Abstract
The aim of this study was the application of the electronic nose for detection of volatile release from dark chocolate flavored with orange essential oil during the storage. The detection of volatile in chocolate without and with different concentrations of orange oil (0–10 ppm) was performed using polyaniline (Pani) gas sensor array with different dopants. Orange oil and chocolate were analyzed in relation to water activity, moisture, and acidity. The chocolate maintained unchanged the water activity; however, the moisture and acidity presented alterations during the storage. It was verified a decrease in resistance response of the sensor array with the chocolate during the storage time. The sensor doped with TSA (toluenesulfonic acid) presented the higher sensitivity of the array. Principal component analysis (PCA) revealed five distinct groups corresponding to the volatiles released during the storage time (0, 20, 40, 60, and 100 days). This work demonstrates that the electronic nose technology with Pani gas-sensing array can be effective and successfully applied to discriminate different concentrations of orange essential oil during the dark chocolate storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The great competitiveness in the food market makes the industries improve quality, reduce safety mistakes, and monitor trends of the products. Chocolate stands out for its high consumption, where the global market estimated to ship over 9500 thousand tons in 2024, especially the dark chocolate market reached US$ 45.6 billion in 2018 (IMARC Group 2019). This high consumption is linked to the versatility on the preparation, and it uses in various manufactured products. Dark chocolate presents an intense flavor, which is very appreciated by the bittersweet. Also, it is an excellent source of flavonoids through antioxidant activity (Afoakwa et al. 2008).
Chocolate can be defined as a suspension of solid particles, sugar and cocoa powder, in a continuous fat phase (cocoa butter), which contributes to the aroma, taste, and color of the product (Sasaki et al. 2012). It should be melted quickly at temperatures close to 37 °C, otherwise, promote an aroma/taste release and a waxy residue (Beckett 2008). Chocolate is a source of protein, fat, carbohydrates, vitamins, and minerals.
The aroma is an extremely important sensory property and a major factor that affects quality perception and consumer’s acceptance (Mirković et al. 2018). The use of essential oils in chocolates has advantage as the possibility of replacement of the artificial (flavors, odors, and colors) to natural. Natural products, particularly essential oils, are being targeted as potential preservatives due to the antimicrobial and antioxidant activity. In this context, the use of essential oils in chocolate presents new possibilities for exploring the use of these substances to make foods more natural and healthier (Albak and Tekin 2014; Dwijatmoko 2016; Ilmi et al. 2017).
Orange essential oil (Citrus sinensis) is composed of a mixture of compounds (terpenes, hydrocarbons, and oxidized compounds) considered chemically volatiles (González-Mas et al. 2019). The major compound is the limonene and the major sesquiterpene the valencene (Galvão et al. 2015). These compounds contribute to the aroma profile, gige the organoleptic characteristics of the fruit, and can be used to aromatize other products (Palazzolo et al. 2013; González-Mas et al. 2019). Also, it can be used as antimicrobial substance for food preservation. According to Espina et al. (2011), low concentration (0.2 μL/mL) of orange oil showed synergistic lethal effects on bacterial cells, inactivating more than 5 log units.
In the evaluation of food flavorings, sensory panels are used with trained staff to provide a profile of pleasure and satisfaction of the consumers. The sensory analysis can be limited by human factors as subjectivity and fatigue over long periods. In this sense, the electronic nose can be used to identify volatile compounds in food matrices in real-time, with high detection levels, fastness, and low cost. The electronic nose consists of a gas sensor array composed of different sensors to interact with a wide range of chemical classes and discriminate diverse volatiles compounds (Ballen et al. 2019; Graboski et al. 2018a, 2018b). Conductive polymer-based on gas sensors have been widely used in electronic nose due to their chemical structure and sensitivity. The gas sensors have low cost of fabrication, high sensitivity, and fast response when in contact with volatile molecules, and can be operated at room temperature. The use of different dopants in Pani films is very promising due to the increased conductivity, mechanical properties, and well-defined layered morphology, which have a pronounced influence on its charge-transport characteristics. The in situ polymerization technique can incorporate easily the dopant acid, so a variety of dopants can be used to construct the sensor array. Pani gas sensors have been applied successfully in humidity (Steffens et al. 2009; Steffens et al. 2013), aroma (Tiggemann et al. 2016, 2017), gummy candies (Ballen et al. 2019), female cattle fertile period (Manzoli et al. 2019), and fruits ripening detection (Manzoli et al. 2011).
In the present investigation, electronic nose composed by Pani gas sensor array was applied to detect release of the volatile compounds from dark chocolate with orange essential oil during the storage (1, 30, 40, 60, and 100 days).
