial and ethnic differences in HF incidence and prevalence, it begs the question whether similar patterns are noted for MACM by race/ethnicity. Racial and ethnic data for MACM are beginning to emerge. Multiple groups have failed to find statistical significance for race/ethnicity as a predictor of severity in MACM [18•, 21, 22•], though the majority of patients in these studies were Caucasian persons. In the USA, racial/ethnic MA use data suggests use prevalence in descending order as follows: Native American, white, Hispanic/LatinX, and African American [16]. Data from New Zealand suggests that persons of indigenous Māori ethnicity are over-represented among MACM patients [24•, 25] compared with matched non-ischemic cardiomyopathy controls [24•]. In the Western USA, Native Hawaiian and other Pacific Islanders (NHOPI) are reported to develop HF 10 years earlier than the general population [42]. One study showed that among NHOPI patients, MA use was 23%, and that MA independently predicted HF in this population [43].
Summary and Management Challenges for MACM Patients
MA is an established cause of CM, and MACM is becoming increasingly recognized worldwide. MA use is stabilizing in some areas such as the USA and Australia, but expanding in others such as East and Southeast Asia. Use is also shifting toward more chronic use, especially in the USA [16]. Substance use, and in particular MA use is an independent predictor of emergency room visits and hospital readmissions [44]. Because MACM studies show decreased rates of follow-up in patients who continue to use MA [24•,25, 26••], the role MA plays in affecting HF severity and resource utilization makes this an issue worthy of attention among drug users.
Available studies demonstrate MACM is associated with significant disease severity and morbidity such as severely reduced LVEF, LV dilation, elevated BNP and creatinine, and impaired NYHA functional class [18•, 20, 21, 22•, 23, 24•, 25, 26••, 27•, 42] that is growing in prevalence and representing at least 6.7% of HF admissions in the Western USA [18•, 19]. MACM cohorts are comprised predominantly of young white men in the Western USA [18•, 20, 21, 22•, 23], and young Māori men in New Zealand [24•, 25], though increased use in different areas globally will likely increase understanding of racial/ethnic differences in this disease. Encouragingly, some studies indicate potential for EF recovery with early cessation of MA and medical therapy [18•, 26••, 27•], particularly in patients with short duration of use and lack of myocardial fibrosis [26••], suggesting that early intervention might be impactful.
Identification, engagement, and treatment of patients with MACM remain challenging. Psychosocial interventions such as Contingency Management (CM) and Cognitive Behavioral Therapy (CBT) for the treatment of MA use disorder have limited efficacy and the effects are generally short-lived [45]. Multiple medications are under investigation and may show some promise (bupropion, topiramate, naltrexone, and mirtazapine), but none is currently approved by the FDA for this use [46].
Patients with substance use disorders have strong comorbid association with psychosocial vulnerabilities such as mood disorders, low socioeconomic status (SES), and homelessness [47,48,49] as well as poorer medical adherence rates in some populations [50, 51]. These findings are also observed in MACM patients [19, 24•, 25, 26••]. Therefore, a multidisciplinary approach to addressing social vulnerability, comorbid substance use disorders, and mental health concerns is required.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kalant H, Kalant OJ. Death in amphetamine users: causes and rates. Can Med Assoc J. 1975;112(3):299–304.
Hong R, Matsuyama E, Nur K. Cardiomyopathy associated with the smoking of crystal methamphetamine. JAMA. 1991;265(9):1152–4.
Ayres PR. Amphetamine cardiomyopathy. Ann Intern Med. 1983;98(1):110.
Segawa T, Arita Y, Ogasawara N, Hasegawa S. Hypertensive heart disease associated with methamphetamine abuse. J Cardiol Cases. 2019;19(2):47–50.
Sadeghi R, Agin K, Taherkhani M, Najm-Afshar L, Nelson LS, Abdollahi M, et al. Report of methamphetamine use and cardiomyopathy in three patients. Daru. 2012;20(1):20.
Paratz ED, Cunningham NJ, Macisaac AI. The cardiac complications of methamphetamines. Heart Lung Circ. 2016;25(4):325–32.
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–66.
Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. 2011;306(15):1669–78.
Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391(10120):572–80.
Jackson SL, Tong X, King RJ, Loustalot F, Hong Y, Ritchey MD. National Burden of heart failure events in the United States, 2006 to 2014. Circ Heart Fail. 2018;11(12):e004873.
Dunlay SM, Weston SA, Jacobsen SJ, Roger VL. Risk factors for heart failure: a population-based case-control study. Am J Med. 2009;122(11):1023–8.
Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101(7):1016–22.
Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168(19):2138–45.
Ruppar TM, Cooper PS, Mehr DR, Delgado JM, Dunbar-Jacob JM. Medication adherence interventions improve heart failure mortality and readmission rates: systemic review and meta-analysis of controlled trials. J Am Heart Assoc. 2016;5(6):e002606. https://doi.org/10.1161/JAHA.115.002606.
