Abstract
The goal of this study was to optimize an innovative ultrahigh-pressure–assisted extraction method for phenolic compounds of date palm (Phoenix dactylifera L.) using response surface methodology (RSM). A four-factor-three-level Box–Behnken design (BBD) was used to test the following extraction parameters: ethanol concentration (X1), solvent to sample ratio (X2), temperature (X3), and pressure (X4). Analysis of variance (ANOVA) results revealed that the main treatment X1, X2, and X4 significantly affected extraction yield, while X3 did not. The optimum response for the combination of phenolic compounds and antioxidant activity was obtained with following extraction parameters predicted by the model: X1 = 60.54% (v/v), X2 = 70 mL/g, X3 = 65 °C, and X4 = 600 MPa. These parameters were validated in an additional experiment carried out under the optimal conditions given by experimental results; the experimental values obtained were in close agreement with the predicted values. This suggests that the RSM BBD model described in this work is an efficient quantitative tool to predict the extraction efficiency of polyphenols and antioxidants capacity from date palm fruit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among natural sources, and for thousands of years, date palm (Phoenix dactylifera L., Arecaceae) has played an important role in the development of human societies due to its significant nutritional and economic value (Chandrasekaran and Bahkali 2013). Recently, special attention was given to health benefits of date palms, as well as the identification and quantification of different classes of bioactive compounds it contains (Chaira et al. 2009). Several studies have shown that dates provide many health benefits and display immunostimulant, antimutagenic, antimicrobial, antiinflammatory, anticancer, and gastrohepatoprotective properties (Tang et al. 2013). Previous reports have also suggested that date fruit may provide cardiovascular protection (Al-Yahya et al. 2016). The high polyphenol content of the fruit is most probably responsible for these therapeutic effects (Al-Farsi and Lee 2008); date palm accumulates phenolic compounds to protect themselves from extreme temperature and arid climate (Vinson et al. 2005).
The first and most important step in the recovery and purification of bioactive compounds from plant materials is extraction, which conventionally is made by maceration, distillation, or Soxhlet reflux extraction. These methods are hampered by long extraction periods and low extraction efficiency (Xu et al. 2017). In this respect, an optimized large-scale extraction procedure should have high efficiency in the shortest processing time possible. A new extraction technique is gaining credence for the extraction of target compounds from different plant materials. Ultrahigh-hydrostatic pressure (UHP) extraction is an emerging technology increasingly used in the food industry for several processes like cold pasteurization (Mújica-Paz et al. 2011) and has been recognized as an environment-friendly technology by the US Food and Drug Administration (Joo et al. 2011). UHP extraction technique offers many advantages like short extraction time, high extraction yield, higher purity, high reproducibility, simplified manipulation, and low energy input, as well as reduced solvent consumption. In addition, it can be carried out at lower temperatures, avoiding thermal denaturation, and structural change of thermosensible molecules of an extract (Lee et al. 2011). UHP extraction technique was utilized for the first time in 1994 on onions plant (Butz et al. 1994). Since this study and due to its efficiency, this technique has been employed for the extraction of several bioactive compounds such as phenolic compounds from Pinus densiflora root (Joo et al. 2011), green tea (Xi et al. 2013), fig byproducts (Alexandre et al. 2017), and watercress (Pinela et al. 2018); flavonoids from Laoying tea (Ji et al. 2011); berberine from Cortex phellodendri (Guoping 2012); and ginsenosides from ginseng (Lee et al. 2011) and ginseng root (Chen et al. 2009). In the study of Chen, an additional and important advantage was reported, in addition to other advantages cited above, and which concern mechanical effects of ultrahigh pressure that enhances penetration of solvent into cellular material improving then the release of cell compounds. To prove this phenomenon, Chen et al. (2009) performed scan electron micrography of ginseng root treated with UHP only for 5 min at 200 MPa and 60 °C that showed a big destruction of cell tissues with hollow breaks and very small particles with comparison with untreated or ginseng root treated with heat reflux extraction. This confirm that the damage caused by UHP on cell walls of the plant material is considerable leading to a subsequent change in the surface tension that facilitates then the diffusion and osmotic process and greatly improve extraction of biocompounds (Lee et al. 2011; Zhang et al. 2012). This advantage lead then to another one which is the absence of impurities in the extraction solvent that enhance then the quality of the extract (Wang et al. 2018).
In previous studies, UHP extraction parameters were optimized by a tedious and time consuming one-factor-at-a-time approach (Prasad et al. 2009). Thus, in order to optimize the extraction of polyphenol compounds and antioxidant activity, we optimized the extraction conditions using a response surface methodology (RSM) which is a time- and reagent-saving statistical approach increasingly used in process optimization. By using this method, we tested different solvent concentration, solvent to sample ratio, temperature, and pressure rate (Alexandre et al. 2017) parameters that affect the extraction yield. RSM allow the evaluation of the effects of multiple variables and their interactions on extraction responses. It has been successfully used to optimize the extraction of phenolic compounds from many plants (Belwal et al. 2016; Chen et al. 2018; Espada-Bellido et al. 2017). Generally, there are three most common types of RSM models which are the three-level factorial, the central composite, and the Box–Behnken design (Baş and Boyacı 2007). The latest was used in this study, because it enables the determination of optimum parameters with a minimal number of experiments compared with other designs.
To our knowledge, this is the first study aimed at the optimization of polyphenolic compounds and antioxidant activities extraction from date palm fruit using ultrahigh-pressure extraction technology and response surface methodology. Hence, the objective of this study is to explore and to optimize extraction parameters including ethanol concentration, liquid/solid ratio, temperature, and pressure by using the RSM Box–Behnken design (BBD) tool to obtain the highest polyphenolic content and antioxidant activity from palm dates. Then, we hypothesize that the predictive values obtained by RSM BBD methodology agrees with the experimental values and that this statistical tool is a valid optimization model for the extraction of polyphenolic compounds and antioxidant activities from palm date fruit.
