Abstract
Mycotoxin contamination in agri-food systems has been a serious global concern over the past few decades. Corn is easily contaminated by Ochratoxin A (OTA) and Zearalenone (ZEN), which seriously threaten the survival and health of humans and animals. Herein, a rapid and sensitive method of OTA and ZEN dual quantum dots (QDs) fluorescence immunochromatographic test strip was established in this study. OTA and ZEN fluorescence probes were prepared by carbodiimide. The OTA-OVA, ZEN-BSA and staphylococcal protein A were sprayed on the nitrocellulose filter membrane as the T1 line, T2 line and control line of the dual test strip, which exhibited high sensitivity, accuracy and specificity. For quantitative detection of OTA, the linear regression equation was y = 0.3222x + 0.3834 (R2 = 0.9687), LOD and IC50 were 0.132 ng/mL and 2.296 ng/mL, and the linear detection range was 0.269 ng/mL to 19.588 ng/mL. In addition, for quantitative detection of ZEN, its linear regression equation was y = 0.3077x + 0.3777 (R2 = 0.9648), LOD and IC50 were 0.125 ng/mL and 2.495 ng/mL, and the linear detection range was 0.264 ng/mL to 23.55 ng/mL. Briefly, the OTA and ZEN dual QDs immunochromatographic test strip was favored for the simultaneous detection of OTA and ZEN in corn.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food is one of the basic needs of human survival and development, which can provide basic energy, nutrients and necessary trace elements(Chan 2014). However, food is susceptible to mycotoxin contamination during preparation and storage. Grains and their processed products are an important source of food and feed. Humans and animals face certain health risks when ingesting contaminated food or grains(Kimanya 2015; Selvaraj et al. 2015). Mycotoxin contamination in agri-food systems has been a serious global concern over the past few decades. About 25% of the world's crops are contaminated with mycotoxins, which cause huge losses of billions of dollars per year to agricultural production and industrial products(Brera et al. 1998; Khatoon and Abidin 2018; Streit et al. 2013). Even if the production, storage and processing are standardized, it is difficult to completely avoid the contamination of mycotoxins in crops and their agricultural products(Bellver Soto et al. 2014; Molinie et al. 2005). Currently, more than 300 mycotoxins have been discovered and identified (Alshannaq and Yu 2017; Huwig et al. 2001). Ochratoxin A (OTA) and Zearalenone (ZEN) are two common mycotoxins found in grains. According to the GB2761-2017 document of China, the maximum level (ML) of OTA and ZEN for Cereals and their products in China is 5 µg/kg and 60 µg/kg, respectively(Zhang et al. 2017).
Ochratoxin A (OTA) is a fungal secondary metabolite mainly produced by Aspergillus ochraceous and Penicillium verrucosum(Anli and Alkis 2010). OTA was classified as a potential carcinogen in category 2B by the International Agency for Research on Cancer (IARC) in 1993(Pfohl-Leszkowicz et al. 1993). OTA has multiple toxicities including nephrotoxicity, carcinogenicity, teratogenicity, genotoxicity and immunotoxicity, and exposure to OTA can cause DNA damage in the kidney, liver, testis, spleen and lymphocytes, thymocytes and fibroblasts(Bragulat et al. 2019; Niaz et al. 2020; Ostry et al. 2017; Palma et al. 2007). Zearalenone (ZEN) is mainly produced by some Fusarium species and listed as a group III carcinogen by IARC(Maaroufi et al. 1996; Zhang et al. 2017). ZEN has estrogenic activity and disrupts the reproductive system of humans and animals(D’Mello et al. 1999; Gromadzka et al. 2008; Krska and Josephs 2001; Massart et al. 2008). In addition, ZEN also has hepatotoxicity, hematological toxicity, immunotoxicity and genotoxicity(Gromadzka et al. 2008). As an important grain and feed, corn is easily contaminated by OTA and ZEN(Giorni et al. 2019; Lee and Ryu 2017). Therefore, the contamination of mycotoxins poses a serious challenge to food safety. The establishment of a rapid and accurate diagnosis method for mycotoxins has important application value and social significance for improving food safety and reducing the harm of toxins.