Whiteford HA, Ferrari AJ, Degenhardt L, Feigin V, Vos T. The global burden of mental, neurological and substance use disorders: an analysis from the Global Burden of Disease Study 2010. PLoS One. 2015;10(2):e0116820.
World Drug Report 2019 (United Nations publication, Sales No. E.19.XI.8). Booklet 4 Stimulants. p35–58. Accessed 10/20/2019.
2018 National drug threat assessment. US Department of Justice Drug Enforcement Administration. p59–76. Accessed 10/20/2019.
• Sliman S, Waalen J, Shaw D. Methamphetamine-associated congestive heart failure: increasing prevalence and relationship of clinical outcomes to continued use or abstinence. Cardiovasc Toxicol. 2016;16(4):381–9 Sliman et al. demonstrated in a 6-year retrospective review of 3705 heart failure admissions that prevalence of methamphetamine-associated congestive heart failure increased over that time from 1.8 to 5.6% of total heart failure admissions. For patients for whom longitudinal data was available, the authors determined that methamphetamine cessation was associated with improvement in NYHA functional class as well as ejection fraction.
Nishimura M, Ma J, Fox S, Toomu A, Mojaver S, Juang DK, et al. Characteristics and outcomes of methamphetamine abuse among veterans with heart failure. Am J Cardiol. 2019;124(6):907–11.
Kiel RG, Ambrose J, Khatri B, et al. The prevalence and presentation of methamphetamine associated cardiomyopathy: a single center experience. J Am College Cardiol. 2015;65(10S):A942.
Neeki MM, Kulczycki M, Toy J, Dong F, Lee C, Borger R, et al. Frequency of methamphetamine use as a major contributor toward the severity of cardiomyopathy in adults ≤50 years. Am J Cardiol. 2016;118(4):585–9.
• Zhao SX, Kwong C, Swaminathan A, Gohil A, Crawford MH. Clinical Characteristics and outcome of methamphetamine-associated pulmonary arterial hypertension and dilated cardiomyopathy. JACC Heart Fail. 2018;6(3):209–18 Zhao et al. sought to identify risk factors for the development of methamphetamine-associated cardiomyopathy through a retrospective analysis of 296 patients with MACM and 356 methamphetamine-using controls with structurally normal hearts. They identified male sex, alcohol use disorder, and hypertension as independent risk factors in the development of MACM.
Richards JR, Harms BN, Kelly A, Turnipseed SD. Methamphetamine use and heart failure: prevalence, risk factors, and predictors. Am J Emerg Med. 2018;36(8):1423–8.
• Wang TKM, Kueh SA, Sutton T, Gabriel R, Lund M, Looi JL. Poor outcomes in methamphetamine-associated cardiomyopathy-a growing health issue in New Zealand. N Z Med J. 2019;132(1502):55–66 Wang et al. retrospectively reviewed 13 years of MACM admissions compared with age-matched non-ischemic cardiomyopathy patients and found increased severity of heart failure on presentation (measured by reduced ejection fraction and prevalence of cardiogenic shock), as well as a trend toward increased mortality and rehospitalization in the MACM group. They also noted that the majority of MACM patients were of Māori ethnicity, whereas other groups have found the predominant racial/ethnic group in MACM patients to be white.
Kueh SA, Gabriel RS, Lund M, et al. Clinical characteristics and outcomes of patients with amphetamine-associated cardiomyopathy in South Auckland, New Zealand. Heart Lung Circ. 2016;25(11):1087–93.
•• Schürer S, Klingel K, Sandri M, et al. Clinical characteristics, histopathological features, and clinical outcome of methamphetamine-associated cardiomyopathy. JACC Heart Fail. 2017;5(6):435–45 Schurer et al. performed a retrospective analysis of 30 patients hospitalized with MACM with available ejection fraction and endomyocardial biopsy data. They found that extent of myocardial fibrosis on biopsy positively correlated with duration of methamphetamine use. Prospective follow-up analysis showed that EF improvement was seen in patients who abstained from methamphetamine use and adhered to medical therapy, and extent of fibrosis was independently associated with EF improvement.
• Voskoboinik A, Ihle JF, Bloom JE, Kaye DM. Methamphetamine-associated cardiomyopathy: patterns and predictors of recovery. Intern Med J. 2016;46(6):723–7 Voskoboinik et al retrospectively analyzed 20 patients diagnosed with MACM and determined a reverse takutsubo pattern of cardiomyopathy, smaller left atrium and left ventricle, and shorter duration of methamphetamine use are associated with improvement in EF on follow-up.
Islam MN, Kuroki H, Hongcheng B, Ogura Y, Kawaguchi N, Onishi S, et al. Cardiac lesions and their reversibility after long term administration of methamphetamine. Forensic Sci Int. 1995;75(1):29–43.
Yi SH, Ren L, Yang TT, Liu L, Wang H, Liu Q. Myocardial lesions after long-term administration of methamphetamine in rats. Chin Med Sci J. 2008;23(4):239–43.