Materials and Methods
Plant Materials
Palm dates were collected from the crops of 2017/2018 at the arid region of Kebili (Tunisia). Samples of palm date Degelt Nour variety were pitted and washed with distilled water. Then, samples were cut and kept in the freezer (− 80 °C) overnight. After that, samples were lyophilized and then ground using a household grinder to obtain a fine powder. The lyophilized samples were kept at − 20 °C until extraction.
Chemicals and Reagents
Ethanol (100 %) used for extraction were obtained from Les Alcools du Commerce Inc. (Boucherville, Canada). Deionized water was prepared using a MilliQ water purification system from Millipore (Etobicoke, Canada). Folin–Ciocalteu reagent, sodium carbonate (Na2CO3), gallic acid, and DPPH (2,2-diphenyl-1-picrylhydrazyl) were purchased from Sigma–Aldrich (Canada), 4-Dimethylaminocinnamaldehyde (DMAC) was from Sigma–Aldrich (St Louis, MO, USA), procyanidin A2 (HPLC; purity > 99%) was purchased from Extrasynthèse, Genay Cedex, France.
Ultrahigh Hydrostatic Pressure Extraction
The ultrahigh pressure apparatus was purchased from ISO-LAB' model S-IL-085-O9-AO (Stansted, Essex, UK). The principle of UHP is based on increasing the pressure (usually 100 to 1000 MPa) of a pressure transmitter liquid. A homogeneous pressure is then applied at every point of the product to minimize its deformation. The treatment time varies according to the applications. The process can be used with or without heat. The specifications of this equipment were 500 mL, effective volume of the vessel; 900 MPa, maximal working pressure; 30 mm, vessel inner diameter; water and glycol (20/80, v/v), pressure transmitting media.
For the extraction process with UHP, 1 g of the dried palm date powder was placed in a sterile polyethylene bag containing aqueous ethanol in desired proportion and volume. The bag was sealed after removing bubbles from the inside and loaded into a pressure tank which was equipped with release pressure valves and temperature controllers at the top and bottom of the vessel to keep desired extraction conditions. The pressure tank was pressurized with the fluid by an ultrahigh pressure booster pump. According to the experimental design shown in Table 1, the extraction conditions were ethanol concentration (X1, 0–100 %), liquid/solid ratio (X2, 10–70 mL/g), temperature (X3, 25–65 °C), and pressure (X4, 100–600 MPa). After extraction, the mixture was filtered through a filter paper. The extract was centrifuged at 4000×g for 10 min, and the supernatant was stored at − 20 °C until analysis.
Determination of the Total Phenolic Compounds
Total phenolic content (TPC) of different extracts was determined using Folin–Ciocalteu reagent slightly modified by Belwal et al. (2016) using gallic acid as a standard. Briefly, 10 μL of extract was mixed with 25 μL of Folin–Ciocalteu reagent (1 M) and 80 μL of a 7.5% (w/v) Na2CO3 solution; finally, 85 μL of distilled water was added. The microplate was incubated in the dark at 30 °C for 30 min. The absorbance was recorded at 745 nm against a blank. A standard curve was prepared using gallic acid (0.02, 0.04, 0.06, 0.08, 0.10, and 0.12 μg/mL). The TPC in each sample was expressed as milligrams of gallic acid equivalents per 100 g of dry weight (mg GAE/100 g DW). All measurements were performed in triplicate.
Determination of the Proanthocyanidin Content
Proanthocyanidins (PACs) were determined using 4-dimethylaminocinnamaldehyde (DMAC) colorimetric method (Prior et al. 2010). Briefly, 70 μL of PAC’s extract sample was mixed with 210 μL of DMAC solution. The microplate was incubated for 25 min. The absorbance against a blank was recorded at 640 nm. A standard curve was prepared using procyanidin A2. The PACs content in each sample was expressed as milligrams of procyanidin A2 equivalents per 100 g of dry weight (mg procyanidin A2/100g). All measurements were performed in triplicate.
DPPH Radical Scavenging Activity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity was assessed according the method of Hatano et al. (1988). Briefly, 1 mL of extract was mixed with 250 μL of freshly prepared DPPH solution (0.2 mM in methanol). The mixtures were shaken vigorously and incubated for 30 min at room temperature in the dark. The absorbance was measured at 517 nm against a blank. The radical scavenging activity represented as percentage of inhibition was expressed as percentage of DPPH radical elimination calculated according to the following equation:
Acontrol is the absorbance of the control, and Asample is the absorbance of the samples. All tests were run in triplicate.
Experimental Design
Selection of Variables
It is known that extraction methods, temperature, solvent to sample ratio, and solvent concentration are important factors that significantly affect the extraction yield and phytochemical contents such as phenolic compounds (Celli et al. 2015; He et al. 2016). Therefore, for this study, we selected the following variables to optimize: ethanol concentration (v/v, %) (X1), solvent to sample ratio (mL/g) (X2), extraction temperature (°C) (X3), and extraction pressure (MPa) (X4). Acetone, ethanol, and methanol are the most common solvents for extraction; however, due to its low toxicity and its food grade and green manufacturing, ethanol was selected for extraction in this study (Celli et al. 2015). Ethanol respects principles of green extraction enumerated by Chemat et al. (2012). It is a short-chain alcohol that can be produced by fermentative processes from renewable sources especially sugar-rich materials such as sugar beet and cereals and is known to result in good quality of extracts. It is less flammable than other solvents like hexane, and it is completely biodegradable. Its low toxicity allows the administration of ethanolic extracts on cells or in animals to test their effects without harmful consequences (Chemat et al. 2012; Scharlack et al. 2017). In addition, ethanol has a good solubility rate than other solvents especially when it is mixed with water and this contributes to a higher degree of biocompounds extraction (Wang et al. 2018). The input variables were low, medium, and high levels, and the time of extraction (10 min) were selected based on preliminary experiments carried out in our laboratory. Each variable coded at its three levels (− 1, 0, 1) represents lower, middle, and higher value with X1 (0, 50, and 100 %), X2 (10, 40, and 70 mL/g), X3 (25, 45, and 65 °C), and X4 (100, 350, and 600 MPa).
