Abstract
Atherosclerosis is a chronic inflammatory condition complicating cholesterol accumulation within the artery wall. Inflammation is believed to play an important role in the formation, progression, and ultimately the rupture of atherosclerotic plaques (the principle event leading to most myocardial infarctions and strokes). An enhanced understanding of the inflammatory process within the atheroma may therefore facilitate risk stratification and treatment strategies. Molecular imaging techniques such as PET/CT have the ability to quantify arterial inflammation and assess the high-risk features of atheromas thus may be useful for identifying patients who are at higher risk for an atherothrombotic event. In this review, we focus on the potential of FDG-PET/CT as a tool to measure arterial inflammation, enhance risk stratification, and to evaluate novel therapies directed against atherosclerotic disease. Additionally, this review will provide a discussion on current challenges as well as future directions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis and its complications are the primary causes of cardiovascular morbidity and mortality worldwide [1]. The formation and development of an atherosclerotic lesion is a complex process that includes chronic inflammation as a central pathologic component [2]. Monocyte-derived macrophages play a key role both in the incipient phases of plaque formation as well as in the progression of mature plaques [3, 4]. Imaging modalities such as conventional angiography, ultra sonography (US), computed tomography (CT), and magnetic resonance imaging (MRI) provide clinically useful information on plaque structure or luminal narrowing [5–7]. However, this structural data provides an incomplete assessment of atherothrombotic risk, in part, since stenosis is only a modest predictor of future complications as most acute coronary events occur at sites of mild to moderate obstruction [8, 9•]. The evaluation of the arterial wall inflammation, in addition to assessment of structure and narrowing may therefore provide clinically important insights. Such structural and biological imaging can be achieved via combined PET/CT or PET/MR imaging.
Inflammation and Atherosclerosis
The development of atherosclerotic lesions is a complex process, with chronic inflammation and lipid accumulation as central interconnected pathologic components. Endothelial damage or dysfunction participates as a stimulus for the accumulation of lipids and inflammatory cells in the affected area. Inflammatory cells such as monocytes and T lymphocytes attach to the vascular endothelium via vascular cells adhesion molecules (e.g., VCAM-1) [10]. The monocytes subsequently transmigrate across the intimal layer and become intimal macrophages. There, they internalize lipoprotein particles thru scavenger receptors and develop into lipid-laden foam cells, an ongoing process that can eventually lead to apoptosis and necrosis thus contributing to the growth of the lipid-rich core. The foam cells produce pro-inflammatory cytokines (TNF, IL-1, IL-6), promoting a chronic inflammatory response which leads to the recruitment of additional macrophages into the plaque [11]. They further produce matrix metalloproteinases (MMPs) thus contributing to the weakening of the plaque fibrous cap. Additionally, macrophages secrete tissue factor, which can promote thrombus formation after plaque rupture [12]. Accordingly, macrophages contribute to the formation, progression and rupture of plaques, and also accentuate the thrombosis that ensues.
The important relationship between inflammation and atherosclerotic events is further supported by clinical evidence. Multiple prospective studies performed on apparently healthy subjects have found that individuals with increased plasma biomarkers of inflammation (such as IL-6, soluble P-selectin, CD40, or MIC-1) have an increased risk of CV events [13–15]. The biomarker with the highest predictor value for future events was proved to be high-sensitivity CRP. Various large prospective studies have shown that elevated levels of hs-CRP are predictive of an increased risk for future cardiovascular events [16, 17].
Molecular Imaging with FDG PET/CT
PET/CT imaging, using 18F-fluorodeoxyglucose (FDG) has proven to be clinically invaluable as a diagnostic tool in oncology. It is widely used in the process of staging tumors, monitoring response to treatment, and detecting disease recurrence [18, 19]. Additionally, FDG-PET/CT has gained a role in the clinical evaluation of different inflammatory and infectious diseases like FUO, device infection, cardiac sarcoidosis, and endocarditis [20].