Material and Methods
Chocolate Samples
Orange essential oil (Citrus sinenses var. Dulcis, Quinarí, Brazil) and dark chocolate (Harald, Brazil) were commercially purchased. According to the manufacturer, the dark chocolate contains the following ingredients: sugar, cocoa butter, cocoa powder, soybean lecithin, and polyglycerol polyricinoleate. The amount of orange essential oil added to the chocolate was based on preliminary tests and taking into account the antimicrobial activity (Espina et al. 2011). Also, the orange oil used is considered by the United States Food and Drug Administration (Electronic Code of Federal Regulations e-CFR 2019) generally regarded as safe oil (GRAS).
The dark chocolate flavored with orange essential oil was produced in an acclimatized room (20 °C). Initially, the dark chocolate was melted in a microwave oven (Consul, Brazil) at 45 °C and tempered. Next, the concentrations of orange oil (2.5, 5.0, 7.5, and 10 ppm, w/w) were added to the dark chocolate. Also, a control sample (without orange oil) was produced. The chocolate samples were molded in bars (10 g) using a polyethylene mold, cooled at 5 °C in a refrigerator (Consul, Brazil), packaged in polyethylene terephthalate (PET), wrapped in aluminum foil ,and maintained at 18 °C. The samples were stored in a place protected from light for 100 days. The samples were produced in three similar batches.
Electronic Nose
The electronic nose apparatus used in this work was described previously by Graboski et al. (2018). The apparatus was composed of a glass chamber (1 L), magnetic stirrer and heater (Fisatom, 714 model, Brazil), digital timer (Cronobio - SW2018), temperature and humidity commercial sensor (Sensirion Kit EK-H5, SHT2), glass holder (samples), and a unit with the gas sensor array. The data of the gas sensors (resistance) were obtained with a NOVUS acquisition system (Field Logger model).
The sensor array consisted of four doped Pani films. The gas sensors were obtained on a substrate of tracing paper (Schoellershammer 90/95 g). The graphite-based interdigitated electrodes were produced by the line patterning technique, according to Manzoli et al. (2011) and Steffens et al. (2010) over the tracing paper substrate. The sensitive layer (Pani film) was obtained by the in situ synthesis, in the emeraldine state, according to Graboski et al. (2018). The dopants used were chloride acid (HCl, Sigma-Aldrich, Germany), canforsulfonic acid (CSA, Sigma-Aldrich, Germany), toluenesulfonic acid (TSA, Sigma-Aldrich, Germany), and dodecylbenzenesulfonic acid (DBSA, Fluka) (Manzoli et al. 2011).
The response of the sensor array (resistance) to the volatiles from orange essential oil was evaluated at different concentrations (2.5; 5.0; 7.5, 10.0, and 12.5 ppm). These concentrations were inserted into the sampling chamber through a syringe (Hamilton). The response was obtained alternately in synthetic air flow (White Martins) for 5 min (baseline), and for 5 min in the volatile compounds (orange essential oil). Thus, each measurement was performed with synthetic air flow (100 mL/min) inserted in the glass chamber to obtain the baseline, next followed by the addition of the volatiles to be analyzed by the array of sensors (Ballen et al. 2019). The resistance values obtained in the e-nose not were submitted to any signal processing. This experimental sequence was repeated for all concentrations of orange essential oil. Also, to each orange essential oil concentration evaluated, the experiments were performed in triplicate.
The application of the electronic nose in the detection of the volatiles from dark chocolate flavored with orange essential oil was performed in the same way that the essential oil. The same procedure was repeated for all concentrations in increasing order of the amount of essential oil (0, 2.5, 5.0, 7.5, 10.0 ppm). For each analysis, 5 g of chocolate was used. The sample was inserted in a glass chamber, maintained at 35 °C, and submitted to agitation in a magnetic stirrer at 33 rpm. The release of volatile from dark chocolate flavored with orange essential oil was evaluated by the electronic nose during the storage time (1, 20, 40, 60, and 100 days). In the experiments, it was used one sample of each batch of chocolate, so there are three samples evaluated.
The Pani gas sensors with different dopants (HCl, TSA, CSA, and DBSA) were characterized in relation to sensitivity, limit of detection, and response time. The sensitivity and limit of detection were obtained from the analytical calibration curve (concentrations versus the maximum resistance). The response time was calculated as the time required for changing the sensor resistance (reached 90% of the equilibrium value) after the insertion of the volatile in the chamber.
Composition Analysis
The moisture, acidity, and water activity analyses were performed according to AOAC methodology (AOAC 2005). The chocolates with and without addition of orange oil were evaluated during 100 days of storage. The orange oil was also evaluated in full form. All the samples were analyzed in triplicate.
The moisture was determined by direct drying in an air recirculating oven (Fanem, model 320-SE, Brazil). Around 3.0 g of sample was weighed and dried at 105 °C for 4 h until constant weight.
Acidity (meq NaOH/100 g) was determined by the titration method using 0.1 M sodium hydroxide solution (Dinamica, Brazil).
Water activity was determined using a water activity meter (Lobtouch Novasina, China). The samples were cut in half and shredded into small pieces. Next, were placed in the sample holder of the equipment to perform the measurements. The experiments were conducted at 25 °C.