Box–Behnken Design (BBD) for Extraction Optimization
A response surface methodology (RSM) was used for this study since it allows the optimization of multiple variables experiments and to statistically assess the optimum process parameters in a minimum number of runs, contrary to traditional approaches that test single parameter and observe the effect of one factor at a time on the variable. In addition, these conventional methods are unable to detect the interactions between the different factors tested (Ferreira et al. 2007).
In general, Box–Behnken design is constructed in such a way that 2k + 2 k + 1 experiments are required for the study where k represents the number of factors to be studied and with one experiment chosen randomly and repeated three times. Thus, for our study, a total of 27 experiments were conducted, 16 of which corresponded to a complete factorial design, eight experiments as star points and three in the middle factor’s fields (Table 1).
The mean values of dependent parameters obtained from the triplicates were fitted to a second order polynomial model as follows:
where Y is the response variable, Xi and Xj are independent variables, and k is the number of tested variables (k = 4). The regression coefficient is defined as β0 for the intercept and βi for linear, βii for quadratic, and βij for cross-product terms.
The adequacy of the model was verified using the determination coefficient R2, the adjusted determination coefficient Adj.R2 and the lack of fit test. The statistical significance was supported by a Ftest and their corresponding P value at the 5% significance level. Analysis based on the response surface and the desirability function was used to validate the optimum extraction parameters. The present model was validated with an experiment, run in triplicate, using the optimal conditions predicted by the model, and the average values of the validation experiment were compared with the predicted values (Dong et al. 2009).
Statistical Analysis
The analysis of variance (ANOVA) (Table 2) was carried out to determine individual linear, quadratic and interaction regression coefficients using Statgraphics Centurion XV (Version 15.2.06) and the fitness of the polynomial equation to the responses were estimated using the coefficient of determination (R2). The significance of all the terms of the polynomial equation was analyzed statistically by computing the F value at p < 0.05. Statgraphics Centurion XV (Version 15.2.06) was also used to generate 3D response surface graphs.
Derringer’s Desirability Function
The optimum parameters maximizing all responses together in a single experiment were obtained using Derringer’s desirability function. This method converts each response into an individual desirability function di, ranging from 0 to 1 (from the lowest to the highest desirability). These individual functions are then combined into an overall desirability function, D where D is the overall desirability, di is the individual response desirability, and wi is a response weight (Hu et al. 2008; Islam et al. 2012):
Results and Discussion
Fitting the Model
In the present study, a Box–Behnken design (BBD) was employed. The experimental conditions and results of 27 runs are presented in Table 1. A second order polynomial equation was applied to build a mathematical model to identify the optimum conditions for each response. The results of ANOVA are shown in Table 2. The significance of coefficient was tested using the p value.
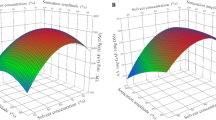
The lack of fit of each model was not significant (p > 0.05), indicating that the developed model adequately explains the relationship between the independent variables and responses. The values of determination coefficients (R2) and adjusted determination coefficients (Adj. R2) were close to 1, which indicate a high degree of correlation between the experimental and predicted values. The generated response surface 3D graphs corresponding to each response show the interactive effects of the variables (Figs. 1, 2, 3, 4, and Supplementary material file)
Effect of the Variables on the Phenolic Compounds Content
Total Phenolic Content
The linear effect of ethanol concentration (X1) and its quadratic component (X12) on TPC were significant (Table 2). The relationship between TPC and variables was described as the following second order polynomial equation:
The nonsignificant value of the lack of fit (F = 4) indicates that the model had a good fit prediction (R2 = 0. 8393; Adj.R2 = 0. 6518) (Table 2).
Response surface models adequately predicted the effects of parameters on total phenolics content of palm date extract. The third and fourth variables were assigned to be constant at the optimized setting while three-dimension surface plots were shown by two independent variables. As shown in Fig. 1a, when the temperature (X3) and pressure (X4) were fixed at their optimum point (65 °C and 600 MPa respectively), we predicted that maximum total phenolic extraction could be achieved when ethanol concentration (X1) was near to 50% with a maximum of solvent to sample ratio of 70 ml/g. This could be explained by the fact that a medium ethanol concentration may improve the solvent polarity and solubilize more polyphenols such as polar and moderately polar compounds (Alberti et al. 2014). TPC increased to a maximum of ethanol concentration of 47.80%, and then decreased. In a previous comparative study, experiments demonstrated that the extraction of polyphenols from green tea leaves based on high hydrostatic pressure process increased with the increase of the percentage of ethanol in the solvent to reach its maximum at 50% and then decreased above this proportion (Xi et al. 2009). So, an equilibrated mixture between water and ethanol named “aqueous ethanol” extracts more efficiently molecules of polyphenols by increasing their solubility, and their diffusion and mass transfer rates leading then to high polyphenol extractability (Richter et al. 1996). In addition, ethanol when is present at a medium concentration in water may affect the penetrability of plant cells by disrupting and breaking the architecture and the organization of phospholipids that make up the lipid bilayer of membranes (Gurtovenko and Anwar 2009) allowing then a better extraction and diffusion of phenolic compounds. Our observation is in good agreement with the previous studies (Sang et al. 2017; Xi et al. 2013; Xi and Wang 2013) which showed that a percentage of 50% in ethanol concentration is the optimum point for improving total phenolic extraction. However, other studies showed that 85% and 75% of ethanol is the adequate proportion for the extraction of phenolic compounds from Litchi fruit pericarp and propolis, respectively (Prasad et al. 2009; Shouqin et al. 2005). This difference could be explained by the fact that on the one hand matrix differ between plants materials and the technique of extraction employed may influence the percentage of ethanol required for polyphenols dissolution, and on the other hand, the presence of specific phenolic groups in plant require a specific ethanol concentration for good extractability like for example quercetin whose yield extraction is high at a percentage of ethanol in water above 70% because of its strong solubility in alcohols (Yang and Zhang 2008).