FDG is a radiolabeled glucose analogue, which is employed as a molecular probe to track cellular activity. 18F-FDG enters the cells thru glucose transporters and is phosphorylated by hexokinase to FDG-6-phosphate. While glucose becomes glucose-6-phosphate and further participates in glycolysis, FDG, once phosphorylated, becomes FDG-6-phosphate and is unable to move forward thru the glycolytic pathways or exit the cell, so it is trapped within the macrophages at a rate proportional to the glycolysis rate [21]. Thus, tissue uptake of FDG provides an index of glycolytic activity.
Glycolysis is of particular importance to macrophages. Macrophages have higher basal glycolytic rates compared to most cell types and are reliant on external glucose for metabolism as they are unable to store glycogen [22]. Importantly, macrophage glycolysis is substantially upregulated after pro-inflammatory (M1) but not alternative (M2) activation [23]. As a consequence, pro-inflammatory (M1) macrophages avidly accumulate FDG [24]. The relatively hypoxic environment within tumors, atheroma, and inflamed tissues leads to additional upregulation of macrophage pro-inflammatory activation, with stimulation of TNF alpha production and other potent pro-inflammatory cytokines [25]. As a result, FDG uptake is further stimulated. Macrophage FDG uptake may in part be responsible for the substantial success of FDG in clinical oncological imaging. Indeed, within tumors, tumor-associated macrophages (TAMs) can manifest higher FDG accumulation than the cancerous cells themselves [20]. Furthermore, within the atheroma, abundant cytokines exist in association with oxidized lipids. Modified LDL provides an additional stimulus for macrophage glycolysis and FDG uptake [26] whereby Ox LDL increases reactive oxygen species (ROS) production and HIF-1 alpha expression, which in-turn enhance macrophage glycolysis [27•].
Arterial FDG Uptake Provides a Noninvasive Index of Inflammation
Several preclinical studies have shown a direct correlation between arterial FDG uptake and histologically measured degree of inflammation [28, 29]. The first prospective study using FDG-PET to evaluate atherosclerotic tissue in humans was performed by Rudd et al. in 2002. In that study of patients with symptomatic carotid atherosclerosis, 18-FDG accumulation was significantly higher in symptomatic lesions compared to asymptomatic contralateral lesions [30]. A separate study, in which patients with carotid stenosis were imaged just prior to carotid endarterectomy, showed that the FDG signal correlates with macrophage density measured histologically in the subsequently excised carotid specimens [31]. Those studies thus established that FDG-PET imaging can be used to noninvasively assess the severity of inflammation in carotid plaques in patients. Other studies showed that in clinically stable patients, the arterial FDG signal is high reproducible [32, 33].
Relationship to Disease Progression and Event Risk
Arterial FDG uptake appears to provide insights about an individual’s risk for atherosclerotic disease. The degree to which FDG accumulates within the arterial wall is related to the individual’s cardiovascular risk factors and Framingham risk score [34]. Furthermore, several studies demonstrate that arterial FDG uptake is greatest in atherosclerotic plaques that contain structural features that are associated with a higher risk of rupture [35–37]. Figueroa et al. demonstrated that FDG uptake is greatest in plaques with high-risk features, defined as positive remodeling, luminal irregularity, or low attenuation [36, 38].
Importantly, several studies have shown the arterial FDG signal provides insights about the rate of atherosclerotic disease progression within the underlying arterial segment. Fayad et al. demonstrated that FDG uptake within the carotid arteries is associated with the subsequent rate of plaque expansion of the underlying atheroma (using MRI to measure disease progression over 2 years in humans) [39••]. Abdelbaky et al. observed that arterial FDG uptake correlates with the subsequent rate of calcium deposition in the underlying arterial segment (another measure of plaque progression, by CT) [40]. Accordingly, high arterial FDG accumulation identifies arterial locations where atherosclerotic disease is most likely to progress.