Currently, enzyme-linked immunosorbent assay, high-performance liquid chromatography, colloidal gold-based immunochromatographic test strip and fluorescence resonance energy transfer effect have been developed to detect OTA or ZEN(Cha et al. 2012; Shao et al. 2018; Sun et al. 2016; Wu et al. 2019; Zhao et al. 2017). In addition, fluorescence immunochromatography based on quantum dots(QDs) has advantages of high throughput, accuracy, rapidity and low cost, so it has attracted much attention in recent years(Huang et al. 2016; Mahmoudi et al. 2019a). QDs were main label of fluorescent probes because of their stability and photoluminescence brightness(Li et al. 2019). Furthermore, QDs can improve the sensitivity and consume fewer immunoreagents of fluorescence immunochromatographic test strips (Beloglazova et al. 2011; Foubert et al. 2017). In view of the universality of multiple mycotoxin contamination, the single target immunochromatographic test strip is difficult to meet the detection needs of actual samples(Liu et al. 2020). Therefore, the development of dual or multiple immunochromatographic test strips for simultaneous detection of multiple targets in agricultural products is the main development direction of rapid immunoassay in the future.
This study aimed to establish rapid detection methods of novel OTA and ZEN dual fluorescence immunochromatographic test strips based on QDs. The dual fluorescence immunochromatographic test strip not only provided technical support for the batch and rapid detection of OTA and ZEN in corn but also provided a certain reference for the establishment of rapid detection methods for other small molecule toxins.
Materials and methods
Materials
Water-soluble QDs were purchased from Jiayuan Quantum Dots Co., Ltd. (Wuhan, China). OTA, ZEN, deoxynivalenol (DON) and T-2 toxin were purchased from Pribolab Pte. Ltd. (Singapore). Ochratoxin C (OTC) and ochratoxin D (OTD) were obtained from Henan Academy of Agricultural Sciences. Bovine serum albumin (BSA), ovalbumin (OVA), 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and nitrocellulose membrane were purchased from Merck KGaA (Darmstadt, Germany). Methanol, ethanol, acetonitrile, and chloroform were purchased from Zhiyuan Chemical Reagent Co., Ltd. (Tianjin, China). Glass fiber, rubber plate and absorbent paper were purchased from Kinbio Tech.Co., Ltd. (Shanghai, China). Staphylococcal Protein-A (SPA) was purchased from Solarbio Science & Technology Co., Ltd. (Beijing, China).
Instruments
The Dispense Platform and Guillotine Cutter were purchased from BioDot, Inc. (USA). The Nanodrop 2000c Spectrophotometer, Nanopure Water Purifier, ultralow temperature refrigerator, CO2 incubator and drying oven were purchased from Thermo Scientific (Waltham, USA). The UV lamp was purchased from Crystal Technology & Industries, Inc. (CTI, USA). The high-speed centrifuge was purchased from Eppendorf (Hamburg, Germany). The homogenizer was purchased from Joyoung Co., Ltd. (Shandong, China). The multimode reader was purchased from Molecular Devices (USA). The fluorescence immunochromatographic test strip reader was purchased from Nanjing Microdetection Bio-Tech Co., Ltd. (Nanjing, China).
Preparation of OTA and ZEN fluorescence probes
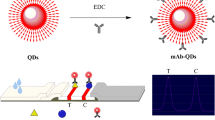
OTA and ZEN fluorescence probes were both prepared by the EDC activation method (Taranova et al. 2015). Briefly, the carboxyl groups of water-soluble QDs were firstly activated by EDC solution. Anti-OTA mAb or anti-ZEN mAb was added into the QDs solution and stirred for 30 min. Subsequently, the anti-OTA mAb or anti-ZEN mAb was combined with water-soluble QDs through amide bonds (Fig. 1a). Afterward, BSA solution was added into the above-mixed solution for obstructing 30 min. Finally, OTA and ZEN fluorescence probes were stored at 4 °C for further experiments. In the preliminary work(Zhou et al. 2021), we optimized the synthesis conditions, including the pH value and the molar ratio of monoclonal antibodies and EDC to reduce the unbound antibodies during the coupling process. Fluorescent probes were synthesized under optimized conditions in this study.
Identification of bioactivity of OTA and ZEN fluorescence probes
To confirm whether OTA and ZEN fluorescence probes were successfully prepared, the bioactivity of fluorescence probes (anti-OTA mAb-QDs and anti-ZEN mAb-QDs) was analysed by OTA and ZEN dual quantum dot fluorescence immunochromatographic test strips (Fig. 1b). This study took out the blank dual test strips without fluorescence probe, then add pure water-soluble QDs, anti-OTA mAb-QDs and anti-ZEN mAb-QDs to the dual test strips in turn. After waiting for a few minutes, the colour development of the dual test strips was observed under UV Lamp. Therefore, the immunoreactivity of fluorescent probes could be evaluated based on the result of antigen and antibody binding.