Total Proanthocyanidins
PACs, also called condensed tannins, are oligomers and polymers of monomeric flavan-3-ols, such as catechin and epicatechin (Prior et al. 2010).
In Table 2 the ANOVA revealed significant linear effects of solvent to sample ratio (X2) and quadratic ethanol concentration (X12). The PACs content was expressed by the following second order polynomial equation:
The data fitted well to the model (R2 = 0. 8140; Adj.R2 = 0. 5971), and the F for lack of fit was nonsignificant (F = 2.85) (Table 2).
Response surface models were used to study the effects of parameters and their interactions on total PACs extraction from palm date. The third and fourth variables were assigned to be constant at the optimum setting while surface plots of three-dimensions were shown by two independent variables. As shown in Fig. 2a, when the temperature (X3) and pressure (X4) were fixed at their optimum points (25 °C and 600 MPa respectively), maximum PACs were extracted at an ethanol concentration of 54.57 % and solvent to solid ratio of 69.51 ml/g. PACs content increased with the increase of ethanol concentration from 0 to 54.57 % and decrease above this percentage. This effect which is similar to TPC may be attributed to the adequate polarity of the solvent at medium concentration of ethanol which induces PACs extraction by the principle of dissolution (Tabaraki et al. 2012). Similar results were reported in the extraction of anthocyanins from red pear peels (Wang et al. 2016). As described for TPC, this is due to the adequate polarity of the solvent when ethanol is present at medium proportion and which enhance dissolution and distribution of PACs in the solvent (Sang et al. 2017). Our findings are in agreement with a previous study (Ghafoor et al. 2009) that showed an optimum of 52.35% in ethanol concentration for maximizing total anthocyanins in grape seeds. Another recent study (Janceva et al. 2018) that focused on the optimization of the extraction of proanthocyanidins showed also the same trend. It is important to note that the amount of anthocyanins present in the solvent depends highly on their chemical structure and also their stability which is directly proportional to the content of OH- and OCH3- moieties and acyl groups, as described in previous study (Corrales et al. 2008) that showed a high content of acetylglucoside and coumaroylglucoside anthocyanins after high hydrostatic pressure (HHP) treatment. Moreover, HHP technique leads to an acidic environment by reducing pH of the solvent due to the release of phenolics and deprotonation of some molecules of the extract. Then, acidic solution gives facility to extract acylated anthocyanins which are stable at acidic pH (Corrales et al. 2008). So, this could explain the presence of some specific PACs after extraction which depends highly on the technique of treatment. Concerning solvent to sample ratio, PACs yield increased by increasing this parameter. This may be explained by the fact that higher solvent to sample ratio allow solvent to penetrate into the sample cell enhancing the PACs extraction yield (He et al. 2016). In addition, some studies showed that a high solvent to sample ratio provide better diffusion with elevated coefficient (Cacace and Mazza 2003) that enhance the solubility of compounds (Letcher 2004).
Effect of Extraction Variables on Antioxidant Activity
Antioxidant activity explored by DPPH radical scavenging activity showed a significant effect of ethanol concentration (X1), its quadratic (X12), solvent to sample ratio (X2), its quadratic (X22), and pressure (X4). The correspondent fitted second order polynomial equation is:
F for lack of fit was nonsignificant (F = 5.80) suggested that the model was fit with very good prediction (R2 = 0.9603; Adj.R2 = 0.9140) (Table 2)
According to the three-dimensional response surface plots displayed in Fig. 3a, DPPH radical scavenging activity increases with increasing ethanol concentration to reach its maximum at 95% of ethanol. This indicates that the more proportion of organic solvent is, the best antiradical activity is observed. This result is not in accordance with the result obtained for TPC that require 50% of ethanol for optimum extraction. This could be explained by the fact that 50% of ethanol allow the extraction of abundant polyphenolic compounds in palm date fruit in term of quantity with polar, weak-polar, and non-polar compounds; however, 95% of ethanol permit the extraction of effective phenolic compounds in term of quality. Sun et al. (2015) have demonstrated in a previous study that different ethanol concentration in the solvent ranged from 25 to 100% showed different phenolic profiles and antioxidant properties. Another study showed that antioxidant activity is due to some specific phenolic compounds called key phenolics (Gregoris and Stevanato 2010). Similar result was found on Limnophila aromatica antioxidant activity that showed the highest DPPH radical scavenging activity with 100% of ethanol (Do et al. 2014). Other studies have shown similar trend with different plant materials like pineapple crude extract (Alothman et al. 2009) and defatted wheat germ (Zhu et al. 2011). For solvent to sample ratio variable, the maximum of DPPH radical scavenging activity is observed at 31 ml/g. This could be explained by the fact that a medium ratio of solvent to sample enhances the focus of the surface of contact between plant material and solvent stimulating then the dissolution rate and solubility of key antioxidant components (Xu et al. 2017). Solvent to sample ratio could depends on the type of antioxidant component. For example, an optimum