Moreover, a body of research has emerged showing that arterial FDG uptake may improve prediction of cardiovascular disease (CVD). In a cohort of patients with cancer, Rominger et al. [41] observed that a higher arterial FDG signal was associated with a high risk of subsequent CVD event. Figueroa et al. recently extended those findings. In a study of 513 cancer-free patients who underwent FDG-PET and computed tomography (CT) imaging, aortic target to background ratio (TBR) strongly predicted subsequent CVD independent of traditional risk factors during the 6-year follow-up period. The addition of FDG uptake to FRS scores substantially improved risk discrimination. Moreover, FDG data resulted in the net accurate reclassification of 27 % of individuals over Framingham risk score. Of note, arterial TBR was inversely associated with the timing of CVD. The study concluded that arterial FDG uptake improved incident CVD prediction beyond FRS and provided information on the potential timing of such events [42•].
Thus far, limited prospective data are available regarding the ability of arterial FDG measures to predict subsequent atherothrombotic events. A small prospective study recently showed in individuals presenting with a stroke that increased FDG uptake in carotid arteries was associated with an increased risk of stroke recurrence [43]. Several large prospective studies that are evaluating FDG PET/CT imaging are currently underway: the BioImage and the PESA studies, studies that might clarify the utility of noninvasive imaging approaches for predicting cardiovascular outcomes in asymptomatic individuals [44, 45].
FDG-PET/CT Imaging Assessment of Therapies
The observations that 18-FDG-PET imaging provides a reproducible measure of arterial inflammation and is predictive of disease progression and of clinical events provide justification to use this modality to assess anti-atherosclerotic treatments. To that end, the imaging approach has been applied to the study of dozens of drug classes, in both humans and animal models. A question of substantial importance to the field is to what degree do the arterial PET/CT imaging findings in drug treatment trails predict the clinical efficacy of the drugs tested (Fig. 1). To answer this question, one would need to compare the findings of FDG-PET/CT imaging studies to the findings of clinical endpoint studies using the same drug. At this time, there are four drug classes for which arterial FDG-PET/CT imaging data and clinical events data are available: 1. statins, 2. thiazolidinediones (TZDs), 3. cholesterylester transfer protein (CETP) antagonists, and 4. lipoprotein-associated phospholipase A2 (LPPLA2) antagonists. Below is a review of the imaging and clinical endpoint trial findings for those drug classes.
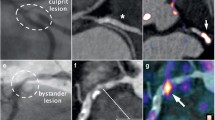
a Arterial FDG PET/CT images. Axial and coronal sections of CT and PET/CT images of carotid atherosclerosis with severe luminal narrowing are shown. The top images show a carotid arterial plaque associated with severe stenosis, high-risk morphological features, and high FDG uptake. The lower panel also shows severe stenosis, but without high-risk morphological features or high FDG uptake (modified with permission from Figueroa et al., Circ Cardiovascular Imaging 2012) [36]. b Arterial FDG uptake independently predicts CVD risk. In a study of over 500 individuals who underwent FDG-PET/CT imaging, arterial FDG uptake (here divided into tertiles of FDG activity) provided an independent prediction of CVD risk. Moreover, the net reclassification index (NRI), which represents the fraction of individuals whose risk was accurately reclassified by PET/CT imaging was a robust 27 % (modified with permission from Figueroa et al., JACC CV Imaging 2013) [42]
-
1.
Statin therapy
Statins reduce plasma LDL cholesterol, and several human studies have shown that statins reduce circulating markers of inflammation such as hsCRP [46, 47] thus supporting the hypothesis that statins may have important anti-inflammatory effects. FDG-PET/CT was used in several clinical trials to more directly study the anti-inflammatory effects of statins on the arterial wall. The first such study, by Tahara et al., compared the effects of low-dose simvastatin with dietary management using an unblinded, single-center study design. After a 3-month period, there was a significant reduction in arterial FDG uptake in the simvastatin-treated patients compared to diet-treated comparator group [48]. In a more recent double-blind, multicenter trial, adults with risk factors or with established atherosclerosis were randomized to atorvastatin 10 mg versus 80 mg. FDG uptake within the artery wall was assessed using PET/CT. The study demonstrated that FDG uptake was rapidly reduced (starting at 4 weeks). Moreover, arterial inflammation fell nearly twice as much in the high-dose (vs. low-dose) atorvastatin group [49]. Accordingly, the findings of the FDG-PET/CT imaging studies are consistent with findings from several clinical endpoint trials, which have repeatedly shown a reduction in CVD events with statin therapy and also showed a substantial clinical benefit for atorvastatin 80 mg over atorvastatin 10 mg [50].