Preparation of OTA and ZEN dual quantum dots fluorescence immunochromatographic test strips
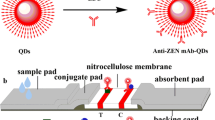
First of all, SPA, OTA-OVA and ZEN-BSA solutions were sprayed on the NC membrane in turn, which was used as the C line, T1 line and T2 line of dual QDs fluorescence immunochromatographic test strips. Then, the NC membranes were dried immediately for 4 h in an air blast drying oven at 40 °C. Secondly, sample pads and bonding pads were pre-treated with solutions: 10 mmol/L PBS, 1.0% BSA (w/v), 0.05% trehalose (w/v), 0.25% Tween-20 (v/v) and 0.1% NaN3 (w/v). After soaking, they were dried at 60 °C for 4 h. The OTA and ZEN dual QDs fluorescence immunochromatographic test strips were assembled as follows: Firstly, the absorbent paper, NC membrane, bonding pad and sample pad were affixed to the plastic baseboard, overlapping them by 2 mm. Secondly, the plastic baseboards were cut into test strips with a width of 2.79 mm. Finally, the test strips were put into special plastic cartridges and stored under sealed, dark, dry and normal temperature conditions for standby.
Qualitative detection of OTA and ZEN
The corn samples were prepared according to previous research (Yang et al. 2022). OTA and ZEN standards were added into corn extraction for preparation of the sample solution. The final OTA concentrations were 0, 0.05, 0.25, 1.25, 2.5, 5, 10, 25, 50 and 100 ng/mL, and the final ZEN concentrations were 0, 0.1, 0.5, 1, 2.5, 5, 10, 25, 50 and 100 ng/mL. For qualitative detection of OTA and ZEN by dual QDs fluorescence immunochromatographic test strips, the corn sample solutions were pipetted onto sample pads (Fig. 2). After incubating for 10 min, the colour development of dual test strips was observed under a UV lamp. The visual detection threshold was the lowest concentration of OTA and ZEN which made the T1 line and T2 line fluorescence invisible to the naked eye (Wu et al. 2019).
Quantitative detection of OTA and ZEN
For Quantitative detection of OTA and ZEN by dual QDs fluorescence immunochromatographic test strips, the fluorescence intensity of the T1 line (FIT1), T2 line (FIT2), C line (FIC), values of FIT1/FIC and FIT2/FIC were analyzed by fluorescence immunochromatographic test strip reader (Fig. 2). According to the relationship between the competition inhibition rate and the concentration of OTA or ZEN, the competition inhibition curves of OTA and ZEN were established by using GraphPad Prism 8, respectively. The abscissa is the logarithm value of OTA or ZEN concentration with 10 as the base. The ordinate is their competition inhibition rate (1-B/B0), where B is the FIT1/FIC or FIT2/FIC values of dual QDs fluorescent immunochromatographic test strips under different OTA or ZEN concentrations. B0 is the FIT1/FIC or FIT2/FIC value of the dual test strip when detecting OTA or ZEN negative samples (Guo et al. 2019). The limit of detection (LOD) is calculated based on the 10% competitive inhibition rate of OTA or ZEN, and the linear detection range is calculated based on the 20%—80% competitive inhibition rate of OTA or ZEN (Wang et al. 2019).
Results
Evaluation of immunoreactivity of OTA and ZEN fluorescent probes
The immunoreactivity of OTA and ZEN fluorescent probes was characterized by the immunochromatographic assay. There was no red fluorescence at the C line, T1 line and T2 line of the dual QDs fluorescent immunochromatographic test strip after adding only QDs solution. This indicated that QDs could not react with SPA, OTA-OVA and ZEN-BSA. However, red fluorescence appeared on the C line and T1 line of the dual QDs fluorescent immunochromatographic test strip when only the anti-OTA mAb-QDs solution was added. This indicated that anti-OTA mAb QDs could react with SPA and OTA-OVA. When only the anti-ZEN mAb QDs solution was added, red fluorescence appears at the C line and T2 line of the dual QDs fluorescent immunochromatographic test strip. This indicated that anti-ZEN mAb QDs can react with SPA and ZEN-BSA. In addition, red fluorescence appears on the C, T1 and T2 lines of the dual QDs fluorescent immunochromatographic test strip when OTA and ZEN fluorescent probes existed at the same time (Fig. 3a). Therefore, the anti-OTA mAb-QDs and anti-ZEN mAb-QDs exhibited the immunoreactivity.
