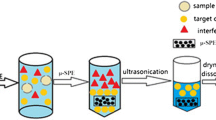
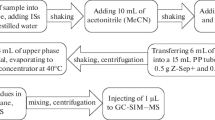
Abstract
This paper reports an analytical method to simultaneously determine the level of organophosphate ester residues in different cereal-based baby foods. The samples were extracted with acidic acetonitrile and n-hexane, using mixed adsorbent (PSA:Florisil:alumina-B, 2:2:1, w/w/w) for clean-up during the extraction procedure. This method combines sample extraction and purification into one step, reducing preparation time. The target analytes were confirmed and quantified with ultra-high-performance liquid chromatography–tandem mass spectrometry. The limits of quantitation were 0.04–0.75 ng g−1 and the recovery rate was 73.2–107.0%. The proposed method was applied for the analysis of real samples obtained from domestic markets in China. It provides a new approach to detection of pollutants in complex food samples.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organophosphate esters (OPEs) are widely used as additive flame retardants and plasticizers in a variety of products (Zhou et al. 2017), but weak physical bonding results in poor stability (Zheng et al. 2021). Therefore, in the production, transportation, and use of these consumer goods, OPEs are easily released into the environment (Malarvannan et al. 2015; Wang et al. 2015). Many studies have shown that OPEs have been detected in the air (Malarvannan et al. 2015); water (Pantelaki and Voutsa 2021); sediment (Bekele et al. 2019; Zheng et al. 2014); soil (Tang et al. 2021); dust (Cao et al. 2019); grains, vegetables, and fruits (Poma et al. 2018; Zhang et al. 2016); and even human breast milk (Kim et al. 2014). Ingestion in the diet is considered a potential major pathway for human exposure to OPEs (Zhang et al. 2016). Some studies indicate that components of OPEs have significant biological effects including kidney toxicity, neurotoxicity, reproductive toxicity, and carcinogenicity (Patisaul et al. 2021; Wang et al. 2022). For example, tris(2-chloroisopropyl) phosphate (TCPP) and tricresyl phosphate (TCP) cause developmental toxicity, neurotoxic effects, and other detrimental effects in fish, animals, and humans (Bekele et al. 2021; van der Veen and Boer 2012). As a result of these toxic effects, the US Consumer Product Safety Commission warned manufacturers and consumers in 2017 to prohibit all halogenated OPEs in children’s products (Bekele et al. 2021).
As a special group, infants are vulnerable to health threats caused by exposure to OPEs. At present, most studies concerning infants’ OPE intake were focused on the contamination of OPEs in human milk and infant formulas. According to the previous study, the mean concentration of Σ14OPEs (sum of 14 OPEs) in Belgian baby food was up to 12.2 ng g−1 (Poma et al. 2018). Meanwhile, the concentration of Σ14OPEs was ranged from 0.79 to 159 ng g−1 in infant formulas which collected in Beijing (Chen et al. 2022). Meanwhile, feeding the baby supplementary food was also an important way for infants to take OPEs. The concentrations of Σ14OPEs in the baby supplementary food samples were 4.42–115 ng g−1 (median: 19.5 ng/g). The median estimated dietary intakes of the Σ14OPEs were from 429 to 470 ng/kg bodyweight/day for infants via supplementary food BSF feeding. (Chen et al. 2022). Data concerning OPEs in foods were very limited, but the contamination of OPEs in food should not be neglected.
Monitoring OPE content in baby food to control infants’ OPE intake is an effective method of preventing, or at the least reducing, their exposure. Analytical methods with sufficient sensitivity and specificity to detect OPE residues in food are needed. At present, pretreatment of OPEs in foodstuffs to prepare them for testing consists of two steps: extraction and purification. The extraction methods include ultrasound-assisted extraction (Ding et al. 2018) and accelerated solvent extraction (ASE) (Zheng et al. 2014), while the purification methods include solid-phase extraction (SPE) (Brandsmaet al. 2015), solid-phase microextraction (SPME) (Gao et al. 2014), dispersed solid-phase extraction (d-SPE) (Ding et al. 2018) and gel permeation chromatography (Kim et al. 2014). Liquid chromatography–tandem mass spectrometry (LC–MS) (Hou et al. 2017), gas chromatography–tandem mass spectrometry (GC–MS) (Bekele et al. 2019), or gas chromatography–flame photometric detector (GC-FPD) (Gao et al. 2014) can be chosen for subsequent testing. Extraction and purification are divided into two steps, increasing sample pretreatment time, while the selected-ASE method proposed here for extraction and purification is completed in one step. The selected-ASE method involves in-cell extraction and purification, and is able to efficiently extract the targeted compounds and remove potentially interfering matrix compounds (Subedi et al. 2015). The selected-ASE has been used in determining levels of hazardous substances such as antibiotics (Zhu et al. 2018), pesticides (Wang et al. 2020), triclosan (Canosa et al. 2007), and persistent organic pollutant (Gbeddy et al. 2020) in environmental samples.