-
2.
Thiazolidinediones
Thiazolidinediones (TZDs) are used in the treatment of type 2 diabetes and effectively reduce plasma glucose and hemoglobin A1c levels. A study by Mizoguchi et al. evaluated the ability of the TZD pioglitizone to reduce arterial FDG uptake. In that study, individuals with diabetes or glucose intolerance were randomized to TZDs vs. another glucose-lowering strategy (the sulfonylurea glimepiride), and both groups were treated to achieve a similar degree of glucose control over a 16-week period. While both treatments reduced fasting plasma glucose and hemoglobin A1c values comparably, pioglitazone, but not glimepiride, decreased atherosclerotic plaque inflammation. Further, compared with glimepiride, pioglitazone significantly increased high-density lipoprotein cholesterol level. High-sensitivity C-reactive protein was decreased by pioglitazone, whereas it was increased by glimepiride [51]. In concert with those imaging findings, clinical trials with TZDs have similarly shown a benefit of pioglitazone over sulfonylureas [52].
-
3.
Cholesterylester Transfer Protein Antagonists
Cholesterylester transfer protein (CETP) transfers cholesterol from HDL cholesterol to very low-density or low-density lipoproteins. Inhibition of CETP results in higher HDL levels. In 2011, the randomized, double-blind multicenter dal-PLAQUE trial employed MRI and FDG-PET/CT imaging of the artery wall to evaluate the effects of dalcetrapib on atherosclerostic structure and inflammation. The study demonstrated that dalcetrapib did not impact any of the prespecified PET/CT endpoints despite a substantial rise in the HDL cholesterol levels. In concert with those findings, the 16,000-patient clinical endpoint trial evaluating dalcetrapib, the dal-OUTCOMES study, subsequently showed that the drug does not alter the incidence of CV events [53].
-
4.
Lipoprotein-Associated Phospholipase A2 Antagonists
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is a vascular-specific inflammatory enzyme that is associated with a substantially increased risk of CVD events. Rilapladib, a novel LPPLA2 antagonist, was recently studied in a randomized, double-blinded, multicenter imaging trial using FDG-PET/CT and MR imaging [54]. That study showed that inhibition of LPPLA2 did not result in a reduction in atherosclerotic inflammation. In concert with those findings, two studies (that randomized nearly 32,000 individuals with atherosclerotic disease) subsequently showed no benefit for LPPLA2 inhibition for reducing atherothrombotic events [55, 56].
Accordingly, from the studies to date, there appears to be directional concordance between changes in atherosclerotic inflammation by FDG-PET/CT and changes in the risk of atherothrombotic events. Further study is ongoing, which will refine our understanding of the manner by which imaging can predict therapeutic efficacy.
Current Challenges and Future Directions
The 18F-FDG/PET imaging of the coronary arteries is complicated by several technical factors. The small size of the arteries and cardiac and respiratory motion during image acquisition and high-background myocardial uptake jointly collude to make measurement of the coronary signal challenging. Several groups have sought to address some of these limiting factors. Dietary modification has been used to address the problem of high myocardial activity, which otherwise can overwhelm the signal emanating from the adjacent epicardial arteries. One often-employed approach is to prescribe a high-fat, low-carbohydrate diet prior to imaging. This approach shifts myocardial metabolism from glycolysis to mitochondrial oxidation of fatty acids and has shown moderate success [57, 58]. Additionally, the use of motion-compensated reconstructions can improve image quality [59]. Despite the fact that several groups have demonstrated the ability to measure coronary signals [57, 60], coronary imaging using FDG as a tracer remains challenging and awaits further technological advances.