Characterization and optimization of fluorescence probes and complete antigen. a The bioactivity of anti-OTA mAb-QDs and anti-ZEN mAb-QDs. 1: QDs; 2: anti-OTA mAb-QDs; 3: anti-ZEN mAb-QDs; 4: anti-OTA mAb-QDs and anti-ZEN mAb-QDs. b Optimization of the dilution of fluorescence probes, and the spray concentration of OTA-OVA and ZEN-BSA. C Line: SPA; T1 line: OTA-OVA; T2 line: ZEN-BSA
Optimization of fluorescent probes and complete antigen
The fluorescent probes and complete antigens could affect the sensitivity and detection range of dual QDs fluorescent immunochromatographic test strips. Therefore, the dilution of OTA and ZEN fluorescence probes, and the spray concentration of OTA-OVA and ZEN-BSA were optimized by checkerboard titration in this study. The spray concentration of OTA-OVA was set to 0.1, 0.25 and 0.5 mg/mL, the spray concentration of ZEN-BSA was set to 0.2, 0.4 and 0.8 mg/mL, and the dilution of the fluorescent probe was set to twofold, fourfold and eightfold. When the spray concentration of OTA-OVA and ZEN-BSA was 0.5 mg/mL and 0.8 mg/mL, and the dilution of OTA and ZEN fluorescent probe was fourfold, the red fluorescence at C line, T1 line and T2 lines of the dual QDs fluorescent immunochromatographic test strip were clearly visible under UV lamp, and the background of the dual tests trip was clear, and the colour development was also good (Fig. 3b).
Optimization of detection conditions of the dual test strip
To improve the efficiency of detection, the reaction time of OTA and ZEN dual QDs fluorescent immunochromatographic test strips was optimized in this study. The dual test strip gradually appeared red fluorescence when the reaction time was 1 min. The colour development of the C line, T1 line and T2 line of the dual test strip displayed well when the reaction time was 5–20 min. However, the non-specific colour development of the dual test strip increased, and the colour development background gradually deepened with time which was difficult to accurately identify (Fig. 4a). In addition, the relative fluorescence intensity of the dual test strip was judged by multi-channel fluorescence immunochromatographic test strip reader. FIT1, FIT2 and FIC gradually increased within 30 min, while their ratios FIT1/FIC and FIT2/FIC tended to be stable after 10 min of reaction (Fig. 4b). Therefore, the reaction time of the dual test strip was set to 10 min, and then the qualitative and quantitative detection of OTA and ZEN were interpreted and analysed. In the early stage of this study, a series of optimizations have been carried out for different pH values of the sample solution, methanol concentration in the sample diluent, and corn matrix effect(Yang et al. 2022; Zhou et al. 2021). In detail, the pH value of the sample solution was 7.4, the methanol concentration of the sample diluent was 5%, and the dilution of corn matrix extract was (1:9, v/v).
Optimization of the immunoreaction time of the dual QDs fluorescence immunochromatographic test strip. a: C Line: SPA; T1 line: OTA-OVA; T2 line: ZEN-BSA. b: FIT1: the fluorescence intensity of the T1 line; FIT2: the fluorescence intensity of the T2 line; FIC: the fluorescence intensity of the C line
Detection of OTA and ZEN by the dual test strip
In this study, OTA and ZEN were quantitatively detected by dual QDs fluorescent immunochromatographic test strips. According to the linear relationship between the competitive inhibition rate and the concentration of OTA and ZEN, the competitive inhibition curves of OTA and ZEN were established, respectively (Fig. 5). For OTA, the linear regression equation was y = 0.3222x + 0.3834 (R2 = 0.9687), LOD and IC50 were 0.132 ng/mL and 2.296 ng/mL, and the linear detection range was 0.269 ng/mL to 19.588 ng/mL (Fig. 5a). In addition, for quantitative detection of ZEN, its linear regression equation was y = 0.3077x + 0.3777 (R2 = 0.9648), LOD and IC50 were 0.125 ng/mL and 2.495 ng/mL, and the linear detection range was 0.264 ng/mL to 23.55 ng/mL (Fig. 5b). Furthermore, for the qualitative detection of OTA and ZEN, the dual test strips were exposed to a UV lamp and observed with the naked eye. The red fluorescent band at the T1 line and T2 line of the dual test strip was invisible to the naked eye when the concentration of OTA and ZEN in the sample solution was 10 ng/mL and 25 ng/mL (Fig. 6). Therefore, the threshold values of the dual test strip for visual detection of OTA and ZEN were 10 ng/mL and 25 ng/ mL, respectively.