In this study, the selected accelerated solvent extraction apparatus to extract and purify the sample, and used ultra-high-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) with high selectivity and sensitivity to simultaneously determine the levels of 12 kinds of OPEs including 5 non-halogenated alky-OPEs, 3 halogenated alky-OPEs, and 4 aryl-OPEs in cereal-based baby food.
Materials and Methods
Reagents and Materials
Standards of tri(2-butoxyethyl) phosphate (TBEP), tris(2-chloroethyl) phosphate (TCEP), tributyl phosphate (TBP), triethyl phosphate (TEP), trimethyl phosphate (TMP), triphenyl phosphate (TPPA), tris(2-chloroisopropyl) phosphate (TCPP), tri-n-propyl phosphate (TPP), tris(1,3-dichloro-2-propyl) phosphate (TDCPP), cresyl diphenyl phosphate (CDPP), 2-ethylhexyl-diphenyl phosphate (EHDPP), and tricresyl phosphate (TCP) were purchased from Dr. Ehrenstorfer, GmbH (Germany). Isotopically labeled compounds, d15-TEP, d21-TPP, and d15-TPPA, were purchased from A ChemTek Inc (USA).
HPLC-grade acetonitrile and methanol were obtained from Merck (Germany), HPLC-grade acetic acid was purchased from Scharlau (Spain) and ammonium formate was purchased from Acros Organics (USA). Analytical grade n-hexane was purchased from Shanghai Lingfeng Chemical Reagent Co., LTD (China). Primary secondary amine sorbent (PSA, 40 μm particle size) and Florisil (60–100 mesh) were purchased from Agela Technologies (China). Diatomaceous earth (80–100 mesh) was purchased from Thermo Scientific (USA). Alumina-B was from Aladdin Industrial (China). Deionized water (18 Mcm) was purified by the Milli-Q water purification system (USA).
Stock standard solutions (100–300 mg L−1) of analytes and internal standards were prepared in methanol. A series of working solutions were prepared by appropriate dilution of the stock solution before use.
Preparation of Samples and Selective Accelerated Liquid Extraction Procedure
A total of 15 samples of cereal foods for babies (rice cereals, noodles, and biscuits) were collected from supermarkets in Zhejiang Province (China). They were ground in a mortar or grinder, screened through an Φ 0.5–mm sieve, covered with aluminum foil, and stored at − 20℃ until analysis. During sample collection, storage, transfer, and extraction, the plastic or rubber products were avoided.
The target compounds were extracted and purified by ASE using an ASE 350 system (Thermo Scientific, USA) with the adsorbents packed in the extraction cells. The glass fiber filter paper, 2.0 g diatomaceous earth, 125 mg mixed adsorbent (PSA:Florisil:alumina-B, 2:2:1, w/w/w), a 1.0 g sample spiked with internal standards of OPEs, and 3.0 g of diatomaceous earth in each 34-mL stainless steel extraction cell. The target was extracted twice with 0.5% formic acid acetonitrile: n-hexane (v/v) at 80℃, 1500 psi with the static extraction of 5 min. Then, the extract was condensed to dryness using a Turbovap®LV (Biotage, Sweden) with a nitrogen stream. The residue was dissolved in 4.0 mL methanol:water (1:1, v/v), then centrifuged at 10,000 rpm for 3 min, and the supernatant was collected and filtered through a 0.22-μm PTFE filter for analysis.