ratio of 40:1 was showed in a previous study for the recovery of rutin and quercetin from Euonymus alatus (Yang and Zhang 2008). Similar significant effects on antioxidant activities with ultrahigh-pressure extraction method were observed in other previous studies (Belwal et al. 2016; Xi and Wang 2013). A medium solvent to solid ratio is useful for large-scale research because it minimizes the cost of the process that allow then more assays with many possible combinations of parameters. It is also worthwhile to note that solvent to sample ratio and ethanol concentration depends highly on the size of particles of raw material that modifies the extraction of phenolic components in an inverse proportionality trend (Oreopoulou et al. 2019). In a previous study (Majeed et al. 2016), it was demonstrated that for particle size parameter, in a range from 20 to 110 micron, the optimum of antioxidant activity was obtained with a size of 20 micron. So, this crucial parameter could explain differences of results between researches. Figure 3c shows that pressure has a slight effect on DPPH radical scavenging activity which attains its maximum at 100 MPa and remains stable as a plateau phase at high pressures. This agrees with the findings previously mentioned (Butz et al. 1994) which indicated that 100 MPa may allow the extraction of bioactive compounds from plant cells by breaking intracellular vacuoles and cell membranes. The principle of ultrahigh hydrostatic pressure (UHP) extraction is to create a large differential pressure between the inside and the outside of plant cell membrane which is rapid and achieved in few seconds from a pressure of 100 MPa (Corrales et al. 2008). A previous study demonstrated that 243 MPa was the optimum pressure to extract berberine from Cortex Phellodendri (Guoping 2012). In another study that focused on the comparison between UHP extraction and conventional extraction, authors showed that 200 MPa, which is the minimum value chosen for the pressure range, provided a higher extraction yield than the conventional extraction for 24 h and ultrasonic extraction for 30 min (Prasad et al. 2009). Moreover, between 200 and 500 MPa, there was a slight increase of the extraction yield which joins our observation concerning the plateau phase beyond 100 to 600 MPa. Our results are not in agreement with some other studies that showed an optimum pressure of 490 MPa (Xi and Wang 2013), 498 MPa (Xi et al. 2013) or 600 MPa (Pinela et al. 2018) for antioxidant activity. This difference may be due to many parameters that could affect UHP extraction such as UHP apparatus specifications, the method of sample preparation like grinding and milling, the particle size which may modify the effect of pressure on the cell membrane permeability, the pH of the solvent which decreases at high pressures (Prasad et al. 2009), and also the different steps of sample treatment after UHP extraction that could affect quantification of polyphenols compounds and antioxidant activity. More specific studies that take into account all of these parameters should be done in the future to better elucidate these results.
Model Validation
Responses for phenolic contents (TPC and PACs) and antioxidant activity (DPPH) were simultaneously optimized by the desirability function. Derringer’s desirability function was used to predict the parameters allowing optimum level for all responses in a single extraction by a multivariate analysis (Derrien et al. 2017). The results indicate that the maximum overall desirability D = 0.83 (on a scale of 0 to 1) can be achieved with the following conditions: ethanol concentration (X1, 60.54 %), solvent to sample ratio (X2, 70 mL/g), extraction temperature (X3, 65 °C), and pressure (X4, 600 MPa) (Fig. 4). Under these optimal conditions, the predicted values are TPC (261.06 mgGAE/100 g DW), PACs (9.58 mg procyanidin A2 equivalent/100 g DW), and % DPPH (95%). To validate the adequacy of the model equations, an experiment was carried out in triplicate under the optimal conditions predicted by the Derringer’s desirability model. Results obtained were as follows: TPC (257.93 ± 2.77 mg GAE/100 g DW), PACs (9.29 ± 0.25 mg procyanidin A2 equivalent/100 g DW), and % DPPH (93 ± 0.63). The experimental values are in close agreement with the predicted values (Table 3) confirming that the model was adequate to optimize the common extraction parameters for all responses (Supplementary material file).
Conclusion
Optimization of extraction procedure for phenolic compounds and antioxidant activity of palm date extract was successfully examined using response surface methodology. This is the first study on optimization of polyphenols extraction condition from palm date. The optimized conditions were validated and found to fit very well to the experimental values. The extraction of date’s phenolics depends highly on solvent concentration (X1) and sample to solvent ratio (X2). Pressure significantly affects DPPH radical scavenging activity which represents an important variable of antioxidant capacity measurement. Ultrahigh hydrostatic pressure is thus a good extraction technology that provides novel approaches for the extraction of medicinal plants. The optimum extraction conditions were as follows: ethanol concentration (60.54%), solvent to sample ratio (70 mL/g), extraction temperature (65 °C), and pressure (600 MPa). The results of the model validation experiments agreed with the predicted values. This optimized extraction method could be utilized for further isolation of bioactive compounds contributing to the further research of palm date fruit as a healthy food and drug.
