Abstract
Several cases of mislabeling of the hen farming system of production on the eggs’ package have been reported by different research groups worldwide. The objective of this study was to evaluate the ability of a portable NIR instrument to classify egg samples non-destructively, from different provenances or production systems (e.g. cage, cage-free, free-range and organic) in Australia. Intact whole egg samples were purchased from local supermarkets where the label in each of the packages was used as identification of the layers’ feeding system as per the Australian legislation and standards. The spectra of the intact whole egg samples were obtained using a portable NIR spectrophotometer within the 950–1600-nm wavelength range. Principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were used to analyse the NIR data. The results obtained in this study indicated that 100% of the egg samples were correctly classified using NIR spectroscopy non-destructively. It was concluded that NIR spectroscopy is a promising tool for the non-invasive, rapid and inexpensive analytical verification of the provenance or the production system of whole egg samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food fraud has been a common problem since ancient times due to profitable reasons or to mask the unusual appearance as well as taste of perishable foods (Johnson et al. 2018; Puertas and Vázquez 2019). In recent years, Spink and Moyer (2011) defined food fraud as a collective term that includes the deliberate and intentional substitution, addition, tampering or misrepresentation of food, food ingredients or food packaging; or false or misleading statements made about a product for economic purpose. At present, numerous fraudulent practices have been identified and might be minimised using modern analytical techniques and instrumentation (Oliveri and Downey, 2012; Puertas and Vázquez 2019). Nevertheless, expansion of food supply chains around the world and the prevalence and impact of food fraud have increased over the recent years in many countries (Johnson et al. 2018; Puertas and Vázquez 2019).
Internationally, several cases of misrepresentation or mislabeling of the hen farming method of production on the eggs’ label or package have been reported (Joint Research Centre 2018a, 2018b, 2018c; Puertas and Vázquez 2019). Likewise, several analytical techniques have been developed and utilised as tools to detect issues associated with egg fraud along the food supply and value chains (Soltani and Omid 2015; Abín et al. 2018; Puertas and Vázquez 2019; Loffredi et al. 2021; Van Ruth et al. 2011, 2013). In most European countries, organic and free-range productions have increased compared to other countries due to consumer preferences, and the high demanding for high-quality foods, produced under more environmentally and animal welfare–friendly conditions (Abín et al. 2018; Puertas and Vázquez 2019; Van Ruth et al. 2011, 2013).
In Australia, cage eggs are those produced from hens that are housed in cages inside large, climate-controlled sheds. The Model Code of Practice for the Welfare of Animals stipulates that the minimum space allowance per hen in a cage farming system is 550 cm2 per bird, though the average cage size ranges from 1800 to 11,000 cm2, with a 10-cm feeder space and a minimum cage height of 40 cm. Cage hens are also referred to as battery hens as the parallel rows inside sheds look like rows of cells inside a battery (https://www.australianeggs.org.au/farming/cage-eggs). Free-range eggs are those sourced from hens that have access to an outdoor range during the day but are housed securely and comfortably in sheds at night. To be classified as a free-range egg farm, the hens must have meaningful and regular access to an outdoor range during daylight hours (https://www.australianeggs.org.au/farming/cage-eggs). Organic eggs are defined as those that come from hens that are free to roam in an outdoor area during the day and are housed safely and securely in sheds at night. The main difference between organic and free-range eggs is that organic eggs are produced without the use of any chemicals (e.g. antibiotics). In Australia, the organic eggs represent about 2% of the total supermarket egg sales. Australian Certified Organic is the largest certifier supported by the major supermarkets and its certification can be seen on about 80% of all organic products (https://www.australianeggs.org.au/farming/cage-eggs). When buying organic eggs, it is important and standard practice for the consumer to look out for an organic certification body logo or symbol to ensure the farm meets national organic production standards. Other government-approved organic certification organisations include AUS-QUAL, Bio-Dynamic Research Institute, NASAA Certified Organic, Organic Food Chain and Southern Cross Certified Australia (https://www.australianeggs.org.au/farming/cage-eggs). However, it is still challenging to identify and trace the provenance or the systems of production of the eggs from unknown practices.