To reduce the risk of contamination as much as possible, all of the vessels were washed with distilled water, methanol, and n-hexane three times before use, and glass wares were heated to 450℃ and kept for 4 h in the muffle furnace. There were 10 samples to be analyzed, and a procedure blank was run in sequence to check for background contamination.
Instrumentation and Chromatographic Conditions
An LC30A (Shimadzu, Japan) system equipped with an AB SCIEX (USA) 6500Q mass spectrometry was used to quantify OPEs. Chromatographic separation was carried out using a BEH-C18 column (2.1 × 100 mm, 1.7 μm; Waters, USA) at 40℃. Methanol and 2 mmol L−1 aqueous ammonium acetate consisting of 0.1% formic acid were used as mobile phases A and B at a constant flow rate of 0.4 mL min−1. The gradient elution program was as follows: the initial mobile phase containing 20% A was held for 1 min, increased linearly to 95% in 1 min and held for 2 min, then decreased to 20% in 0.1 min and held for an additional 1.9 min. The injection volume of the sample was set to 5 μL. With regard to the mass spectrometer, a positive electrospray ionization source mode was used. Ion-spray voltage was 4500 V, ion source temperature was 500 °C, gas 1 and gas 2 pressure were both 50 psi, curtain gas pressure was 40 psi. The selected reaction monitoring (SRM) mode was performed on each analyte, and the dwell time was 3 ms. The following aspects of MRM are shown in Table 1: m/z transitions, declustering potentials, and collision energies.
Quantification and Method Validation
The method was validated by assessing the matrix effects, linear range, recovery, intra-day and inter-day precision, the limit of detection (LOD), and limit of quantification (LOQ) for each analyte. Quantification was based on linear regression calibration curves constructed with an internal standard approach at concentrations of 0.04, 0.2, 0.4, 2.0, 4.0, 40.0, 200, and 400 ng g−1. Matrix effects by comparing the slopes of the matrix-matched calibration curve and standard calibration curves. If the ratio between the two was less than 100%, this indicated that the matrix effect was mainly suppressive; if the ratio was more than 100%, it was clear that the matrix mainly had an enhancing effect. Analyte recovery and precision of the method were evaluated by analyzing blank mixture samples (rice cereals:noodles:biscuits, 1:1:1, w/w/w) that had been spiked with the analytes at three concentrations (2, 20, and 100 ng g−1) before the extraction procedure (with six parallel samples prepared at each concentration). The fortified samples were extracted, purified, and analyzed by selected-ASE-UPLC-MS/MS. The intra-day and inter-day precision values were assessed using the relative standard deviations (RSDs) of the results for spiked samples analyzed on the same day and different days, respectively. The LOD and LOQ were defined as the concentrations giving signal-to-noise ratios of 3 and 10 in the matrix, respectively.
Simplex Centroid Mixture Design and Statistical Analysis
A three-component simplex centroid mixture design (Design Expert 10 software) was used to identify the optimum purification materials. A total of 14 groups of experiments with 10 points were performed (Table S1). Each group was carried out in triplicate, and the sum of the 12 target analytes for the same group mean values were used to evaluate the influence of various factors on the experimental results (Kowalski et al. 2002; Zhang et al. 2021). The Statsoft Statistica 19.0 software was used to draw the fitted curves, and LINGO 15.0 software was used to identify extreme values. Besides, Excel 2016 and Origin 2018 software were used for data calculations and statistical analysis.
Results and Discussion
Optimization of Selected Accelerated Solvent Extraction Conditions
The experimental parameters such as extraction solvent and extraction temperature were optimized using the one-variable-at-a-time method. A simplex centroid mixture design was used to optimize the sorbents. All of the optimization experiments were performed at a spiking level of 20 ng g−1. Recovery of the target compounds was calculated using matrix-matched calibrations and evaluated under different conditions.