Chromatography analysis of orange essential oil followed the methodology described by Beneti et al. (2011) and analyzed in a gas chromatograph (CG-2010 Plus model; Shimadzu, Brazil) equipped with a polar column (Rtx-Wax, 30 m × 0.25 mm × 0.25 μm) and a flame ionization detector. The following temperatures were used: 60 °C (8 min), 60–180 °C (15 °C/ min), 230 °C (10 min), injector temperature at 230 °C, detector at 275 °C, with a 50:1 split ratio. Nitrogen and synthetic air (White Martins, 99.99% purity) were used as carrier gases, with a 1.5-mL/min flow rate. A diluted sample (1 μL) in dichloromethane (1:10; Merck, Germany) was injected. The sample components were identified by comparing their mass spectra.
Statistical Analysis
The mean composition results of the dark chocolate were evaluated by analysis of variance (ANOVA) at a 95% confidence level using Statistica 5.0 software.
The electronic nose responses obtained in triplicate at different concentrations of orange essential oil (2.5, 5, 7.5, and 10 ppm (w/v)) were treated using the principal component analysis (PCA) statistical method using OriginPro9.0 (© Origin Lab Corporation) software. The resistance values obtained during the storage time of the chocolate (1, 20, 40, 60, and 100 days) were also evaluated by PCA. By the PCA analysis, the signal of each Pani sensor of the array was transformed into variables, which are linear combinations of the original signals. These variables, called scores, were represented on a two-dimensional plane. In this plot, the similarities were evaluated between the dark chocolate samples measured by the distances between them in the plane. The closer together was described as more similar. Also, the discrimination of the days of storage is done by the score plot of PCA and the distribution of each sensor was done by the loading plot. The PCA algorithm was implemented in software for the different sensors (doped with HCl, CSA, TSA, and DBSA) by calculating the eigenvectors of a covariance matrix of data.
Results and Discussion
Orange Oil and Chocolate Composition
The orange oil presented water activity value of 0.54 ± 0.001, acidity of 1.32 ± 0.04 meq NaOH/100 g, and moisture of 78.18 ± 0.136%. Oils with low acidity value indicate an excellent storage life and can be used safely in food products (Kumar 2014; Putnik et al. 2017). The low acidity of the oils indicates a little magnitude of hydrolytic deterioration. Similar values of acidity were found in bergamot oilseed (Sicari et al. 2017), and lower than 2.0 in Brazilian citrus seed oils as rangpur lime and “sicilian” lemon (Reda et al. 2005).
According to da Silva et al. (2013) in a study of the chemical composition of the essential oil of Citrus sinensis L. and Citrus aurantiun L., the moisture content was 81.56 and 88.97%, respectively. These results were above to those found in the present study.
The results of chromatography analysis of orange essential oil are presented in Fig. 1. It can be observed from the peak area that d-limonene is the major substance, corresponding to 93.2%. Other identified substances were the myrcene, α-pinene, n-decanal, and n-octanal in a low amount. Limonene is a monocyclic monoterpene, 4-isoprenyl-1-methyl-cyclohexene (Badee et al. 2011), the main volatile constituent of citrus peel (Lu et al. 2014) and an aromatic substance (Nikfar and Behboudi 2014; Zhang et al. 2014).
Orange essential oil (Citrus sinensis) is classified as a mixture of terpenes, hydrocarbons, and oxidized compounds. According to the International Organization for Standardization (ISO 2019), the essential oil of sweet orange must have a minimum of 93% and a maximum of 96% of limonene. The other remaining compounds refer to a mixture of other terpenes, and aliphatic aldehydes (Galvão et al. 2015).
In the food matrices, the essential oils can act as antioxidants and antibacterial, besides reproducing the flavor and odor of the plant used (Frassinetti et al. 2011; Chouhan et al. 2017). D-limonene presents biological activities, as antioxidant (Murali and Saravanan 2012) and antimicrobial (Lennartsson et al. 2012). So, it is considered a very interesting substance for addition to food stored at room temperature.
Table 1 shows the results of the water activity of dark chocolate without (0 ppm) and with different concentrations (2.5 to 10 ppm) of essential orange oil during the storage. In general, no significant difference (p > 0.05) was observed between the days of storage for the dark chocolates with and without addition of orange oil. The textural modifications suffered by foods, including chocolate, are directly linked to the structural changes over the storage time. Water is one of the main elements responsible for maintaining the texture characteristics of the food product due to the plasticizing effect. Thus, during the chocolate storage, the water activity remained unchanged, without structural changes such as the transition between the vitreous and gummy states (de Leite et al. 2005).
The water activity value must be lower than 0.60 for confectionery and chocolate to avoid the microbial proliferation and deterioration (Beuchat et al. 2013; Hartel et al. 2018). So, the water activity values found in the present study were lower than 0.55, making the product shelf-stable. Relative equilibrium is important to predict conditions of the product without chemical and microbiological deterioration during the storage (Sandulachi 2012).