Quantitative detection of OTA and ZEN with dual QDs fluorescence immunochromatographic test strips. a: The quantitative standard curve of OTA. b: The quantitative standard curve of ZEN. a, b: B: the value of FIT/FIC of the dual test strip (positive); B0: the value of FIT/FIC of the dual test strip (negative); 1-B/B0: the competitive inhibition rate
Evaluation of the Performance of dual test strip
The specificity of dual QDs fluorescent immunochromatographic test strips was evaluated in this study. The concentrations of OTC, OTD, DON and T-2 were 1000 ng/mL, and the concentrations of OTA and ZEN were 10 ng/mL and 25 ng/mL, respectively. The dual test strips showed obvious red fluorescent bands at the T1 line and T2 line when detecting high concentrations of OTC, OTD, DON and T-2 (Fig. 7a). In addition, their FIT/FIC values had no significant difference from those of the negative control (PBS). However, in the determination of low concentration OTA and ZEN, their FIT/FIC values had significantly decreased (Fig. 7b). Therefore, the OTA and ZEN dual QDs fluorescent immunochromatographic test strips have good specificity.
Evaluation of the specificity of the dual test strip. a: For the visual observation, the concentration of OTA and ZEN were 10 ng/mL and 25 ng/mL, respectively, and the concentration of DON, T-2, OTC, and OTD both was 1000 ng/mL. b: The analysis by multi-channel fluorescence immunoassay analyzer, FIT/FIC: the ratio of the relative fluorescence intensity of the T line and the C line of the dual test strip
Furthermore, the accuracy and precision of the dual test strip were evaluated through the spiking recovery experiment of OTA and ZEN. The recovery rate of OTA for intra-assay was 88.67%-96.53%, and the CV was 3.53%-7.67%. The recovery of OTA for inter-assay was 89.78%—95.33%, and the CV was 5.95–8.62%. The recovery rate of ZEN for intra-assay was 90.73%-99.56%, and the CV was 3.28%-7.23%. The recovery of ZEN for inter-assay was 89.74%-95.23%, and the CV was 4.02%-9.24% (Table 1). Therefore, the rapid detection method of OTA and ZEN dual QDs fluorescent immunochromatographic test strips had good accuracy and precision.
Discussion
Compared with traditional detection methods such as ELISA, HPLC and LC–MS, the main advantages of dual quantum dot fluorescent immunochromatographic test strips are convenience, rapidity and efficiency and low cost (Mahmoudi et al. 2019b). The dual test strip can simultaneously detect two targets, thus reducing the cost, time and sample volume of the entire detection and analysis (Duan et al. 2019). Mycotoxins are secondary metabolites produced by fungi. These toxic metabolites are transmitted through the food chain, causing acute or chronic toxicity to humans. Given the universality of multiple mycotoxin contamination, the single target immunochromatographic test strip is difficult to meet the detection needs of actual samples(Liu et al. 2020). Therefore, the development of dual or multiple immunochromatographic test strips for simultaneous detection of multiple targets in agricultural products is the main development direction of rapid immunoassay in the future. Achieving upgrading from rough qualitative to accurate quantitative, the OTA and ZEN dual QDs immunochromatographic test strips effectively avoid the threats and risks caused by mixed contamination of mycotoxins and ensure the safety of food quality.
Conclusion
The red fluorescent probes anti-OTA mAb-QDs and anti-ZEN mAb-QDs were successfully prepared in this study. The SPA was used as the C-line output signal of the OTA and ZEN dual test strip, and a rapid immunological detection method system for OTA and ZEN was established. Furthermore, its sensitivity, specificity, accuracy and precision were confirmed. The dual test strip favored the detection of OTA and ZEN in corn. Overall, the OTA and ZEN dual QDs fluorescent immunochromatographic test strip provided technical support for the national food security strategy, and also provided a reference for rapid detection and analysis of other toxins and small molecular substances.