Optimization of the Extraction Solvent
As the boiling point of a solvent depends on the pressure, the optimal extraction pressure of the accelerated solvent extractor to prevent the extraction solvent from boiling is 1500 psi (Richter et al. 1996). Thus, in this study, a constant pressure of 1500 psi was used. The extraction solvent is an important factor affecting extraction efficiency in the process of ASE. In general, physical and chemical properties such as boiling point, polarity, density, and toxicity of the extraction solvent should be considered when selecting the solvent. In addition, according to the principle of similarity compatibility, the selected extraction solvent should have similar polarity to the target compound. To extract the maximum target compounds with minimum interference, acetonitrile, methanol, and n-hexane were assessed. Due to the marked differences in the physicochemical properties of OPEs, recovery of the selected compounds under the same conditions was quite different. N-hexane was the best solvent for TCP and TPPA, but recovery of TEP and TMP was below 30%. As TEP and TMP have strong polarity, their recovery was increased by enhancing solvent polarity. Methanol and acetonitrile, as polar solvents, resulted in lower recovery of EHDPP, TCP, and CDPP. Clearly, it is important to select the appropriate extraction solvent to satisfy the wide polarity range of compounds. Therefore, the mixed solvents acetonitrile-n-hexane and acetonitrile (1:1, containing 0.5% formic acid, v/v)-n-hexane (1:1, v/v) were investigated. Results showed that the acetonitrile (containing 0.5% formic acid)–n-hexane within the target especially for TMP’s recovery increased significantly, and therefore acidified acetonitrile and n-hexane were selected as the extraction solvent for the following experiments.
Optimization of the Extraction Cycles
This study explored the effects of different numbers of extraction cycles (1, 2, and 3) on recovery of the target compounds. As shown in Fig. 1, recovery of all the compounds was increased with more extraction cycles. But there were no significant differences between two and three extraction cycles. When raising the number of extraction cycles, the introduced fresh solvent in each cycle increased the total volume of solvent, thus promoting extraction of the target compounds. Because more extraction cycles would have resulted in extraction of not only more target compounds, but more interfering compounds, and increased the time and cost, two extraction cycles were chosen as the optimal number for use in further experiments.
Optimization of the Extraction Temperature
Extraction temperature affects the viscosity of extraction solvent and the solubility of the target compound, and also affects extraction efficiency. The influence of extraction temperature on recovery of the target compounds at four different extraction temperatures (60, 80, 100, and 120 °C) was explored. In the beginning, recovery of most of the target compounds increased with increasing extraction temperature, with the exception of TBEP and TBP. However, when the extraction temperature was 80℃, recovery of TCEP, TEP, TPP, and TDCPP (73.1–86.4%) was significantly higher than at 60 °C (68.8–80.4%), 100 °C (69.3–79.6%), or 120 °C (67.0–81.0%) (Fig. 2). The other compounds were less affected by temperature. Thus, 80 °C was chosen as a feasible extraction temperature.
Optimization of Adsorbent
Sample purification commonly determines a target compound’s signal response. A highly efficient clean-up adsorbent can remove interfering compounds while retaining most of the target compound. PSA, Florisil, and alumina-B are commercially available and are the most commonly used purification materials for organophosphorus esters in the food matrix (Guo et al. 2016; Zhang et al. 2016). PSA can effectively remove impurities such as fatty acids, sugars, and some polar pigments common in the food matrix (Yin et al. 2022), Florisil preferentially adsorbs polar components in the nonpolar matrix, and alumina-B is suitable for adsorbing lipid compounds (Yang et al. 2020). Considering the presence of lipids, proteins, pigments, carbohydrates, fatty acids, and other co-extraction matrix components in the food matrix which could cause interference (Poma et al. 2018), the type of sorbent has a significant influence on sample purification. Thus, the simplex centroid mixture design was used to select the most suitable absorbent combination. The fitting function for this study was as follows: Recovery = 0.7194 × A + 0.6924 × B + 0.7429 × C + 0.1771 × A × B − 0.5782 × A × C + 0.1305 × B × C + 7.46 × A × A × B × C + 3.25 × A × B × B × C − 1.45 × A × B × C × C, where A, B, and C are PSA, Florisil, and alumina-B mass fractions, respectively. According to the selected regression equation, the recovery was modeled, and the contour diagram and response surface diagram was shown in Fig. 3. Contour lines are ellipses, indicating strong interaction among factors and significant influence of each absorbent on recovery. The recovery rate increases with the increase of PSA and Florisil, but decreases after reaching a certain value. Alumina-B showed the opposite trend. In order to determine the rationality of the mode, square difference analysis was carried out (shown in Table S2). The R2 was 0.9842, the p-value was 0.0004 < 0.01, indicating that the height of the model is significant, but the lack of fit p-value was 0.6920 > 0.05, indicating that the established equation has good fitting and high reliability. The response surface is arched and the recovery has a maximum value (Fig. 3). At A = 0.4243876, B = 0.3957150, and C = 0.1798974, the function gave the maximum recovery (assuming that A + B + C = 1 and A, B, and C were all ≥ 0). For ease of operation, the optimum adsorbent ratio was defined as a 2:2:1 w/w/w mixture of PSA, Florisil, and alumina-B.