Table 2 presents the results of moisture of chocolate during the storage time. It can be observed a significant difference (p < 0.05) between the 1 and 20 days of storage. In samples stored for 60 and 100 days, no significant difference was verified (p > 0.05). The increase in the moisture values during the storage can due to the migration and diffusion of water into dry products (Ghosh et al. 2004). The addition of orange oil caused an increase in the moisture content with significative difference (p < 0.05) between 1 and 40 days of storage, and after 60 days, the moisture content remained unchanged.
Low moisture values (0.5 to 3%) in foods present low spoilage during storage (Minifie 1989). So, values outside the technical recommendations can cause large losses in chemical stability, microbiological deterioration, or changes in overall food quality. Hygroscopicity is an undesirable property, in which the sugar has the ability to absorb moisture from the environment. This hygroscopic characteristic of sugars directly affects the candies industries, causing losses in the product quality (Ghosh et al. 2005). So, the process should be carried out in a climate-controlled environment and package the product under appropriate conditions to maintaining the moisture content of chocolate bars (Ghosh et al. 2005).
The acidity results showed a significant difference (p < 0.05) between all days of storage (Table 3). The chocolates with 7.5 ppm of orange oil did not show significant difference (p > 0.05) between 1 and 20 days of storage. Chocolates with 10.0 ppm of orange essential oil presented high acidity. The acidity is due to the original oil acidity (1.32 ± 0.04 meq NaOH/100 g), and the increased acidity during storage may be associated with the hydrolysis of sucrose by the citric acid (Dar and Sharma 2011).
Electronic Nose
The maximum resistance response of Pani gas sensor array with different dopants (DBSA, CSA, TSA, and HCl) at different concentrations (2.5, 5.0, 7.5, 10.0, and 12.5 ppm) of orange essential oil is shown in Fig. 2. All gas sensors of the array distinguished the different concentrations of orange essential oil. All sensors presented saturation in the resistance signal at 12.5 ppm; thus, this concentration was excluded from the experiments.
Table 4 shows the sensitivity and limit of detection values for Pani gas sensors with different dopants exposed to orange essential oil. These values were obtained from the calibration curve (Fig. 2b). Pani sensors doped with TSA and CSA showed the high limit of detection and sensitivity for orange oil (Table 4). The response time demonstrates that the Pani HCl was faster in the detection of the orange oil (Table 4). The volatiles of orange oil come principally from limonene, considering that it is a major compound with considerable volatility (133 Pa steam pressure at 14 °C) that contribute to the citrus and floral aroma (Högnadóttir and Rouseff 2003). The good sensitivity of the sensors is attributed to the high surface area of the film. Also, it can be correlated to the conductivity of the dopant used (CSA > DBSA>TSA > HCl) that depend on interchain mobility, and the chain length (DBSA>CSA > TSA > HCl) exerts more force against ordering and closing of the Pani chains (Sinha et al. 2009).
The size of the counter-ion may affect the mobility of charges present in the polymer chain and the free volume between them (Sinha et al. 2009), which influences the sensitivity and charge-transport properties. The addition of protons into Pani by the dopant (acid) has important effects on the conductivity. According to Kizildag and Ucar (2014) CSA is more acidic than DBSA, so the Pani film doped by CSA acid presents higher conjugation length and conductivity.
The fast response time of HCl sensor can be due to the morphology of the Pani film which allows the volatile molecules to easily diffuse into and/or out of the film, in relation to the other dopants. This was also observed by Qi et al. (2014) when used Pani deposited on a porous substrate. The possible reason for the fast response is the presence of lighter dopant ions (Cl−) in Pani doped with HCl, which promotes higher charge mobility. Tiggemann et al. (2017) also observed faster response time (3–11 min) for HCl-doped Pani sensor in relation to CSA and DBSA sensors in the detection of artificial aromas (strawberry, grape, and apple).
The electronic nose experiments with chocolate were performed at 35 °C and relative humidity of 56% (± 4). The response was evaluated initially with dark chocolate without orange essential oil (0 ppm). Gas sensors presented response to chocolate without the addition of orange oil. Dark chocolate has volatile chemical compounds like pyrazines, amines and amides, acids, esters, and hydrocarbons (Afoakwa et al. 2008; Fernández-Murga et al. 2011) that was detected by the sensor array.
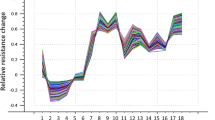
In Fig. 3, the resistances of the sensors decrease with the increase of the concentrations of orange essential oil in the chocolates. TSA, CSA, and HCl-doped sensors presented the low resistance to volatiles, which may be related to the dopant structure (Guenet 2008). The conductivity can be related to the length of the alkyl side chain, and the transport of electrons can be associated to the interchain transfer and intrachain coherence length (Zheng et al. 1996). The sudden drop on the resistance signal could be attributed to the swelling of the Pani doped films when in contact with the volatile compounds that diffuse on the matrix. This response mechanism can increase the interchain distance in the Pani film and affects the flow of the electrons.