References
Al-Farsi MA, Lee CY (2008) Nutritional and functional properties of dates: a review. Crit Rev Food Sci Nutr 48(10):877–887. https://doi.org/10.1080/10408390701724264
Al-Yahya M, Raish M, AlSaid MS, Ahmad A, Mothana RA, Al-Sohaibani M, Al-Dosari MS, Parvez MK, Rafatullah S (2016) ‘Ajwa’ dates (Phoenix dactylifera L.) extract ameliorates isoproterenol-induced cardiomyopathy through downregulation of oxidative, inflammatory and apoptotic molecules in rodent model. Phytomedicine 23(11):1240–1248. https://doi.org/10.1016/j.phymed.2015.10.019
Alberti A, Zielinski AAF, Zardo DM, Demiate IM, Nogueira A, Mafra LI (2014) Optimisation of the extraction of phenolic compounds from apples using response surface methodology. Food Chem 149:151–158. https://doi.org/10.1016/j.foodchem.2013.10.086
Alexandre EMC, Araújo P, Duarte MF, de Freitas V, Pintado M, Saraiva JA (2017) High-pressure assisted extraction of bioactive compounds from industrial fermented fig by-product. J Food Sci Technol 54(8):2519–2531. https://doi.org/10.1007/s13197-017-2697-2
Alothman M, Bhat R, Karim AA (2009) Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem 115(3):785–788. https://doi.org/10.1016/J.FOODCHEM.2008.12.005
Baş D, Boyacı İH (2007) Modeling and optimization I: usability of response surface methodology. J Food Eng 78(3):836–845. https://doi.org/10.1016/j.jfoodeng.2005.11.024
Belwal T, Dhyani P, Bhatt ID, Rawal RS, Pande V (2016) Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem 207:115–124. https://doi.org/10.1016/j.foodchem.2016.03.081
Butz P, Koller WD, Tauscher B, Wolf S (1994) Ultra-high pressure processing of onions: chemical and sensory changes. LWT Food Sci Technol 27(5):463–467. https://doi.org/10.1006/FSTL.1994.1093
Cacace JE, Mazza G (2003) Mass transfer process during extraction of phenolic compounds from milled berries. J Food Eng 59(4):379–389. https://doi.org/10.1016/S0260-8774(02)00497-1
Celli GB, Ghanem A, Brooks MSL (2015) Optimization of ultrasound-assisted extraction of anthocyanins from haskap berries (Lonicera caerulea L.) using response surface methodology. Ultrason Sonochem 27:449–455. https://doi.org/10.1016/j.ultsonch.2015.06.014
Chaira N, Smaali MI, Martinez-Tomé M, Mrabet A, Murcia MA, Ferchichi A (2009) Simple phenolic composition, flavonoid contents and antioxidant capacities in water-methanol extracts of Tunisian common date cultivars ( Phoenix dactylifera L.). International Journal of Food Sciences and Nutrition, 60(sup7), 316–329. https://doi.org/10.1080/09637480903124333
Chandrasekaran M, Bahkali AH (2013) Valorization of date palm (Phoenix dactylifera) fruit processing by-products and wastes using bioprocess technology – review. Saudi J Biol Scie 20(2):105–120. https://doi.org/10.1016/J.SJBS.2012.12.004
Chemat F, Vian MA, Cravotto G (2012) Green extraction of natural products: concept and principles. In International Journal of Molecular Sciences (Vol. 13, Issue 7, pp. 8615–8627). MDPI AG. https://doi.org/10.3390/ijms13078615
Chen R, Meng F, Zhang S, Liu Z (2009) Effects of ultrahigh pressure extraction conditions on yields and antioxidant activity of ginsenoside from ginseng. Sep Purif Technol 66(2):340–346. https://doi.org/10.1016/j.seppur.2008.12.026
Chen S, Zeng Z, Hu N, Bai B, Wang H, Suo Y (2018) Simultaneous optimization of the ultrasound-assisted extraction for phenolic compounds content and antioxidant activity of Lycium ruthenicum Murr. fruit using response surface methodology. Food Chem 242(August 2017):1–8. https://doi.org/10.1016/j.foodchem.2017.08.105
Corrales M, Toepfl S, Butz P, Knorr D, Tauscher B (2008) Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: a comparison. Innovative Food Sci Emerg Technol 9(1):85–91. https://doi.org/10.1016/J.IFSET.2007.06.002
Derrien M, Badr A, Gosselin A, Desjardins Y, Angers P (2017) Optimization of a green process for the extraction of lutein and chlorophyll from spinach by-products using response surface methodology (RSM). LWT Food Sci Technol 79:170–177. https://doi.org/10.1016/j.lwt.2017.01.010
Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, Ju Y-H (2014) Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal 22(3):296–302. https://doi.org/10.1016/J.JFDA.2013.11.001
Dong C-H, Xie X-Q, Wang X-L, Zhan Y, Yao Y-J (2009) Application of Box-Behnken design in optimisation for polysaccharides extraction from cultured mycelium of Cordyceps sinensis. Food Bioprod Process 87(2):139–144. https://doi.org/10.1016/J.FBP.2008.06.004
Espada-Bellido E, Ferreiro-González M, Carrera C, Palma M, Barroso CG, Barbero GF (2017) Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem 219:23–32. https://doi.org/10.1016/j.foodchem.2016.09.122
Ferreira SLC, Bruns RE, Ferreira HS, Matos GD, David JM, Brandão GC, da Silva EGP, Portugal LA, dos Reis PS, Souza AS, dos Santos WNL (2007) Box-Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta 597(2):179–186. https://doi.org/10.1016/J.ACA.2007.07.011