Islam MN, Jesmine K, Kong sn Molh A, Hasnan J. Histopathological studies of cardiac lesions after long term administration of methamphetamine in high dosage--part II. Leg Med (Tokyo). 2009;11(Suppl 1):S147–50.
Kaye S, Darke S, Duflou J, Mcketin R. Methamphetamine-related fatalities in Australia: demographics, circumstances, toxicology and major organ pathology. Addiction. 2008;103(8):1353–60.
Jafari Giv M. Exposure to amphetamines leads to development of amphetamine type stimulants associated cardiomyopathy (ATSAC). Cardiovasc Toxicol. 2017;17(1):13–24.
Akhgari M, Mobaraki H, Etemadi-aleagha A. Histopathological study of cardiac lesions in methamphetamine poisoning-related deaths. Daru. 2017;25(1):5.
Lopez JE, Yeo K, Caputo G, Buonocore M, Schaefer S. Recovery of methamphetamine associated cardiomyopathy predicted by late gadolinium enhanced cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2009;11:46.
Pujol-lópez M, Ortega-paz L, Flores-umanzor EJ, Perea RJ, Bosch X. Cardiac magnetic resonance as an alternative to endomyocardial biopsy to predict recoverability of left ventricular function in methamphetamine- associated cardiomyopathy. JACC Heart Fail. 2017;5(11):853–4.
Zamanian RT, Hedlin H, Greuenwald P, Wilson DM, Segal JI, Jorden M, et al. Features and outcomes of methamphetamine-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2018;197(6):788–800.
Bibbins-domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360(12):1179–90.
Chang PP, Wruck LM, Shahar E, Rossi JS, Loehr LR, Russell SD, et al. Trends in hospitalizations and survival of acute decompensated heart failure in four US communities (2005-2014): ARIC study community surveillance. Circulation. 2018;138(1):12–24.
Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168(19):2138–45.
Blair JE, Huffman M, Shah SJ. Heart failure in North America. Curr Cardiol Rev. 2013;9(2):128–46.
Rathore SS, Foody JM, Wang Y, Smith GL, Herrin J, Masoudi FA, et al. Race, quality of care, and outcomes of elderly patients hospitalized with heart failure. JAMA. 2003;289(19):2517–24.
Yeo KK, Wijetunga M, Ito H, Efird JT, Tay K, Seto TB, et al. The association of methamphetamine use and cardiomyopathy in young patients. Am J Med. 2007;120(2):165–71.
Mau MK, Seto TB, Kaholokula JK, Howard B, Ratner RE. Association of modifiable risk factors and left ventricular ejection fraction among hospitalized Native Hawaiians and Pacific Islanders with heart failure. Hawaii J Med Public Health. 2014;73(12 Suppl 3):14–20.
Nishimura M, Bhatia H, Ma J, et al. The impact of substance abuse on heart failure hospitalizations. Am J Med. 2020;133(2):207–213.e1.
Lee NK, Rawson RA. A systematic review of cognitive and behavioural therapies for methamphetamine dependence. Drug Alcohol Rev. 2008;27(3):309–17.
Courtney KE, Ray LA. Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend. 2014;143:11–21.
Couwenbergh C, Van den Brink W, Zwart K, Vreugdenhil C, Van Wijngaarden-cremers P, Van der Gaag RJ. Comorbid psychopathology in adolescents and young adults treated for substance use disorders: a review. Eur Child Adolesc Psychiatry. 2006;15(6):319–28.
Gauffin K, Vinnerljung B, Fridell M, Hesse M, Hjern A. Childhood socio-economic status, school failure and drug abuse: a Swedish national cohort study. Addiction. 2013;108(8):1441–9.
Guina J, Nahhas RW, Goldberg AJ, Farnsworth S. PTSD symptom severities, interpersonal traumas, and benzodiazepines are associated with substance-related problems in trauma patients. J Clin Med. 2016;5(8):70.
Moore DJ, Blackstone K, Woods SP, Ellis RJ, Atkinson JH, Heaton RK, et al. Methamphetamine use and neuropsychiatric factors are associated with antiretroviral non-adherence. AIDS Care. 2012;24(12):1504–13.
Montoya JL, Georges S, Poquette A, Depp CA, Atkinson JH, Moore DJ, et al. Refining a personalized mHealth intervention to promote medication adherence among HIV+ methamphetamine users. AIDS Care. 2014;26(12):1477–81.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Race and Ethnicity Disparities
Rights and permissions
About this article
Cite this article
Tobler, D., Albert, M. Heart Failure in Vulnerable Populations: The Emerging Evidence of Methamphetamine-Associated Cardiomyopathy. Curr Cardiovasc Risk Rep 14, 21 (2020). https://doi.org/10.1007/s12170-020-00653-5
Published:
DOI: https://doi.org/10.1007/s12170-020-00653-5