The resistance results of the sensor array decreased with the storage time of dark chocolate with different concentrations of orange essential oil. The loss of volatile compounds of the dark chocolate with storage is related to their constituents that can either block the volatile outflow through the barrier or increase the viscosity (Nightingale et al. 2012). Also, this result can be associated with the moisture values, because an increase of moisture was observed during the first and 40 days of storage.
The response to the volatile substances and concentrations occurs because each dopant used provides a kind of selectivity, which is called molecular recognition (Manzoli et al. 2010). Thus, sensors doped with different acids (CSA, TSA, DBSA, and HCl) exhibited different behaviors in the presence of the same specific substance. The TSA-doped sensor showed high sensitivity to the volatiles of the dark chocolate with orange oil (Fig. 4). The sensitivity values decrease for all sensors during the storage due to the volatile release.
Gas sensor responses were analyzed by principal component analysis (PCA) at different concentrations of orange essential oil (0, 2.5, 5.0, 7.5, and 10.0 ppm) added to the chocolate (Fig. 5a). The PCA showed 96.6% of the total information collected by the matrix in PC1 and PC2. PC1 presented the largest amount of information (89.67%); therefore, the concentration analysis should be based on this axis, although the small contribution of PC2 (6.93%) should be considered for analysis. The high discrimination was observed between chocolate without orange oil (0 ppm) and the concentration of 10.0 ppm, being distinctly represented in the lower quadrant. Already, the concentrations 2.5, 5.0, and 7.5 ppm were discriminated but were closer.
The resistance results obtained by electronic nose experiments with chocolate during the storage (1, 20, 40, 60, and 100 days) were also evaluated by PCA (Fig. 6a). 97.77% of the total information was collected by the matrix (PC1 and PC2), and the PC1 has the largest amount of information (94.93%). The results demonstrate that the sensors’ array used was able to discriminate the storage time of chocolate, being more evident a difference between 1, 20, and 100 days of storage. Lower discrimination was verified between 40 and 60 days.
The analysis of the contribution of each gas sensor (doped with CSA, TSA, DBSA, and HCl) of electronic nose array in the discrimination of different concentrations of orange essential oil (0, 2.5, 5.0, 7.5, and 10.0 ppm) and different days of storage was evaluated on the loading plot, presented in Figs. 5b and 6b, respectively. The loadings’ plot showed the distribution of sensors in the positive side of the principal components. The sensors with high sensitivities (CSA and TSA) to the volatiles presented longer projections on the axes in relation to sensors doped with DBSA and HCl.
In literature works, the electronic nose was also applied in the discrimination of gummy candy volatiles during storage. The gummy candies were aromatized artificially flavored with apple, strawberry, and grape (Ballen et al. 2019). The developed electronic nose demonstrates great potential in the investigation and monitoring of volatiles and can be a powerful tool for the food industries to help in food quality control.
The results showed that the Pani gas sensor array doped with different acids was able to detect the volatile release from the dark chocolate during storage, presenting an excellent response to the concentrations studied (0, 2.5, 5.0, 7.5, and 10.0 ppm). In addition, dark chocolate with orange essential oil can be used as a flavoring agent, enhancing the health benefit with its biological activities as an antioxidant, anti-inflammatory, and antimicrobial.
Conclusions
Dark chocolate with orange essential oil indicated that there was no significant difference (p > 0.05) between the days of storage in the water activity analysis. However, it showed an increase in moisture and acidity values during the storage time. The chromatographic analysis of the orange essential oil demonstrates that d-limonene is the major substance present in the sample.
The sensors doped with CSA and TSA had the highest sensitivities, and thus felt more the presence of volatiles in orange oil. When the sensors were exposed with dark chocolate with different concentrations of orange essential oil, the best response was the sensor using the TSA dopant, followed by CSA, DBSA, and HCl. It was observed that there was a decrease in the response of the sensors during the storage days, related to the loss and release of volatiles.
Principal component analysis for different concentrations and storage time of the dark chocolate with orange oil showed good discrimination of orange essential oil at different concentrations. So, the different acid-doped Pani sensor array is a potential technique in the detection of volatiles during the food storage.