Data Availability
No datasets were generated or analysed during the current study.
References
Alshannaq A, Yu JH (2017) Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int J Environ Res Public Health 14(6):632. https://doi.org/10.3390/ijerph14060632
Anli E, Alkis IM (2010) Ochratoxin A and Brewing Technology: A Review. J I Brewing 116(1):23–32. https://doi.org/10.1002/j.2050-0416.2010.tb00394.x
Bellver Soto J, Fernandez-Franzon M, Ruiz MJ, Juan-Garcia A (2014) Presence of ochratoxin A (OTA) mycotoxin in alcoholic drinks from southern European countries: wine and beer. J Agric Food Chem 62(31):7643–7651. https://doi.org/10.1021/jf501737h
Beloglazova NV, Goryacheva IY, Niessner R, Knopp D (2011) A comparison of horseradish peroxidase, gold nanoparticles and qantum dots as labels in non-instrumental gel-based immunoassay. Microchim Acta 175(3–4):361–367. https://doi.org/10.1007/s00604-011-0682-4
Bragulat MR, Abarca ML, Castella G, Cabanes FJ (2019) Intraspecific variability of growth and ochratoxin A production by Aspergillus carbonarius from different foods and geographical areas. Int J Food Microbiol 306:108273. https://doi.org/10.1016/j.ijfoodmicro.2019.108273
Brera C, Miraglia M, Colatosti M (1998) Evaluation of the impact of mycotoxins on human health: Sources of errors. Microchem J 59(1):45–49. https://doi.org/10.1006/mchj.1998.1583
Cha SH, Kim SH, Bischoff K, Kim HJ, Son SW, Kang HG (2012) Production of a highly group-specific monoclonal antibody against zearalenone and its application in an enzyme-linked immunosorbent assay. J Vet Sci 13(2):119–125. https://doi.org/10.4142/jvs.2012.13.2.119
Chan M (2014) Food safety must accompany food and nutrition security. Lancet 384(9958):1910–1911. https://doi.org/10.1016/s0140-6736(14)62037-7
D’Mello JPF, Placinta CM, Macdonald AMC (1999) Fusarium mycotoxins: a review of global implications for animal health, welfare and productivity. Anim Feed Sci Tech 80(3–4):183–205. https://doi.org/10.1016/s0377-8401(99)00059-0
Duan H, Li Y, Shao YN, Huang XL, Xiong YH (2019) Multicolor quantum dot nanobeads for simultaneous multiplex immunochromatographic detection of mycotoxins in maize. Sensor Actuat B-Chem 291:411–417. https://doi.org/10.1016/j.snb.2019.04.101
Foubert A, Beloglazova NV, De Saeger S (2017) Comparative study of colloidal gold and quantum dots as labels for multiplex screening tests for multi-mycotoxin detection. Anal Chim Acta 955:48–57. https://doi.org/10.1016/j.aca.2016.11.042
Giorni P, Bertuzzi T, Battilani P (2019) Impact of Fungi Co-occurrence on Mycotoxin Contamination in Maize During the Growing Season. Front Microbiol 10:1265. https://doi.org/10.3389/fmicb.2019.01265
Gromadzka K, Waskiewicz A, Chelkowski J, Golinski P (2008) Zearalenone and its metabolites: occurrence, detection, toxicity and guidelines. World Mycotoxin J 1(2):209–220. https://doi.org/10.3920/WMJ2008.x015
Guo L, Shao Y, Duan H, Ma W, Leng Y, Huang X, Xiong Y (2019) Magnetic Quantum Dot Nanobead-Based Fluorescent Immunochromatographic Assay for the Highly Sensitive Detection of Aflatoxin B1 in Dark Soy Sauce. Anal Chem 91(7):4727–4734. https://doi.org/10.1021/acs.analchem.9b00223
Huang XL, Aguilar ZP, Xu HY, Lai WH, Xiong YH (2016) Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: A review. Biosens Bioelectron 75:166–180. https://doi.org/10.1016/j.bios.2015.08.032
Huwig A, Freimund S, Kappeli O, Dutler H (2001) Mycotoxin detoxication of animal feed by different adsorbents. Toxicol Lett 122(2):179–188. https://doi.org/10.1016/s0378-4274(01)00360-5