Ghafoor K, Choi YH, Jeon JY, Jo IH (2009) Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape ( Vitis vinifera ) seeds. J Agric Food Chem 57(11):4988–4994. https://doi.org/10.1021/jf9001439
Gregoris E, Stevanato R (2010) Correlations between polyphenolic composition and antioxidant activity of Venetian propolis. Food Chem Toxicol 48(1):76–82. https://doi.org/10.1016/j.fct.2009.09.018
Guoping L (2012) Optimization for ultrahigh pressure extraction of berberine from Cortex Phellodendri by central composite design-response surface methodology. J Med Plant Res 6(23):3963–3970. https://doi.org/10.5897/JMPR11.1092
Gurtovenko AA, Anwar J (2009) Interaction of ethanol with biological membranes: the formation of non-bilayer structures within the membrane interior and their significance. J Phys Chem B 113(7):1983–1992. https://doi.org/10.1021/jp808041z
Hatano T, Kagawa H, Yasuhara T, Okuda T (1988) Two new flavonoids and other constituents in licorice root. Their relative astringency and radical scavenging effects. Chem Pharm Bull 36(6):2090–2097. https://doi.org/10.1248/cpb.36.2090
He B, Zhang LL, Yue XY, Liang J, Jiang J, Gao XL, Yue PX (2016) Optimization of ultrasound-assisted extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem 204:70–76. https://doi.org/10.1016/j.foodchem.2016.02.094
Hu Z, Cai M, Liang H-H (2008) Desirability function approach for the optimization of microwave-assisted extraction of saikosaponins from Radix Bupleuri. Sep Purif Technol 61(3):266–275. https://doi.org/10.1016/J.SEPPUR.2007.10.016
Islam MA, Alam MR, Hannan MO (2012) Multiresponse optimization based on statistical response surface methodology and desirability function for the production of particleboard. Compos Part B 43(3):861–868. https://doi.org/10.1016/J.COMPOSITESB.2011.11.033
Janceva S, Lauberte L, Arshanitsa A, Akishin J, Dizhbite T, Telysheva G (2018) Optimization of proanthocyanidins extraction from bark of local hardwood. Key Eng Mater 762:163–168. https://doi.org/10.4028/www.scientific.net/KEM.762.163
Ji H, Zhang L, Du A, Yang M, Wang Q (2011) Effects of ultrahigh pressure extraction conditions on yields and antioxidant activities of flavonoid from Laoying Tea. Int J Food Eng 7(5). https://doi.org/10.2202/1556-3758.2320
Joo CG, Lee KH, Park C, Lee BC (2011) Antioxidative activities and composition analysis of Pinus densiflora root by ultra high pressure extraction. J Ind Eng Chem 17(4):712–716. https://doi.org/10.1016/J.JIEC.2011.05.018
Lee H-S, Lee HJ, Yu HJ, Ju DW, Kim Y, Kim C-T, Kim C-J, Cho Y-J, Kim N, Choi S-Y, Suh HJ (2011) A comparison between high hydrostatic pressure extraction and heat extraction of ginsenosides from ginseng (Panax ginseng CA Meyer). J Sci Food Agric 91(8):1466–1473. https://doi.org/10.1002/jsfa.4334
Letcher, T. M. (Trevor M. (2004). Chemical thermodynamics for industry. Royal Society of Chemistry. https://books.google.fr/books?id = omLPJJ0MLJMC&pg = PA170&lpg = PA170&dq = Quickly+screen+solvent+for+organic+solids&source = bl&ots = 0qsHOQZ0VO&sig = pZqc-rizQhRJe7Z7OjAE6rdgNTU&hl = fr&sa = X&ved = 2ahUKEwigsd-QuOfdAhWM4IUKHZ6MDSUQ6AEwAHoECAgQAQ#v = onepage&q = Quickly screen solvent for organic solids&f = false
Majeed M, Hussain AI, Chatha SAS, Khosa MKK, Kamal GM, Kamal MA, Zhang X, Liu M (2016) Optimization protocol for the extraction of antioxidant components from Origanum vulgare leaves using response surface methodology. Saudi J Biol Scie 23(3):389–396. https://doi.org/10.1016/J.SJBS.2015.04.010
Mújica-Paz H, Valdez-Fragoso A, Samson CT, Welti-Chanes J, Torres JA (2011) High-pressure processing technologies for the pasteurization and sterilization of foods. Food Bioprocess Technol 4(6):969–985. https://doi.org/10.1007/s11947-011-0543-5
Oreopoulou A, Tsimogiannis D, Oreopoulou V (2019) Extraction of polyphenols from aromatic and medicinal plants: an overview of the methods and the effect of extraction parameters. Polyphenols in Plants:243–259. https://doi.org/10.1016/B978-0-12-813768-0.00025-6
Pinela J, Prieto MA, Barros L, Carvalho AM, Oliveira MBPP, Saraiva JA, Ferreira ICFR (2018) Cold extraction of phenolic compounds from watercress by high hydrostatic pressure: process modelling and optimization. Sep Purif Technol 192(October 2017):501–512. https://doi.org/10.1016/j.seppur.2017.10.007
Prasad NK, Yang B, Zhao M, Wang BS, Chen F, Jiang Y (2009) Effects of high-pressure treatment on the extraction yield, phenolic content and antioxidant activity of litchi (Litchi chinensis Sonn.) fruit pericarp. Int J Food Sci Technol 44(5):960–966. https://doi.org/10.1111/j.1365-2621.2008.01768.x
Prior RL, Fan E, Ji H, Howell A, Nio C, Paynef MJ, Reed J (2010) Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J Sci Food Agric 90(9):1473–1478. https://doi.org/10.1002/jsfa.3966
Richter BE, Jones BA, Ezzell JL, Porter NL, Avdalovic N, Pohl C (1996) Accelerated solvent extraction: a technique for sample preparation. Anal Chem 68(6):1033–1039. https://doi.org/10.1021/ac9508199
Sang, J., Sang, J., Ma, Q., Hou, X. fang, & Li, C. qin. (2017). Extraction optimization and identification of anthocyanins from Nitraria tangutorun Bobr. seed meal and establishment of a green analytical method of anthocyanins. Food Chem, 218, 386–395. https://doi.org/10.1016/j.foodchem.2016.09.093