As the amount of the purification material also influences extraction efficiency, the purification effects of 75 mg, 100 mg, 125 mg, and 150 mg of the purification materials were investigated. When the amount was increased from 75 to 100 mg, there was no obvious variation in recovery of the compounds. However, when the amount was increased from 100 to 125 mg, recovery increased, and when the highest amount (150 mg) was used, recovery of most compounds actually decreased significantly. Ultimately, 125 mg were selected as the optimal amount.
Method Validation and Comparison
Co-extracts present in the food matrix can cause changes in the baseline of chromatograms and the responses of target compounds, so such effects from the matrix were assessed. Most of the target compounds only experienced a weak matrix inhibition effect and the slope ratio of the two curves was between 83.2 and 97.9%, which indicates that the method was not affected by matrix effects and gave satisfactory purification performance. However, to ensure that the analyte concentrations were as accurate as possible, the isotope internal standard method was used for quantitative analysis to compensate for possible matrix effects and pretreatment losses. For TCEP and TDCPP, the linear ranges were 0.4–400 ng g−1, while the linear ranges of the remaining 10 targets were 0.04–400 ng g−1. Good linearity was obtained and the linear correlation coefficients of the target compounds were all greater than 0.99. Table 2 shows that recovery of the target compounds ranged from 73.2 to 107.0% at the three spiking levels examined. With regard to the precision of the method, the RSDs were satisfactory (shown in Table 2). The LOQs for the test compounds were 0.04–0.75 ng g−1 (shown in Table 2). The recoveries and RSDs at the spiked at LOQ concentrations were also tested (shown in Table 2). These results confirmed that the LODs and LOQs achieved with the method were sufficient to determine concentrations of organic phosphate ester pollutants in cereal-based baby food samples.
Additionally, the analytical performance of the proposed method with that of other reported ASE methods for foodstuffs was compared (Table 3). In these methods, the OPEs were extracted first, and then SPE was applied mostly for clean-up. The proposed method simplifies sample preparation because extraction and purification occur in one step. In addition, selected-ASE is an automated approach, which is easily to execute and can process up to 24 samples at a time. In the experiments described above, the sensitivity and precision of this method was comparable to previously reported methods.
Real Sample Analysis
The proposed method was applied to 15 real samples (five rice cereals, five noodles, and five biscuits) purchased in retail markets in Zhejiang to evaluate the presence of OPEs in commonly available cereal-based baby foods (Table S3). Most of the OPEs were detected in the studied samples, except TPP, TCP, and TMP. Seventy-three percent of the samples contained at least one OPE. The concentration of ΣOPEs in cereal-based baby foods analyzed was ND-3.84 ng g−1, which was relatively lower than the concentration in cereals reported in the previous literature with the concentration of ND-21.0 ng/g (Zhao et al. 2019). TEP and EHDPP were detected in seven samples, with ranges of 0.18–2.30 ng g−1 and 0.21–1.22 ng g−1, respectively. The number of samples in which TCPP was detected was 2, 2, and 1 for rice cereal, biscuit, and noodle samples, respectively. The high detection rate of TCPP may be due to the food packaging, which has been suggested as a source of TCPP (Han et al. 2019).