Khatoon A, Abidin ZU (2018) Mycotoxicosis – diagnosis, prevention and control: past practices and future perspectives. Toxin Rev 39(2):99–114. https://doi.org/10.1080/15569543.2018.1485701
Kimanya ME (2015) The health impacts of mycotoxins in the eastern Africa region. Curr Opin Food Sci 6:7–11. https://doi.org/10.1016/j.cofs.2015.11.005
Krska R, Josephs R (2001) The state-of-the-art in the analysis of estrogenic mycotoxins in cereals. Fresenius J Anal Chem 369(6):469–476. https://doi.org/10.1007/s002160100715
Lee HJ, Ryu D (2017) Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-occurrence. J Agric Food Chem 65(33):7034–7051. https://doi.org/10.1021/acs.jafc.6b04847
Li RX, Meng CZ, Wen Y, Fu W, He PL (2019) Fluorometric lateral flow immunoassay for simultaneous determination of three mycotoxins (aflatoxin B-1, zearalenone and deoxynivalenol) using quantum dot microbeads. Microchim Acta 186(12):748. https://doi.org/10.1007/s00604-019-3879-6
Liu Z, Hua Q, Wang J, Liang Z, Li J, Wu J, Li X (2020) A smartphone-based dual detection mode device integrated with two lateral flow immunoassays for multiplex mycotoxins in cereals. Biosens Bioelectron 158:112178. https://doi.org/10.1016/j.bios.2020.112178
Maaroufi K, Chekir L, Creppy EE, Ellouz F, Bacha H (1996) Zearalenone induces modifications of haematological and biochemical parameters in rats. Toxicon 34(5):535–540. https://doi.org/10.1016/0041-0101(96)00008-6
Mahmoudi T, de la Guardia M, Shirdel B, Mokhtarzadeh A, Baradaran B (2019a) Recent advancements in structural improvements of lateral flow assays towards point-of-care testing. Trac-Trends in Analytical Chemistry 116:13–30. https://doi.org/10.1016/j.trac.2019.04.016
Mahmoudi T, de la Guardia M, Shirdel B, Mokhtarzadeh A, Baradaran B (2019b) Recent advancements in structural improvements of lateral flow assays towards point-of-care testing. Trends Analyt Chem 116:13–30. https://doi.org/10.1016/j.trac.2019.04.016
Massart F, Meucci V, Saggese G, Soldani G (2008) High growth rate of girls with precocious puberty exposed to estrogenic mycotoxins. J Pediatr 152(5):690–695. https://doi.org/10.1016/j.jpeds.2007.10.020
Molinie A, Faucet V, Castegnaro P, Pfohl-Leszkowicz A (2005) Analysis of some breakfast cereals on the French market for their contents of ochratoxin A, citrinin and fumonisin B-1: development of a method for simultaneous extraction of ochratoxin A and citrinin. Food Chem 92(3):391–400. https://doi.org/10.1016/j.foodchem.2004.06.035
Niaz K, Shah SZA, Khan F, Bule M (2020) Ochratoxin A-induced genotoxic and epigenetic mechanisms lead to Alzheimer disease: its modulation with strategies. Environ Sci Pollut Res Int 27(36):44673–44700. https://doi.org/10.1007/s11356-020-08991-y
Ostry V, Malir F, Toman J, Grosse Y (2017) Mycotoxins as human carcinogens-the IARC Monographs classification. Mycotoxin Res 33(1):65–73. https://doi.org/10.1007/s12550-016-0265-7
Palma N, Cinelli S, Sapora O, Wilson SH, Dogliotti E (2007) Ochratoxin A-induced mutagenesis in mammalian cells is consistent with the production of oxidative stress. Chem Res Toxicol 20(7):1031–1037. https://doi.org/10.1021/tx700027j
Pfohl-Leszkowicz, A., Grosse, Y., Castegnaro, M., Nicolov, I. G., Chernozemsky, I. N., Bartsch, H., . . . Dirheimer, G. (1993). Ochratoxin A-related DNA adducts in urinary tract tumours of Bulgarian subjects. IARC Sci Publ(124), 141–148. https://www.ncbi.nlm.nih.gov/pubmed/8225477.