References
Afoakwa EO, Paterson A, Fowler M, Ryan A (2008) Flavor formation and character in cocoa and chocolate: a critical review. Crit Rev Food Sci Nutr 48:840–857. https://doi.org/10.1080/10408390701719272
Albak F, Tekin AR (2014) The effect of addıtıon of ıngredıents on physıcal propertıes of dark chocolate durıng conchıng. Basic Res J Food Sci Technol 1(7):51–59
Association of Officiating Analytical Chemists (AOAC) (2005) Official methods of analysis of the Association of Official Analytical Chemists (method 900.02, 994.12, 996.06, 996.01). Gaithersburg, Maryland
Badee AZM, Helmy SA, Morsy NFS (2011) Utilisation of orange peel in the production of α-terpineol by Penicillium digitatum (NRRL 1202). Food Chem 126:849–854. https://doi.org/10.1016/j.foodchem.2010.11.046
Ballen SC, Graboski AM, Manzoli A, Steffens J, Steffens C (2019) Monitoring aroma release in gummy candies during the storage using electronic nose. Food Anal Methods 13:1–10. https://doi.org/10.1007/s12161-019-01496-6
Beckett ST (2008) The science of chocolate, 2nd edn. Royal Society of Chemistry, Cambridge
Beneti SC, Rosset E, Corazza ML, Frizzo CD, di Luccio M, Oliveira JV (2011) Fractionation of citronella (Cymbopogon winterianus) essential oil and concentrated orange oil phase by batch vacuum distillation. J Food Eng 102:348–354. https://doi.org/10.1016/j.jfoodeng.2010.09.011
Beuchat LR, Komitopoulou E, Beckers H et al (2013) Low–water activity foods: increased concern as vehicles of foodborne pathogens. J Food Prot 76:150–172. https://doi.org/10.4315/0362-028X.JFP-12-211
Chouhan S, Sharma K, Guleria S (2017) Antimicrobial activity of some essential oils—present status and future perspectives. Medicines 4:58. https://doi.org/10.3390/medicines4030058
da Silva TG, de Cremoneze ML, Argandona EJS, et al (2013) Composição química do óleo essencial da casca de citrus sinensis L. e Citrus aurantium L. In: VII SBOE - Simpósio Brasileiro de Óleos Essenciais. Santarém - Pará
Dar BN, Sharma S (2011) Total phenolic content of cereal brans using conventional and microwave assisted extraction. Am J Food Technol 6:1045–1053. https://doi.org/10.3923/ajft.2011.1045.1053
de Leite JTC, Murr FEX, Park KJ (2005) Transições de fases em alimentos: influência no processamento e na armazenagem. Rev Bras Prod Agroindustriais 7:83–96
Dwijatmoko MI (2016) Effect of cinnamon essential oils addition in the sensory attributes of dark chocolate. Nusant Biosci 8:301–305. doi: https://doi.org/10.13057/nusbiosci/n080227
Electronic Code of Federal Regulations e-CFR (2019) Electronic Code of Federal Regulations e-CFR data is current as of September 16, 2019 - PART 182—Substances generally recognized as safe. In: Electron. Code Fed. Regul. e-CFR. <https://www.ecfr.gov/cgi-bin/text-idx? SID=e956d645a8b4e6b3e34e4e5d1b690209&mc=true&node=pt21.3.182&rgn=div5> Accessed 18 Sep 2019
Espina L, Somolinos M, Lorán S, Conchello P, García D, Pagán R (2011) Chemical composition of commercial citrus fruit essential oils and evaluation of their antimicrobial activity acting alone or in combined processes. Food Control 22:896–902. https://doi.org/10.1016/j.foodcont.2010.11.021
Fernández-Murga L, Tarín JJ, García-Perez MA, Cano A (2011) The impact of chocolate on cardiovascular health. Maturitas 69:312–321. https://doi.org/10.1016/j.maturitas.2011.05.011
Frassinetti S, Caltavuturo L, Cini M, Della Croce CM, Maserti BE (2011) Antibacterial and antioxidant activity of essential oils from citrus spp. J Essent Oil Res 23:27–31. https://doi.org/10.1080/10412905.2011.9700427
Galvão JG, Silva VF, Ferreira SG, França FRM, Santos DA, Freitas LS, Alves PB, Araújo AAS, Cavalcanti SCH, Nunes RS (2015) β-Cyclodextrin inclusion complexes containing Citrus sinensis (L.) Osbeck essential oil: an alternative to control Aedes aegypti larvae. Thermochim Acta 608:14–19. https://doi.org/10.1016/j.tca.2015.04.001
Ghosh V, Duda JL, Ziegler GR, Anantheswaran RC (2004) Diffusion of moisture through chocolate-flavoured confectionery coatings. Food Bioprod Process 82:35–43. https://doi.org/10.1205/096030804322985290
Ghosh V, Ziegler GR, Anantheswaran RC (2005) Moisture migration through chocolate-flavored confectionery coatings. J Food Eng 66:177–186. https://doi.org/10.1016/j.jfoodeng.2004.03.012
González-Mas MC, Rambla JL, López-Gresa MP, Blázquez MA, Granell A (2019) Volatile compounds in citrus essential oils: a comprehensive review. Front Plant Sci 10:1–18. https://doi.org/10.3389/fpls.2019.00012