Near infrared (NIR) spectroscopy has been utilised to determine egg composition and quality where freshness has been one of the most reported parameters related to quality (Narushi 1997; Berardinelli et al. 2005; Kemps et al. 2006; Zhao et al. 2010; Lin et al. 2011; Aboonajmi et al., 2014, 2016; Abdel-Nour et al. 2011; Puertas and Vázquez 2019; Coronel-Reyes et al. 2018; Dong et al. 2017, 2018a, b, 2019). For example, diffuse reflectance Fourier transform near infrared (FT-NIR) spectroscopy was used to predict the thick albumen height, with a determination coefficient of 0.82 (Giunchi et al. 2008). Lin and collaborators (2011) predicted the HU (Haugh units, index of freshness) of eggs and obtained a correlation coefficient of 0.88 by means of an artificial neural network (ANN) model combined with genetic algorithms (GA) using NIR reflectance spectroscopy. The combination of visible (VIS) and NIR transmission spectroscopy was also reported to predict egg freshness (Liu et al. 2007; Mehdizadeh et al. 2014). Yet, no studies have evaluated NIR as a tool for verification of egg provenance or system of production.
The objective of this study was to evaluate the ability of a portable NIR instrument to non-destructively classify intact egg samples from different provenances or production systems in Australia.
Materials and Methods
A total of 130 intact whole egg samples were purchased from local supermarkets where the label in each of the boxes or package was used as identification of the feeding system utilised to feed the layer hens. These systems were defined as cage (n: 36), cage-free (n: 36), free-range (n: 36) and organic (n: 22) and regulated by different organisations such as Eggs Standards of Australia, Australian Eggs and Eggs Farmers of Australia. It is important to note that cage-free and free-range eggs were considered one group, as it is hard to establish the main differences between them.
The NIR spectra of the intact whole egg samples were collected at four locations (the apical, two scans at the equatorial region and at the blunt) using a portable NIR spectrophotometer (MicroNIR 1700, Viavi, Milpitas, CA, USA) working in the 950–1600-nm wavelength range, with a spectral resolution of 10 nm with no moving parts (Viavi Solutions, 2015, Milpitas, CA, USA). The NIR instrument was connected through a USB interface to a notebook computer running proprietary software (MicroNIR Prov 3.1, Viavi, Milpitas, CA, USA) for the acquisition of diffuse reflectance spectra of the samples (Viavi Solutions, 2015, Milpitas, CA, USA). The controlling parameters for spectral data acquisition were set at 50 ms integration time and averaging of 50 scans (MicroNIR Prov 3.1, Viavi, Milpitas, CA, USA). The reference spectra for absorbance/reflectance calculation were collected using Spectralon® every 20 samples. In addition, the intact eggs were cracked and the NIR spectra of the yolk were collected.
Prior to spectrum interpretation and chemometric analysis, the NIR data were transformed using the Savitzky-Golay second derivative (21 smoothing points and second polynomial order) (Bureau et al. 2019; Savitzky and Golay, 1964). Principal component analysis and partial least squares discriminant analysis (PLS-DA) were used to analyse and interpret any trends in the data set as well as to develop a classification model to monitor the origin of the eggs (The Unscrambler X, CAMO Analytics AS). In this study, the NIR data and the information provided in the label of the egg carton or package were combined to develop the PLS-DA models. The data set was organised in a way that each whole egg sample belonging to the cage group was designed with a dummy number (one in this case), where samples sourced from free-range, organic and cage-free eggs were considered one group, and labelled with the dummy number two. This approach was used in both the intact egg and yolk samples analysed. Full cross validation (leave one out) was used to develop and validate both the PCA and PLS-DA models (Bureau et al. 2019; Næs et al. 2002; Williams et al. 2017). The models were evaluated using the coefficient of determination in cross validation (R2), the standard error in cross validation (SECV), the bias, and slope, and the percentage of correct (%CC) and incorrect classified (%IC) samples (Bureau et al. 2019; Næs et al. 2002; Williams et al. 2017).