Scharlack NK, Aracava KK, Rodrigues CEC (2017) Effect of the type and level of hydration of alcoholic solvents on the simultaneous extraction of oil and chlorogenic acids from sunflower seed press cake. J Sci Food Agric 97(13):4612–4620. https://doi.org/10.1002/jsfa.8331
Shouqin Z, Jun X, Changzheng W (2005) High hydrostatic pressure extraction of flavonoids from propolis. J Chem Technol Biotechnol 80(1):50–54. https://doi.org/10.1002/jctb.1153
Sun C, Wu Z, Wang Z, Zhang H (2015) Effect of ethanol/water solvents on phenolic profiles and antioxidant properties of Beijing propolis extracts. Evidence-Based Complementary and Alternative Medicine : ECAM 2015:595393–595399. https://doi.org/10.1155/2015/595393
Tabaraki R, Heidarizadi E, Benvidi A (2012) Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) peel antioxidants by response surface methodology. Sep Purif Technol 98:16–23. https://doi.org/10.1016/j.seppur.2012.06.038
Tang Z-X, Shi L-E, Aleid SM (2013) Date fruit: chemical composition, nutritional and medicinal values, products. J Sci Food Agric 93(10):2351–2361. https://doi.org/10.1002/jsfa.6154
Vinson JA, Zubik L, Bose P, Samman N, Proch J (2005) Dried fruits: excellent in vitro and in vivo antioxidants. J Am Coll Nutr 24(1):44–50 http://www.ncbi.nlm.nih.gov/pubmed/15670984
Wang H, Ma X, Cheng Q, Wang L, Zhang L (2018) Deep eutectic solvent-based ultrahigh pressure extraction of Baicalin from Scutellaria Baicalensis Georgi. Molecules 23(12). https://doi.org/10.3390/molecules23123233
Wang W, Jung J, Tomasino E, Zhao Y (2016) Optimization of solvent and ultrasound-assisted extraction for different anthocyanin rich fruit and their effects on anthocyanin compositions. LWT Food Sci Technol 72:229–238. https://doi.org/10.1016/J.LWT.2016.04.041
Xi J, Shen D, Zhao S, Lu B, Li Y, Zhang R (2009) Characterization of polyphenols from green tea leaves using a high hydrostatic pressure extraction. Int J Pharm 382(1–2):139–143. https://doi.org/10.1016/j.ijpharm.2009.08.023
Xi J, Wang B (2013) Optimization of ultrahigh-pressure extraction of polyphenolic antioxidants from green tea by response surface methodology. Food Bioprocess Technol 6(9):2538–2546. https://doi.org/10.1007/s11947-012-0891-9
Xi J, Xue Y, Xu Y, Shen Y (2013) Artificial neural network modeling and optimization of ultrahigh pressure extraction of green tea polyphenols. Food Chem 141(1):320–326. https://doi.org/10.1016/j.foodchem.2013.02.084
Xu DP, Zheng J, Zhou Y, Li Y, Li S, Li HB (2017) Ultrasound-assisted extraction of natural antioxidants from the flower of Limonium sinuatum: optimization and comparison with conventional methods. Food Chem 217:552–559. https://doi.org/10.1016/j.foodchem.2016.09.013
Yang Y, Zhang F (2008) Ultrasound-assisted extraction of rutin and quercetin from Euonymus alatus (Thunb.) Sieb. Ultrason Sonochem 15(4):308–313. https://doi.org/10.1016/J.ULTSONCH.2007.05.001
Zhang S, Wang Z, Wang T, Zheng N, Li M, Lin J (2012) Optimization of central composite design-response surface methodology in ultra high pressure extraction of Scutellaria baicalensis. J Med Plant Res 6(3):373–378. https://doi.org/10.5897/JMPR11.612
Zhu K-X, Lian C-X, Guo X-N, Peng W, Zhou H-M (2011) Antioxidant activities and total phenolic contents of various extracts from defatted wheat germ. Food Chem 126(3):1122–1126. https://doi.org/10.1016/J.FOODCHEM.2010.11.144
Acknowledgement
The PhD student, Mr Sami SEDRAOUI, received a mobility fellowship from the “Ministry of Higher Education and Scientific Research of Tunisia”.
Author information
Authors and Affiliations
Contributions
Conceptualization: Mr Sami Sedraoui, Prof. Yves Desjardins; Methodology and experiments: Mr Sami Sedraoui, Dr Ashraf Badr; Data analysis: Mr Sami Sedraoui, Dr María Gretel Michel Barba; Writing—original draft preparation: Mr Sami Sedraoui; Writing—review and editing: Prof. Yves Desjardins, Prof. Alain Doyen and Prof. Zouhair Tabka; Supervision: Prof. Yves Desjardins and Prof. Zouhair Tabka.
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Conflict of Interest
Mr. Sami Sedraoui declares that he has no conflict of interest; Dr. Ashraf Badr declares that he has no conflict of interest; Dr. María Gretel Michel Barba declares that she has no conflict of interest; Prof. Alain Doyen declares that he has no conflict of interest; Prof. Zouhair Tabka declares that he has no conflict of interest and Prof. Yves Desjardins declares that he has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 75 kb)
Rights and permissions
About this article
Cite this article
Sedraoui, S., Badr, A., Barba, M.G.M. et al. Optimization of the Ultrahigh-Pressure–Assisted Extraction of Phenolic Compounds and Antioxidant Activity from Palm Dates (Phoenix dactylifera L.). Food Anal. Methods 13, 1556–1569 (2020). https://doi.org/10.1007/s12161-020-01764-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-020-01764-w