One tracer that has been shown to provide a high signal to background ration in the coronaries is F18-sodium fluoride. This tracer localizes to areas of active bone formation and remodeling. The tracer 18F-NaF is incorporated into exposed hydroxyapatite crystals and hence is a marker for active calcium deposition [61]. In a recent prospective clinical trial [62•], NaF-PET was employed for detection of coronary plaque micro-calcifications in subjects with acute myocardial infarction and stable angina. Increased tracer uptake was noted in 93 % of culprit plaques and autoradiography of the endarterectomy specimens confirmed the accumulation of tracer in areas of plaque rupture rich in macrophages and micro-calcifications.
Novel tracers are being actively evaluated for detection of inflammation. Further, several oncologic tracers have been evaluated for use for the detection of atherosclerotic inflammation. Recently, uptake of 68 Ga-DOTATE, a somatostatin analogue with specific binding affinity for somatostatin receptor-2 present on macrophages, was found to correlate with the presence of calcified LAD plaques [63]. The choline tracers: 11C-choline and 18F-FMCH, taken up by activated macrophages and incorporated into cell membranes, as well as 11C-PK 11195, a selective ligand of the translocator protein expressed in activated macrophages, were furthermore used with moderate success. Another tracer, F18-fluorodeoxymannose binds to the mannose receptors present on M2-activated macrophages [64•] and hence can report of a separate aspect of inflammation compared to FDG (which provides information on M1 activated macrophages). Other tracers are targeting separate processes with atherosclerotic plaques, such as hypoxia and microvessel formation. Growth of atherosclerotic plaques is accompanied by neovascularization which represents a high-risk feature for future plaque rupture [65], and instable plaques are associated with a higher microvessel density than stable ones. Tracers with specific affinity for cell surface integrin receptors expressed on new blood cell membranes like 18-F-galacto and 18F-fluciclatide were tested in human atherosclerotic carotid plaques imaging and significantly higher TBR ratios in stenotic areas compared with nonstenotic areas were demonstrated [66]. The development of PET/MR technologies may further address the coronary tree challenges. Derived simultaneously to the PET, the MR acquisition has the potential to provide higher image quality through improved correction algorithms.
Conclusions
Molecular imaging of atherosclerosis with FDG-PET/CT has shown promise in the quantification of plaque inflammation, risk stratification, and prediction of future events and as an efficient reproducible tool in monitoring therapeutic effects of anti-inflammatory medication. Although not without challenges, especially in evaluation of the coronary tree, molecular imaging modalities have advanced at a rapid pace in recent years. Novel tracers and the recent emergence of hybrid PET/MRI imaging provide a new bleeding edge of molecular imaging of atherosclerosis and may provide more profound, clinically useful insights into atherosclerotic plaque biology.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128.
Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451(7181):953–7.
Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–74.
Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–55.
Cai J, Hatsukami TS, Ferguson MS, Kerwin WS, Saam T, Chu B, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. 2005;112(22):3437–44.
Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54(1):49–57.
Obaid DR, Calvert PA, Gopalan D, Parker RA, Hoole SP, West NE, et al. Atherosclerotic plaque composition and classification identified by coronary computed tomography: assessment of computed tomography-generated plaque maps compared with virtual histology intravascular ultrasound and histology. Circ Cardiovasc Imaging. 2013;6(5):655–64.
Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92(3):657–71.
Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. Jama. 2014;312(17):1754–63. Using coronary angiography, this study showed that nonobstructive CAD, compared with no apparent CAD, was associated with a significantly greater 1-year risk of MI and all-cause mortality.
Li H, Cybulsky MI, Gimbrone Jr MA, Libby P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb J Vasc Biol Am Heart Assoc. 1993;13(2):197–204.
Butcher MJ, Galkina EV. Phenotypic and functional heterogeneity of macrophages and dendritic cell subsets in the healthy and atherosclerosis-prone aorta. Front Physiol. 2012;3:44.