Graboski AM, Ballen SC, Manzoli A, Shimizu FM, Zakrzevski CA, Steffens J, Steffens C (2018a) Array of different polyaniline-based sensors for detection of volatile compounds in gummy candy. Food Anal Methods 11:77–78. https://doi.org/10.1007/s12161-017-0977-0
Graboski AM, Galvagni E, Manzoli A, Shimizu FM, Zakrzevski CA, Weschenfelder TA, Steffens J, Steffens C (2018b) Lab-made electronic-nose with polyaniline sensor array used in classification of different aromas in gummy candies. Food Res Int 113:309–315. https://doi.org/10.1016/j.foodres.2018.07.011
Guenet J-M (2008) Polymer-solvent molecular compounds. Elsevier
Hartel RW, von Elbe JH, Hofberger R (2018) Confectionery science and technology. Springer International Publishing
Högnadóttir Á, Rouseff RL (2003) Identification of aroma active compounds in orange essence oil using gas chromatography–olfactometry and gas chromatography–mass spectrometry. J Chromatogr A 998:201–211. https://doi.org/10.1016/S0021-9673(03)00524-7
Ilmi A, Praseptiangga D, Muhammad DRA (2017) Sensory attributes and preliminary characterization of milk chocolate bar enriched with cinnamon essential oil. IOP Conf Ser Mater Sci Eng 193:012031. https://doi.org/10.1088/1757-899X/193/1/012031
IMARC Group (2019) Dark chocolate market: global industry trends, share, size, growth, opportunity and forecast 2019–2024 Research and Markets <https://wwwresearchandmarketscom/reports/4763118/dark-chocolate-market-global-industry-trends> Accessed 18 Sep 2019
ISO (2019) ISO 3140:2019 - Organisation internationale de normalisation. essential oil of sweet orange expressed [Citrus sinensis (L.)]. ICS : 71.100.60 Essential oils <https://www.iso.org/committee/48956/x/catalogue/>
Kizildag N, Ucar N (2014) Investigation of the protonation of polyaniline by CSA and DBSANa+ in DMF and DMSO. In: The fiber society fall 2014 conference. Philedelphia, 2–4
Kumar A (2014) Physico-chemical and natural products investigations of essential oil from the rhizomes of Kaempferia galanga L. Der Chem Sin 5:1–4
Lennartsson PR, Ylitervo P, Larsson C, Edebo L, Taherzadeh MJ (2012) Growth tolerance of zygomycetes mucor indicus in orange peel hydrolysate without detoxification. Process Biochem 47:836–842. https://doi.org/10.1016/J.PROCBIO.2012.02.019
Lu Y, Li H, Zhuang S, Zhang D, Zhang Q, Zhou J, Dong S, Liu Q, Wang P (2014) Olfactory biosensor using odorant-binding proteins from honeybee: ligands of floral odors and pheromones detection by electrochemical impedance. Sensors Actuators B Chem 193:420–427. https://doi.org/10.1016/j.snb.2013.11.045
Manzoli A, Steffens C, Paschoalin RT et al (2010) Funcionalização da superfície de microcantilevers utilizados em microscopia de força atômica com biomoléculas. Circ técnica 53:1–6
Manzoli A, Steffens C, Paschoalin RT, Correa A, Alves W, Leite F, Herrmann P (2011) Low-cost gas sensors produced by the graphite line-patterning technique applied to monitoring banana ripeness. Sensors 11:6425–6434. https://doi.org/10.3390/s110606425
Manzoli A, Steffens C, Paschoalin RT, Graboski AM, de Mello Brandão H, de Carvalho BC, Bellini JL, de Paula Herrmann PS Jr (2019) Volatile compounds monitoring as indicative of female cattle fertile period using electronic nose. Sensors Actuators B Chem 282:609–616. https://doi.org/10.1016/j.snb.2018.11.109
Minifie BW (1989) Chocolate, cocoa and confectionery: science and technology. Springer Netherlands, Dordrecht
Mirković M, Seratlić S, Kilcawley K, Mannion D, Mirković N, Radulović Z (2018) The sensory quality and volatile profile of dark chocolate enriched with encapsulated probiotic lactobacillus plantarum bacteria. Sensors 18:2570. https://doi.org/10.3390/s18082570
Murali R, Saravanan R (2012) Antidiabetic effect of d-limonene, a monoterpene in streptozotocin-induced diabetic rats. Biomed Prev Nutr 2:269–275. https://doi.org/10.1016/j.bionut.2012.08.008
Nightingale LM, Cadwallader KR, Engeseth NJ (2012) Changes in dark chocolate volatiles during storage. In: Journal of Agricultural and Food Chemistry
Nikfar S, Behboudi AF (2014) Limonene. In: Academic Press (ed) Encyclopedia of toxicology. Philip Wexler, Oxford, pp 78–82
Palazzolo E, Laudicina VA, Germanà MA (2013) Current and potential use of citrus essential oils. Curr Org Chem 17:3042–3049. https://doi.org/10.2174/13852728113179990122