Selvaraj JN, Zhou L, Wang Y, Zhao Y-J, Xing F-G, Dai X-F, Liu Y (2015) Mycotoxin detection - Recent trends at global level. J Integr Agr 14(11):2265–2281. https://doi.org/10.1016/s2095-3119(15)61120-0
Shao Y, Duan H, Guo L, Leng Y, Lai W, Xiong Y (2018) Quantum dot nanobead-based multiplexed immunochromatographic assay for simultaneous detection of aflatoxin B1 and zearalenone. Anal Chim Acta 1025:163–171. https://doi.org/10.1016/j.aca.2018.03.041
Streit E, Naehrer K, Rodrigues I, Schatzmayr G (2013) Mycotoxin occurrence in feed and feed raw materials worldwide: long-term analysis with special focus on Europe and Asia. J Sci Food Agric 93(12):2892–2899. https://doi.org/10.1002/jsfa.6225
Sun Y, Xing G, Yang J, Wang F, Deng R, Zhang G, Zhang Y (2016) Development of an immunochromatographic test strip for simultaneous qualitative and quantitative detection of ochratoxin A and zearalenone in cereal. J Sci Food Agric 96(11):3673–3678. https://doi.org/10.1002/jsfa.7550
Taranova NA, Berlina AN, Zherdev AV, Dzantiev BB (2015) “Traffic light” immunochromatographic test based on multicolor quantum dots for the simultaneous detection of several antibiotics in milk. Biosens Bioelectron 63:255–261. https://doi.org/10.1016/j.bios.2014.07.049
Wang Y, Xu J, Qiu Y, Li P, Liu B, Yang L, Zhang C (2019) Highly Specific Monoclonal Antibody and Sensitive Quantum Dot Beads-Based Fluorescence Immunochromatographic Test Strip for Tebuconazole Assay in Agricultural Products. J Agric Food Chem 67(32):9096–9103. https://doi.org/10.1021/acs.jafc.9b02832
Wu JK, Ma JW, Wang H, Qin DM, An L, Ma Y, Wu XJ (2019) Rapid and visual detection of benzothiostrobin residue in strawberry using quantum dot-based lateral flow test strip. Sensor Actuat B-Chem 283:222–229. https://doi.org/10.1016/j.snb.2018.11.137
Yang QB, Qi YH, Zhou JM, Liu HL, Liu YK, Chen YM, Wang AP (2022) Development of Fluorescent Immunochromatographic Test Strip for Qualitative and Quantitative Detection of Zearalenone. Food Anal Methods 15(9):2547–2557. https://doi.org/10.1007/s12161-022-02295-2
Zhang X, Eremin SA, Wen K, Yu X, Li C, Ke Y, Wang Z (2017) Fluorescence Polarization Immunoassay Based on a New Monoclonal Antibody for the Detection of the Zearalenone Class of Mycotoxins in Maize. J Agric Food Chem 65(10):2240–2247. https://doi.org/10.1021/acs.jafc.6b05614
Zhao H, Chen X, Shen C, Qu B (2017) Determination of 16 mycotoxins in vegetable oils using a QuEChERS method combined with high-performance liquid chromatography-tandem mass spectrometry. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 34(2):255–264. https://doi.org/10.1080/19440049.2016.1266096
Zhou J, Yang Q, Liang C, Chen Y, Zhang X, Liu Z, Wang A (2021) Detection of ochratoxin A by quantum dots-based fluorescent immunochromatographic assay. Anal Bioanal Chem 413(1):183–192. https://doi.org/10.1007/s00216-020-02990-1
Funding
This study was supported by a grant from the National Key Research and Development Program of China (2019YFC1604501).
Author information
Authors and Affiliations
Contributions
Jingming Zhou and Qingbao Yang: Conceptualization, Methodology. Qingbao Yang: Experimentation, Data curation, Writing-Original draft preparation, Reviewing and Editing. Huawen Yan: Validation. Enping Liu and Chao Liang: Investigation. Yumei Chen: Visualization. Aiping Wang: Supervision. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics Declarations
All the authors declare that no human participants or animals were involved in this study.
Informed Consent
Informed consent is not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Q., Yan, H., Chen, Y. et al. Rapid detection of OTA and ZEN with dual quantum dots fluorescence immunochromatographic test strip. Food Anal. Methods 17, 1302–1311 (2024). https://doi.org/10.1007/s12161-024-02662-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-024-02662-1