Results and Discussion
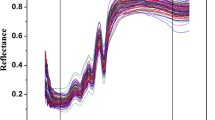
The raw NIR spectra of the intact whole egg samples analysed (not shown) showed only one main absorbance at the wavelength around 1453 nm (O–H overtones) (Workman and Weyer 2008). Thus, pre-treatment of spectra using the Savitzky-Golay second derivative was relevant and utilised to reveal patterns and regions (wavelengths) in the NIR spectra not easily visualised using the raw data. The second derivative spectra of each of the egg group samples analysed are shown in Fig. 1. The main differences in the second derivative between the intact whole egg samples were observed at 1440 nm and 1186 nm. The absorbance at 1440 nm might be associated with N–H (aromatic amines) and C-H combination tones (Workman and Weyer 2008). The absorbance at 1186 nm might be associated with C-H and C-H3 bonds (Workman and Weyer 2008). This band has also been associated with the absorption of pure fatty acids containing cis double bonds (second overtones C-H) as reported by others (Sato et al. 1991; Workman and Weyer 2008). The NIR spectra of the intact cage-free egg samples showed the highest absorbance values around the 1440 nm region, whilst for the intact free-range egg samples the main absorbance value was observed around 1186 nm (Workman and Weyer 2008). Giunchi and collaborators (2008) reported that wavelengths around 1450 nm might also be associated with the first overtone of O–H stretching. These researchers suggested that the increase in the absorbance values of the O–H stretching could be associated with the structural changes of proteins that might occur during the storage of eggs (Giunchi et al. 2008). More recently, Chen and collaborators (2019) indicated that the region between 1400 and 1500 nm can also be associated with the N–H secondary amine. These authors suggested that the NIR spectra of whole eggs originate from the superimposition of various egg constituents (e.g. protein, lipids), determining spectral differences between different types of eggs, despite that most of these spectral characteristics can be considered as being less prominent (Chen et al. 2019). It has been also reported that the colour of the eggshell may play an important role during the collection of the NIR spectra (Chen et al. 2019). A recent study also compared the spectra of white and brown eggs using VIS–NIR (between 500 and 950 nm) where the main differences in the raw spectra were observed around 600–650 nm (VIS region) (Dong et al. 2018a, b and 2019). These authors proposed that the protoporphyrin and carbonate minerals present in the eggshell might influence the transmittance spectrum of the egg samples analysed (Dong et al. 2018a, b). This is very important to consider when the VIS region is included in the analysis. This phenomenon has resulted that Dong and collaborators (2018a, b and 2019) recommend that only white-shelled eggs be analysed, mainly due to brown-shelled eggs having limitations due to the interference of spectral bands in the VIS region. The eggshell colour is mainly related to the hen breeds and the main pigment comes from protoporphyrin IX in haemoglobin whilst eggs with different colours can show no obvious differences in nutritional value or composition (Stadelman and Cotterill 1995; Sim 1998). In this study, all the intact whole egg samples analysed were brown and the VIS region was not collected during the analysis.
Figure 2 shows the score plot of the intact whole egg samples analysed non-destructively using NIR spectroscopy where principal components 1 and 4 were plotted. Overall, the first four principal components explained 99% of the variability in the NIR spectra of the intact whole egg samples analysed. Plotting PC1 (93%) vs PC4 (1%) shows a separation between cage egg and non-cage egg samples, where the late group includes free-range, free-cage and organic free-range samples. Samples were also overlapped and the presence of outlier samples was also observed. The PCA loadings (PC4) indicated that the separation between cage and cage-free egg samples was associated with the information present in wavelengths at 1193 nm (C-H and C-H3 bonds), 1397 nm (C-H combinations and C-H2 bonds) and 1508 nm (N–H) (Workman and Weyer 2008). Separation between intact cage-free, free-range and organic egg samples was explained by the information present in wavelengths at 1180 nm (C-H and C-H3 bonds) and 1434 nm (O–H bonds) (Workman and Weyer 2008) (see Fig. 3).
Table 1 shows the classification rates for the discrimination of the intact whole egg samples according to provenance or system of production using PLS-DA. When comparing cage egg samples vs cage-free egg samples (e.g. free-range, organic, cage-free), 98% of the egg samples were correctly classified belonging to each of the defined groups. When cage and cage-free egg samples selected from the same supermarket were utilised, 100% and 98% correct classifications were also obtained for the egg samples sourced from either cage or cage-free systems, respectively. Similar results were reported for the classification of whole “natural” and commercial eggs (Chen et al. 2019), and the discrimination between free-range and cage yolk samples (Puertas and Vázquez 2019) using a combination of VIS and NIR spectroscopy techniques and chemometrics. The PLS regression coefficients used by the classification models were similar to those described for the PCA reported above. The main differences between the egg samples were observed in the second derivative at 1502 nm and 1180 nm. In addition, wavelengths at 1273 nm, 1341 nm and 1403 nm were also observed (data not shown). The assignation of functional groups and wavelengths was similar to those described in the above sections.
The egg samples were broken and the NIR spectra of the yolk were collected using the same instrument described in the “Materials and Methods” section. The second derivative of the yolk samples is shown in Fig. 4. The PLS-DA classification results based on the yolk samples showed that 96% and 86% of the egg samples were correctly classified, corresponding to free-range or cage egg samples, respectively. Similar results were also reported by Puertas and Vázquez (2019) evaluating egg yolk samples from different origins using a combination of UV–VIS-NIR spectroscopy.