Conclusions
In this study, an analytical method based on selected-ASE–UPLC-MS/MS to determine 12 target compounds in cereal-based baby food samples were established. Acidified acetonitrile and n-hexane were used as the extraction solvents to achieve efficient extraction of target substances in a wide polar range. The selected-ASE process combines sample extraction and purification, reduces preparation time, and provides high-throughput analysis. The analysis results revealed that the method established exhibited good linearity and satisfactory accuracy and precision. Under optimal conditions, recovery of the target compounds ranged from 73.2–107.0%, with LOQs of 0.04–0.75 ng g−1. On the basis of detection results from actual samples, the quantitative multi-residue detection method established here can be applied to determine the level of OPEs in cereal-based baby food samples. It provides a new approach to detecting pollutants in complex food samples.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Bekele TG, Zhao H, Wang Q, Chen J (2019) Bioaccumulation and trophic transfer of emerging organophosphate flame retardants in the marine food webs of Laizhou Bay. North China Environ Sci Technol 53(22):13417–13426. https://doi.org/10.1021/acs.est.9b03687
Bekele TG, Zhao H, Yang J, Chegen RG, Chen J, Mekonen S, Qadeer A (2021) A review of environmental occurrence, analysis, bioaccumulation, and toxicity of organophosphate esters. Environ Sci Pollut Res Int 28(36):49507–49528. https://doi.org/10.1007/s11356-021-15861-8
Brandsma SH, Leonards PE, Leslie HA, de Boer J (2015) Tracing organophosphorus and brominated flame retardants and plasticizers in an estuarine food web. Sci Total Environ 505:22–31. https://doi.org/10.1016/j.scitotenv.2014.08.072
Canosa P, Perez-Palacios D, Garrido-Lopez A, Tena MT, Rodriguez I, Rubi E, Cela R (2007) Pressurized liquid extraction with in-cell clean-up followed by gas chromatography-tandem mass spectrometry for the selective determination of parabens and triclosan in indoor dust. J Chromatogr A 1161(1–2):105–112. https://doi.org/10.1016/j.chroma.2007.05.089
Cao D, Lv K, Gao W, Fu J, Wu J, Fu J, Wang Y, Jiang G (2019) Presence and human exposure assessment of organophosphate flame retardants (OPEs) in indoor dust and air in Beijing, China. Ecotoxicol Environ Saf 169:383–391. https://doi.org/10.1016/j.ecoenv.2018.11.038
Chen N, Fan S, Zhang N, Zhao Y, Yao S, Chen X, Liu X, Shi Z (2022) Organophosphate esters and their diester metabolites in infant formulas and baby supplementary foods collected in Beijing, China: occurrence and the implications for infant exposure. Sci Total Environ 827:154272. https://doi.org/10.1016/j.scitotenv.2022.154272
Ding J, Deng T, Xu M, Wang S, Yang F (2018) Residuals of organophosphate esters in foodstuffs and implication for human exposure. Environ Pollut 233:986–991. https://doi.org/10.1016/j.envpol.2017.09.092
Gao Z, Deng Y, Yuan W, He H, Yang S, Sun C (2014) Determination of organophosphorus flame retardants in fish by pressurized liquid extraction using aqueous solutions and solid-phase microextraction coupled with gas chromatography-flame photometric detector. J Chromatogr A 1366:31–37. https://doi.org/10.1016/j.chroma.2014.09.028
Gbeddy G, Egodawatta P, Goonetilleke A, Ayoko G, Jayarathne A, Chen L, Russell S (2020) Optimized simultaneous pressurized fluid extraction and in-cell clean-up, and analysis of polycyclic aromatic hydrocarbons (PAHs), and nitro-, carbonyl-, hydroxy -PAHs in solid particles. Anal Chim Acta 1125:19–28. https://doi.org/10.1016/j.aca.2020.05.021
Guo X, Mu T, Xian Y, Luo D, Wang C (2016) Ultra-performance liquid chromatography tandem mass spectrometry for the rapid simultaneous analysis of nine organophosphate esters in milk powder. Food Chem 196:673–681. https://doi.org/10.1016/j.foodchem.2015.09.100
Han L, Sapozhnikova Y, Nunez A (2019) Analysis and occurrence of organophosphate esters in meats and fish consumed in the United States. J Agric Food Chem 67(46):12652–12662. https://doi.org/10.1021/acs.jafc.9b01548