Wilcox JN, Smith KM, Schwartz SM, Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci U S A. 1989;86(8):2839–43.
Brown DA, Breit SN, Buring J, Fairlie WD, Bauskin AR, Liu T, et al. Concentration in plasma of macrophage inhibitory cytokine-1 and risk of cardiovascular events in women: a nested case–control study. Lancet. 2002;359(9324):2159–63.
Ridker PM, Buring JE, Rifai N. Soluble P-selectin and the risk of future cardiovascular events. Circulation. 2001;103(4):491–5.
Schonbek U, Varo N, Libby P, Buring J, Ridker PM. Soluble CD 40 and cardiovascular risk in women. Circulation. 2001;104:2266–8.
Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–43.
Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–65.
Antoch G, Vogt FM, Freudenberg LS, Nazaradeh F, Goehde SC, Barkhausen J, et al. Whole-body dual-modality PET/CT and whole-body MRI for tumor staging in oncology. Jama. 2003;290(24):3199–206.
Lardinois D, Weder W, Hany TF, Kamel EM, Korom S, Seifert B, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348(25):2500–7.
Graziosi M, Nanni C, Lorenzini M, Diemberger I, Bonfiglioli R, Pasquale F, et al. Role of (1)(8)F-FDG PET/CT in the diagnosis of infective endocarditis in patients with an implanted cardiac device: a prospective study. Eur J Nucl Med Mol Imaging. 2014;41(8):1617–23.
Kubota R, Kubota K, Yamada S, Tada M, Ido T, Tamahashi N. Microautoradiographic study for the differentiation of intratumoral macrophages, granulation tissues and cancer cells by the dynamics of fluorine-18-fluorodeoxyglucose uptake. J Nucl Med Off Publ Soc Nucl Med. 1994;35(1):104–12.
Babior BM. The respiratory burst of phagocytes. J Clin Invest. 1984;73(3):599–601.
Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P, et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185(1):605–14.
Satomi T, Ogawa M, Mori I, Ishino S, Kubo K, Magata Y, et al. Comparison of contrast agents for atherosclerosis imaging using cultured macrophages: FDG versus ultrasmall superparamagnetic iron oxide. J Nucl Med Off Publ Soc Nucl Med. 2013;54(6):999–1004.
Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364(7):656–65.
De Vries HE, Ronken E, Reinders JH, Buchner B, Van Berkel TJ, Kuiper J. Acute effects of oxidized low density lipoprotein on metabolic responses in macrophages. FASEB J Off Publ Fed Am Soc Exp Biol. 1998;12(1):111–8.
Lee SJ, Thien Quach CH, Jung KH, Paik JY, Lee JH, Park JW, et al. Oxidized low-density lipoprotein stimulates macrophage 18F-FDG uptake via hypoxia-inducible factor-1alpha activation through Nox2-dependent reactive oxygen species generation. J Nucl Med Off Publ Soc Nucl Med. 2014;55(10):1699–705. In this study the authors demonstrated that oxLDL, a key player in atherosclerotic inflammation, is a strong stimulator of macrophage (18)F-FDG uptake and glycolysis through upregulation of GLUT1 and hexokinase.
Lederman RJ, Raylman RR, Fisher SJ, Kison PV, San H, Nabel EG, et al. Detection of atherosclerosis using a novel positron-sensitive probe and 18-fluorodeoxyglucose (FDG). Nucl Med Commun. 2001;22(7):747–53.
Tawakol A, Migrino RQ, Hoffmann U, Abbara S, Houser S, Gewirtz H, et al. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol. 2005;12(3):294–301.
Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105(23):2708–11.
Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48(9):1818–24.
Rudd JH, Myers KS, Bansilal S, Machac J, Pinto CA, Tong C, et al. Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med Off Publ Soc Nucl Med. 2008;49(6):871–8.
Rudd JH, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, et al. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50(9):892–6.