Putnik P, Bursać Kovačević D, Režek Jambrak A, Barba F, Cravotto G, Binello A, Lorenzo J, Shpigelman A (2017) Innovative “green” and novel strategies for the extraction of bioactive added value compounds from citrus wastes—a review. Molecules 22:680. https://doi.org/10.3390/molecules22050680
Qi J, Xu X, Liu X, Lau KT (2014) Fabrication of textile based conductometric polyaniline gas sensor. Sensors Actuators B Chem 202:732–740. https://doi.org/10.1016/j.snb.2014.05.138
Reda SY, Leal ES, Batista EAC, Barana AC, Schnitzel E, Carneiro PIB (2005) Caracterização dos óleos das sementes de limão rosa (Citrus limonia Osbeck) e limão siciliano (Citrus limon), um resíduo agroindustrial. Ciência e Tecnol Aliment 25:672–676. https://doi.org/10.1590/S0101-20612005000400008
Sandulachi E (2012) Water activity concept and its role in food preservation < https://www.researchgate.net/publication/310605656_WATER_ACTIVITY_CONCEPT_AND_ITS_ROLE_IN_FOOD_PRESERVATION/citation/download>
Sasaki M, Ueno S, Sato K (2012) Polymorphism and mixing phase behavior of major triacylglycerols of cocoa butter. Cocoa Butter Relat Compd:151–172. https://doi.org/10.1016/B978-0-9830791-2-5.50009-8
Sicari V, Pellicano TM, Messina F (2017) Comparison of physicochemical characteristics and composition of bergamot oil seed extracted from three different cultivars. Emirates J Food Agric 29:470–475. https://doi.org/10.9755/ejfa.2017-01-240
Sinha S, Bhadra S, Khastgir D (2009) Effect of dopant type on the properties of polyaniline. J Appl Polym Sci 112:3135–3140. https://doi.org/10.1002/app.29708
Steffens C, Manzoli A, Francheschi E, Corazza ML, Corazza FC, Oliveira JV, Herrmann PSP (2009) Low-cost sensors developed on paper by line patterning with graphite and polyaniline coating with supercritical CO2. Synth Met 159:2329–2332. https://doi.org/10.1016/j.synthmet.2009.08.045
Steffens C, Franceschi E, Corazza FC, Herrmann PSP Jr, Oliveira JV (2010) Gas sensors development using supercritical fluid technology to detect the ripeness of bananas. J Food Eng 101:365–369. https://doi.org/10.1016/j.jfoodeng.2010.07.021
Steffens C, Manzoli A, Paschoalin RT, Tiggemann L, Steffens J, Teixeira E, Herrmann PSP (2013) Tracing paper substrate used for development of interdigitated graphite electrode and its application as humidity sensor. Synth Met 183:36–39. https://doi.org/10.1016/j.synthmet.2013.09.015
Tiggemann L, Ballen S, Bocalon C, Graboski AM, Manzoli A, de Paula Herrmann PS, Steffens J, Valduga E, Steffens C (2016) Low-cost gas sensors with polyaniline film for aroma detection. J Food Eng 180:16–21. https://doi.org/10.1016/j.jfoodeng.2016.02.006
Tiggemann L, Ballen SC, Bocalon CM, Graboski AM, Manzoli A, Steffens J, Valduga E, Steffens C (2017) Electronic nose system based on polyaniline films sensor array with different dopants for discrimination of artificial aromas. Innov Food Sci Emerg Technol 43:112–116. https://doi.org/10.1016/j.ifset.2017.08.003
Zhang Z, Vriesekoop F, Yuan Q, Liang H (2014) Effects of nisin on the antimicrobial activity of d-limonene and its nanoemulsion. Food Chem 150:307–312. https://doi.org/10.1016/j.foodchem.2013.10.160
Zheng WY, Levon K, Taka T, Laakso J, Österholm JE (1996) Doping-induced layered structure in n-alkylated polyanilines. Polym J 28:412–418. https://doi.org/10.1295/polymj.28.412
Acknowledgments
The authors would like to thank the National Council for Scientific and Technological Development—Brazil (CNPq), Coordination for the Improvement of Higher Education Personnel—Brazil (CAPES)—Finance Code 001 and Research Support Foundation of the State of Rio Grande do Sul—Brazil (FAPERGS), and Finep for their financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Elisiane Galvagni declares that she has no conflict of interest. Andressa Fritzen declares that she has no conflict of interest. Sandra Cristina Ballen declares that she has no conflict of interest. Adriana Marcia Graboski declares that she has no conflict of interest. Juliana Steffens declares that she has no conflict of interest. Clarice Steffens declares that she has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Galvagni, E., Fritzen, A.A., Graboski, A.M. et al. Detection of Volatiles in Dark Chocolate Flavored with Orange Essential Oil by Electronic Nose. Food Anal. Methods 13, 1421–1432 (2020). https://doi.org/10.1007/s12161-020-01763-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-020-01763-x