Conclusion
This study demonstrated the potential of NIR spectroscopy combined with chemometric data analysis as analytical tools to non-destructively classify intact egg samples according to the system of production (e.g. cage vs cage-free). The proposed method is simple, fast, environmentally friendly and avoids laborious sample pre-treatment. NIR spectroscopy is expected to become an alternative technique to other routine methods used by the industry to assess egg quality. Although the results of the present study are promising, further research is still needed in order to validate the existing classification models, as well as to include other parameters associated with quality such as composition, and shelf life that might influence the classification.
Data Availability
No data available.
References
Abdel-Nour N, Ngadi M, Prasher S, Karimi Y (2011) Prediction of egg freshness and albumen quality using visible/near infrared spectroscopy. Food Bioprocess Technology 4:731–736
Abín R, Laca A, Laca A, Díaz M (2018) Environmental assessment of intensive egg production: a Spanish case study. J Clean Prod 179:160–168
Aboonajmi M, Saberi A, Najafabadi TA, Kondo N (2016) Quality assessment of poultry egg based on visible–near infrared spectroscopy and radial basis function networks. Int J Food Prop 19:1163–1172
Australian Eggs. (https://www.australianeggs.org.au/farming/cage-eggs)
Berardinelli A, Giunchi A, Guarnieri A, Pezzi F, Ragni L (2005) Shell egg albumen height assessment by FT-NIR spectroscopy. Transaction of the ASAE 48:1426–1428
Bureau S, Cozzolino D, Clark CJ (2019) Contributions of Fourier-transform mid infrared (FT-MIR) spectroscopy to the study of fruit and vegetables: a review. Postharvest Biol Technol 148:1–14. https://doi.org/10.1016/j.postharvbio.2018.10.003
Chen H, Tana Ch, Lin Z (2019) Non-destructive identification of native egg by near-infrared spectroscopy and data driven-based class-modeling. Spectrochim Acta Part A Mol Biomol Spectrosc 206:484
Coronel-Reyes J, Ramirez-Morales I, Fernandez-Blanco E, Rivero D, Pazos A (2018) Determination of egg storage time at room temperature using a low-cost NIR spectrometer and machine learning techniques. Comput Electron Agric 145:1–1
Dong X, Li Z, Shen Z, Tang X (2018a) Nondestructive egg freshness assessment from the equatorial and blunt region based on visible near infrared spectroscopy. Spectrosc Lett 51(10):540–546. https://doi.org/10.1080/00387010.2018.1525409
Dong X, Dong J, Li Y, Xu H, Tang X (2019) Maintaining the predictive abilities of egg freshness models on new variety based on VIS-NIR spectroscopy technique. Comput Electron Agric 156:669–676
Dong X, Dong J, Peng Y, Tang X (2017) Comparative study of albumen pH and whole egg pH for the evaluation of egg freshness. Spectrosc Lett 50(9):463–469
Dong X, Li Z, Shen Z, Tang X (2018b) Non destructive egg freshness assessment from the equatorial and blunt region based on visible near infrared spectroscopy. Spectrosc Lett 51(10):540–546
Giunchi A, Berardinelli A, Ragni L, Fabbri A, Silaghi FA (2008) Non-Destructive Freshness Assessment of Shell Eggs Using FT-NIR Spectroscopy Journal of Food Eng 89:142–148
Johnson AE, Sidwick KL, Pirgozliev VR, Edge A, Thompson DF (2018) Metabonomic profiling of chicken eggs during storage using high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Anal Chem 90(12):7489–7494
Joint Research Centre (2018a) 2018a Summary of articles on food fraud and adulteration. JRC Food Fraud Monthly Report January 1–3.
Joint Research Centre (2018b) June 2018b summary of articles on food fraud and adulteration. JRC Food Fraud Monthly Report 1–3.
Joint Research Centre. (2018c) March 2018c summary of articles on food fraud and adulteration. JRC Food Fraud Monthly Report (March) 1–6.