Noh TS, Moon SH, Cho YS, Hong SP, Lee EJ, Choi JY, et al. Relation of carotid artery 18F-FDG uptake to C-reactive protein and Framingham risk score in a large cohort of asymptomatic adults. J Nucl Med Off Publ Soc Nucl Med. 2013;54(12):2070–6.
Choi YS, Youn HJ, Chung WB, Hwang HJ, Lee DH, Park CS, et al. Uptake of F-18 FDG and ultrasound analysis of carotid plaque. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol. 2011;18(2):267–72.
Figueroa AL, Subramanian SS, Cury RC, Truong QA, Gardecki JA, Tearney GJ, et al. Distribution of inflammation within carotid atherosclerotic plaques with high-risk morphological features: a comparison between positron emission tomography activity, plaque morphology, and histopathology. Circ Cardiovasc Imaging. 2012;5(1):69–77.
Graebe M, Pedersen SF, Hojgaard L, Kjaer A, Sillesen H. 18FDG PET and ultrasound echolucency in carotid artery plaques. J Am Coll Cardiol Img. 2010;3(3):289–95.
Graebe M, Pedersen SF, Borgwardt L, Hojgaard L, Sillesen H, Kjaer A. Molecular pathology in vulnerable carotid plaques: correlation with [18]-fluorodeoxyglucose positron emission tomography (FDG-PET). Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. 2009;37(6):714–21.
Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378(9802):1547–59. This trial suggests possible beneficial vascular effects of dalcetrapib, including the reduction in total vessel enlargement.
Abdelbaky A, Corsini E, Figueroa AL, Fontanez S, Subramanian S, Ferencik M, et al. Focal arterial inflammation precedes subsequent calcification in the same location: a longitudinal FDG-PET/CT study. Circ Cardiovasc Imaging. 2013;6(5):747–54.
Rominger A, Saam T, Wolpers S, Cyran CC, Schmidt M, Foerster S, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med Off Publ Soc Nucl Med. 2009;50(10):1611–20.
Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. J Am Coll Cardiol Img. 2013;6(12):1250–9. This study proved that arterial FDG uptake, measured from routinely obtained PET/CT images, substantially improved incident CVD prediction beyond FRS and provided information on the potential timing of such events.
Marnane M, Merwick A, Sheehan OC, Hannon N, Foran P, Grant T, et al. Carotid plaque inflammation on 18F-fluorodeoxyglucose positron emission tomography predicts early stroke recurrence. Ann Neurol. 2012;71(5):709–18.
Fernandez-Ortiz A, Jimenez-Borreguero LJ, Penalvo JL, Ordovas JM, Mocoroa A, Fernandez-Friera L, et al. The Progression and Early detection of Subclinical Atherosclerosis (PESA) study: rationale and design. Am Heart J. 2013;166(6):990–8.
Muntendam P, McCall C, Sanz J, Falk E, Fuster V, High-Risk PI. The BioImage Study: novel approaches to risk assessment in the primary prevention of atherosclerotic cardiovascular disease—study design and objectives. Am Heart J. 2010;160(1):49–57. e1.
Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352(1):20–8.
Corti R, Fuster V, Fayad ZA, Worthley SG, Helft G, Smith D, et al. Lipid lowering by simvastatin induces regression of human atherosclerotic lesions: two years’ follow-up by high-resolution noninvasive magnetic resonance imaging. Circulation. 2002;106(23):2884–7.
Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48(9):1825–31.
Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013;62(10):909–17.
LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352(14):1425–35.
Mizoguchi M, Tahara N, Tahara A, Nitta Y, Kodama N, Oba T, et al. Pioglitazone attenuates atherosclerotic plaque inflammation in patients with impaired glucose tolerance or diabetes a prospective, randomized, comparator-controlled study using serial FDG PET/CT imaging study of carotid artery and ascending aorta. J Am Coll Cardiol Img. 2011;4(10):1110–8.