Hou R, Liu C, Gao X, Xu Y, Zha J, Wang Z (2017) Accumulation and distribution of organophosphate flame retardants (PFRs) and their di-alkyl phosphates (DAPs) metabolites in different freshwater fish from locations around Beijing, China. Environ Pollut 229:548–556. https://doi.org/10.1016/j.envpol.2017.06.097
Kim JW, Isobe T, Muto M, Tue NM, Katsura K, Malarvannan G, Tanabe S (2014) Organophosphorus flame retardants (PFRs) in human breast milk from several Asian countries. Chemosphere 116:91–97. https://doi.org/10.1016/j.chemosphere.2014.02.033
Kowalski SM, Cornell JA, Vining CG (2002) Split-plot designs and estimation methods for mixture experiments with process variables. Technometrics 44:72–79. https://doi.org/10.1198/004017002753398344
Malarvannan G, Belpaire C, Geeraerts C, Eulaers I, Neels H, Covaci A (2015) Organophosphorus flame retardants in the European eel in Flanders, Belgium: occurrence, fate and human health risk. Environ Res 140:604–610. https://doi.org/10.1016/j.envres.2015.05.021
Pantelaki I, Voutsa D (2021) Organophosphate esters in inland and coastal waters in northern Greece. Sci Total Environ 800:149544. https://doi.org/10.1016/j.scitotenv.2021.149544
Patisaul HB, Behl M, Birnbaum LS, Blum A, Diamond ML, Rojello Fernandez S, Hogberg HT, Kwiatkowski CF, Page JD, Soehl A, Stapleton HM (2021) Beyond cholinesterase inhibition: developmental neurotoxicity of organophosphate ester flame retardants and plasticizers. Environ Health Perspect 129(10):105001. https://doi.org/10.1289/EHP9285
Poma G, Sales C, Bruyland B, Christia C, Goscinny S, Van Loco J, Covaci A (2018) Occurrence of organophosphorus flame retardants and plasticizers (PFRs) in Belgian foodstuffs and estimation of the dietary exposure of the adult population. Environ Sci Technol 52(4):2331–2338. https://doi.org/10.1021/acs.est.7b06395
Richter BE, Jones BA, Ezzell JL, Porter NL, Avdalovic N, Pohl C (1996) Accelerated solvent extraction: a technique for sample preparation. Anal Chem 68(6):1033–1039. https://doi.org/10.1021/ac9508199
Subedi B, Aguilar L, Robinson EM, Hageman KJ, Björklund E, Sheesley RJ, Usenko S (2015) Selective pressurized liquid extraction as a sample-preparation technique for persistent organic pollutants and contaminants of emerging concern. Trend Anal Chem 68:119–132. https://doi.org/10.1016/j.trac.2015.02.011
Tang J, Sun J, Ke Z, Yin H, Yang L, Yen H, Li X, Xu Y (2021) Organophosphate esters in surface soils from a heavily urbanized region of Eastern China: occurrence, distribution, and ecological risk assessment. Environ Pollut 291:118200. https://doi.org/10.1016/j.envpol.2021.118200
van der Veen I, de Boer J (2012) Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88(10):1119–1153. https://doi.org/10.1016/j.chemosphere.2012.03.067
Wang J, Xu J, Ji X, Wu H, Yang H, Zhang H, Zhang X, Li Z, Ni X, Qian M (2020) Determination of veterinary drug/pesticide residues in livestock and poultry excrement using selective accelerated solvent extraction and magnetic material purification combined with ultra-high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 1617:460808. https://doi.org/10.1016/j.chroma.2019.460808
Wang R, Tang J, Xie Z, Mi W, Chen Y, Wolschke H, Tian C, Pan X, Luo Y, Ebinghaus R (2015) Occurrence and spatial distribution of organophosphate ester flame retardants and plasticizers in 40 rivers draining into the Bohai Sea, north China. Environ Pollut 198:172–178. https://doi.org/10.1016/j.envpol.2014.12.037
Wang X, Wang L, Li F, Teng Y, Ji C, Wu H (2022) Toxicity pathways of lipid metabolic disorders induced by typical replacement flame retardants via data-driven analysis, in silico and in vitro approaches. Chemosphere 287(Pt 4):132419. https://doi.org/10.1016/j.chemosphere.2021.132419
Yang J, Zahng Q, Su L (2020) Advances in the development of detection techniques for organophosphate ester flame retardants in food. Se Pu 38(12):1369–1380. https://doi.org/10.3724/SP.J.1123.2020.03026