Kemps BJ, Bamelis FR, De Ketelaere B, Mertens K, Tona K, Decuypere EM, De Baerdemaeker JG (2006) Visible transmission spectroscopy for the assessment of egg freshness. J Sci Food Agric 86:1399–1406
Lin H, Zhao J, Sun L, Chen Q, Zhou F (2011) Freshness measurement of eggs using near infrared (NIR) spectroscopy and multivariate data analysis. Innov Food Sci Emerg Technol 12:182–186
Liu Y, Ying Y, Ouyang A, Li Y (2007) Measurement of internal quality in chicken eggs using visible transmittance spectroscopy technology. Food Control 18:18–22
Loffredi E, Grassi S, Alamprese C (2021)Spectroscopic approaches for non-destructive shell egg quality and freshness evaluation: opportunities and challenges Food Control 129
Mehdizadeh SA, Minaei S, Hancock NH, Torshizi MAK (2014) An intelligent system for egg quality classification based on visible-infrared transmittance spectroscopy. Information Processing in Agriculture 1:105–114
Næs T, Isaksson T, Fearn T, Davies T. (2002) A user-friendly guide to multivariate calibration and classification (Second ed., Vol. 6). NIR Chichester. https://doi.org/10.1255/978-1-906715-25-0
Narushin VG (1997) Non-destructive measurements of egg parameters and quality characteristics. Worlds Poult Sci J 53:141–153
Oliveri P, Downey G (2012) Multivariate class modeling for the verification of food authenticity claims. TrAC - Trends in Analytical Chemistry 35:74–86
Puertas G, Vázquez M. (2019) Fraud detection in hen housing system declared on the eggs’ label: an accuracy method based on UV-VIS-NIR spectroscopy and chemometrics Food Chem 288.
Savitzky A, Golay MJ (1964) Smoothing and differentiation of data by simplified least squares procedures. Anal Chem 36(8):1627–1639
Sato T, Kawano S, Iwamoto M. (1991). Near infrared spectral patterns of fatty acid analysis from fats and oils. J Am Oil Chem Soc 68(11):827–833
Sim JS (1998) Designer eggs and their nutritional and functional significance. In: Simopoulos, A.P. (Ed.), The return of omega-3 fatty acids into the food supply, 1. Land-based Animal Food Products and Their Health Effects, pp. 89e101. Basel, Switzerland.
Soltani M, Omid M (2015) Detection of poultry egg freshness by dielectric spectroscopy and machine learning techniques. LWT-Food Sci. Technol. 62 1034e1042.
Spink J, Moyer DC (2011) Defining the public health threat of food fraud. J Food Sci 76(9).
Stadelman WJ, Cotterill OJ (1995) Egg science and technology, 4th edn. Food Product Press, New York
Van Ruth S, Alewijn M, Rogers K, Newton-Smith E, Tena N, Bollen M, Koot A (2011) Authentication of organic and conventional eggs by carotenoid profiling. Food Chem 126(3):1299–1305
Van Ruth SM, Koot AH, Brouwer SE, Boivin N, Carcea M, Zerva CN, Rom S (2013) Eggspectation: organic egg authentication method challenged with produce from ten different countries. Quality Assurance and Safety of Crops and Foods 5(1):7–14
Williams P, Dardenne P, Flinn P (2017) Tutorial: items to be included in a report on a near infrared spectroscopy project. J near Infrared Spectrosc 25(2):85–90. https://doi.org/10.1177/0967033517702395
Workman, J., & Weyer, L. (2008) Practical guide to interpretive near-infrared spectroscopy CRC Press Taylor and Francis Group. Boca Raton
Xu L, Yan SM, Cai CB, Yu XP (2014) Nondestructive discrimination of lead (Pb) in preserved eggs (Pidan) by near-infrared spectroscopy and chemometrics. J Spectrosc 2014:1–7
Zhao J, Lin H, Chen Q, Huang X, Sun Z, Zhou F (2010) Identification of egg’s freshness using NIR and support vector data description. J Food Eng 98:408–414
Author information
Authors and Affiliations
Contributions
Hoffman, L. reviewed and edited the manuscript; Ni, D. analysis and data collection, reviewed the manuscript; Abdulghafar, N. analysis, data collection; Dayananda, B. reviewed and edited the manuscript; Cozzolino, D. data analysis, wrote the main manuscript text, supervision.
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent not applicable.
Conflict of Interest
Hoffman, Louw declares he has not conflict of interest; Ni, Dongdong declares he has not conflict of interest; Abdul Ghafar, Nuratifah declares she has not conflict of interest; Dayananda, Buddhi declares he has not conflict of interest; Cozzolino, Daniel, declares he has not conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hoffman, L., Ni, D., Dayananda, B. et al. Inside the Egg—Demonstrating Provenance Without the Cracking Using Near Infrared Spectroscopy. Food Anal. Methods 15, 3013–3019 (2022). https://doi.org/10.1007/s12161-022-02348-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-022-02348-6