Erdmann E, Dormandy JA, Charbonnel B, Massi-Benedetti M, Moules IK, Skene AM, et al. The effect of pioglitazone on recurrent myocardial infarction in 2,445 patients with type 2 diabetes and previous myocardial infarction: results from the PROactive (PROactive 05) Study. J Am Coll Cardiol. 2007;49(17):1772–80.
Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367(22):2089–99.
Tawakol A, Singh P, Rudd JH, Soffer J, Cai G, Vucic E, et al. Effect of treatment for 12 weeks with rilapladib, a lipoprotein-associated phospholipase A2 inhibitor, on arterial inflammation as assessed with 18F-fluorodeoxyglucose-positron emission tomography imaging. J Am Coll Cardiol. 2014;63(1):86–8.
Investigators S, White HD, Held C, Stewart R, Tarka E, Brown R, et al. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014;370(18):1702–11.
O'Donoghue ML, Braunwald E, White HD, Lukas MA, Tarka E, Steg PG, et al. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. Jama. 2014;312(10):1006–15.
Cheng VY, Slomka PJ, Le Meunier L, Tamarappoo BK, Nakazato R, Dey D, et al. Coronary arterial 18F-FDG uptake by fusion of PET and coronary CT angiography at sites of percutaneous stenting for acute myocardial infarction and stable coronary artery disease. J Nucl Med Off Publ Soc Nucl Med. 2012;53(4):575–83.
Wykrzykowska J, Lehman S, Williams G, Parker JA, Palmer MR, Varkey S, et al. Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J Nucl Med Off Publ Soc Nucl Med. 2009;50(4):563–8.
Rudd JH, Narula J, Strauss HW, Virmani R, Machac J, Klimas M, et al. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: ready for prime time? J Am Coll Cardiol. 2010;55(23):2527–35.
Rogers IS, Nasir K, Figueroa AL, Cury RC, Hoffmann U, Vermylen DA, et al. Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. J Am Coll Cardiol Img. 2010;3(4):388–97.
Dweck MR, Chow MW, Joshi NV, Williams MC, Jones C, Fletcher AM, et al. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol. 2012;59(17):1539–48.
Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, et al. 18F-Fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383(9918):705–13. The authors in this study demonstrated the eficficacy of (18)F-NaF PET-CT imaging to identify and localise ruptured and high-risk coronary plaque.
Rominger A, Saam T, Vogl E, Ubleis C, la Fougere C, Forster S, et al. In vivo imaging of macrophage activity in the coronary arteries using 68Ga-DOTATATE PET/CT: correlation with coronary calcium burden and risk factors. J Nucl Med Off Publ Soc Nucl Med. 2010;51(2):193–7.
Tahara N, Mukherjee J, de Haas HJ, Petrov AD, Tawakol A, Haider N, et al. 2-Deoxy-2-[18F]fluoro-D-mannose positron emission tomography imaging in atherosclerosis. Nat Med. 2014;20(2):215–9. In this study,fluorodeoxymanose PET/CT imaging was used to characterize high risk atherosclerothic plaques with activated macrophages.
Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, et al. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004;110(14):2032–8.
Beer AJ, Pelisek J, Heider P, Saraste A, Reeps C, Metz S, et al. PET/CT imaging of integrin alphavbeta3 expression in human carotid atherosclerosis. J Am Coll Cardiol Img. 2014;7(2):178–87.
Compliance with Ethics Guidelines
Conflict of Interest
Ahmed Tawakol reports grants and personal fees from Takeda, personal fees from Actelion, grants and personal fees from Roche/Genentech, and personal fees from Amgen outside the submitted work. Amorina Ishai has no conflicts relevant to this work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Novel and Emerging Risk Factors
Rights and permissions
About this article
Cite this article
Ishai, A., Tawakol, A. Imaging Plaque Inflammation in Higher-Risk Patients: What Do We Know and What Are We Looking For?. Curr Cardiovasc Risk Rep 9, 31 (2015). https://doi.org/10.1007/s12170-015-0459-0
Published:
DOI: https://doi.org/10.1007/s12170-015-0459-0