Yin Z, Yuan B, Lyu W, Huang Q, Simon JE, Wu Q (2022) Method development and validation for analysis of phenolic compounds in fatty complex matrices using enhanced matrix removal (EMR) lipid cleanup and UHPLC-QqQ-MS/MS. Food Chem 373(Pt A):131096. https://doi.org/10.1016/j.foodchem.2021.131096
Zhang X, Li Z, Wu H, Wang J, Zhao H, Ji X, Xu Y, Li R, Zhang H, Yang H, Qian M (2021) High-throughput method based on a novel thin-film microextraction coating for determining macrolides and lincosamides in honey. Food Chem 346:128920. https://doi.org/10.1016/j.foodchem.2020.128920
Zhang X, Zou W, Mu L, Chen Y, Ren C, Hu X, Zhou Q (2016) Rice ingestion is a major pathway for human exposure to organophosphate flame retardants (OPFRs) in China. J Hazard Mater 318:686–693. https://doi.org/10.1016/j.jhazmat.2016.07.055
Zhao L, Jian K, Su H, Zhang Y, Li J, Letcher RJ, Su G (2019) Organophosphate esters (OPEs) in Chinese foodstuffs: dietary intake estimation via a market basket method, and suspect screening using high-resolution mass spectrometry. Environ Int 128:343–352. https://doi.org/10.1016/j.envint.2019.04.055
Zheng G, Schreder E, Dempsey JC, Uding N, Chu V, Andres G, Sathyanarayana S, Salamova A (2021) Organophosphate esters and their metabolites in breast milk from the United States: breastfeeding is an important exposure pathway for infants. Environ Sci Technol Lett 8(3):224–230. https://doi.org/10.1021/acs.estlett.0c00916
Zheng J, Gao Z, Yuan W, He H, Yang S, Sun C (2014) Development of pressurized liquid extraction and solid-phase microextraction combined with gas chromatography and flame photometric detection for the determination of organophosphate esters in sediments. J Sep Sci 37(17):2424–2430. https://doi.org/10.1002/jssc.201301274
Zhou L, Hiltscher M, Gruber D, Püttmann W (2017) Organophosphate flame retardants (OPFRs) in indoor and outdoor air in the Rhine/Main area, Germany: comparison of concentrations and distribution profiles in different microenvironments. Environ Sci Pollut Res 24(12):10992–11005. https://doi.org/10.1007/s11356-016-6902-z
Zhu M, Zhao H, Xia D, Du J, Xie H, Chen J (2018) Determination of 21 antibiotics in sea cucumber using accelerated solvent extraction with in-cell clean-up coupled to ultra-performance liquid chromatography-tandem mass spectrometry. Food Chem 258:87–94. https://doi.org/10.1016/j.foodchem.2018.03.051
Funding
This work has been financially supported by the Natural Science Foundation of China (No. 3210161286), Natural Science Foundation of Hebei Province of China (No. B2021207002), and Zhejiang Shuren University Basic Scientific Research Special Funds (2020XZ011).
Author information
Authors and Affiliations
Contributions
This paper has multiple authors and our individual contributions were as below statement: Huizhen Wu: conceptualization, data curation, writing original draft, and review. Min Zhou: performed the experiment and instrumental analysis. Binyu Zheng and Ningying Song sample pretreatment Dan Wei: validation and funding acquisition. Chao Lei: sample pretreatment. Nabo Sun: analyze the study data and funding acquisition. Mingrong Qian designed the experiment, supervision, writing—reviewing and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of Interest
Huizhen Wu declares that he has no conflict of interest. Min Zhou declares that he has no conflict of interest. Binyu Zheng declares that he has no conflict of interest. Ningying Song declares that he has no conflict of interest. Dan Wei declares that he has no conflict of interest. Chao Lei declares that he has no conflict of interest. Nabo Sun declares that he has no conflict of interest. Mingrong Qian declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, H., Zhou, M., Zheng, B. et al. Selective Accelerated Solvent Extraction for Multi-residue Analysis of Organophosphate Esters in Cereal-Based Baby Food. Food Anal. Methods 15, 2994–3002 (2022). https://doi.org/10.1007/s12161-022-02347-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-022-02347-7