Abstract
In this work, a graphene oxide-based composite (DABP-GO) was prepared by aminating graphene oxide (GO) with the diazonium salt of 4,4′-diaminobiphenyl (DABP). The DABP-GO composite was characterized by scanning electron microscopy, infrared spectroscopy, and nitrogen adsorption-desorption. The DABP-GO showed to be an effective solid-phase extraction (SPE) adsorbent for carbamate pesticides. A SPE method with the DABP-GO as the adsorbent combined with high-performance liquid chromatography with ultraviolet detection was established to determine trace carbamate residues in vegetable samples (benincasahispida and pakchoi). Under the optimal conditions, the linear range was from 1.0 to 40.0 ng g−1. The limits of detection and limits of quantitation of the six carbamates (metolcarb, arprocarb, carbaryl, isoprocarb, fenobcarb, and diethofencarb) were 0.3–0.5 ng g−1 and 0.9–1.5 ng g−1 for benincasahispida sample and 0.5–1.0 ng g−1 and 1.5–3.0 ng g−1 for pakchoi sample, respectively. The relative standard deviations (RSDs) for the intra-day precision at the analyte concentration of 40 ng g−1 are from 2.3 to 8.2% (n = 5) and the inter-day RSDs (n = 5) are from 4.5 to 9.2%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The issues of pesticide residues have caused widespread public concerns due to the heavy use of the pesticides in modern agriculture (Damalas and Eleftherohorinos 2011). In recent years, carbamate insecticides have been increasingly used for the control of insects, fungi, and nematode sand mites. However, the high-dose application of the pesticides can result in the pesticide residue levels in the vegetables higher than their maximum residue limits (MRLs). Since some carbamates are detrimental to the central nervous system and also have carcinogenic and mutagenic effect (Casale et al. 1993), many countries have set the MRLs for carbamates in fruit and vegetables (David et al. 2011). Therefore, effective and quantitative determination of carbamates in food samples at trace levels is necessary.
To meet the requirement of instrumental detection and also to eliminate sample matrix interferences (Lambropoulou and Albanis 2007), sample preparation for the enrichment and isolation of the analytes is often necessary for the determination of trace levels of the pesticide residues in real samples. Currently, a variety of sample preparation methods have been used to enrich carbamates for real sample analysis, such as single-drop microextraction (SDME) (Sarajiand Esteki 2008), dispersive liquid-liquid microextraction (DLLME) (Chen et al. 2010), hollow fiber solid-phase microextraction (HF-SPME) (Song et al. 2013), solid-phase microextraction (SPME) (Cavaliere et al. 2012), magnetic solid-phase extraction (MSPE) (Li et al. 2015), cloud-point extraction (CPE) (Santalad et al. 2009; Rahmani et al. 2018), and solid-phase extraction (SPE) (Wu et al. 2017). Among them, SPE is still a most widely used sample preparation technique.
Adsorbent is the key for an effective extraction of certain analytes in SPE. In recent years, various carbon-based adsorbents such as graphite, carbon nanotubes, fullerene, graphene (G), and graphene oxide (GO) have been used in SPE due to their excellent adsorption capabilities (Fang et al. 2014; Ren et al. 2011; Sitko et al. 2013; Wu et al. 2017; Zhang et al. 2013). Among them, graphene-based adsorbent materials have become a current research hot point due to their excellent mechanical (Papageorgiou et al. 2017), thermal (Ye et al. 2015), and electrochemical properties (Rahmani et al. 2018). Graphene has the excellent properties such as nanosize, thermal and chemical stability, abundant delocalized π electrons, and high specific surface area (theoretical value 2630 m2 g−1) (Sitko et al. 2013). Due to its extremely high theoretical surface area and large delocalized π-electron system, it can adsorb organic molecules by generating strong π–π stacking interactions (Kyzas et al. 2018). Generally, the absorption efficiency depends on the surface area, pore volume, porosity, and functional groups (Ibrahim et al. 2016). Although the theoretical specific surface area of graphene is as high as 2630 m2 g−1, the actual measured value is much smaller than the theoretical one due to the stacking and agglomeration between the layers of graphene (Wu et al. 2016). In order to reduce the stacking effect between the graphene layers, an effective way is to chemically modify the surface of graphene (Wu et al. 2016).
Similar to graphene, graphene oxide (GO) is formed by a monolayer of carbon atoms with high surface area and large π-electron system. However, GO contains a large amount of active oxygen-containing functional groups (hydroxyl, carboxyl group, and epoxy bonds) (Sitko et al. 2013), which make GO soluble in water. Due to the water solubility, the direct application of graphene oxide as an adsorbent is limited. But its oxygen functional groups make it easy to be modified to improve its functionalities, which are helpful for its use as adsorbent to enrich organic contaminants (Li et al. 2018). Many types of chemical reactions can occur with these functional groups, and a variety of different functional groups can be grafted on the surface of GO to achieve the specific functions required (Dreyer et al. 2010). Studies have shown that the functional groups containing nitrogen atoms (such as amino, imine, hydrazine, amidoxime, and imidazole groups) are useful for adsorbing organics containing lone pairs of electrons (Wang et al. 2018). The commonly used methods for GO amination mainly include in situ polymerization, direct compounding, solvothermal synthesis, photoelectric reduction, and diazonium salt reaction (Wang et al. 2018). Among them, the diazonium salt reaction is simple and environmentally friendly for modifying the GO surface with amino groups. The aminated GO can reduce the water solubility of GO and improve its adsorption characteristics for some organic compounds via strong π-π stacking interaction and hydrogen bonds.
Wu et al. (2016) have synthesized a p-phenylenediamine functionalized GO with the aid of the coupling reagents of 1-ethyl-3-(3-dimethyaminopropyl) carbodiimide (EDC) and N-hydroxyl succinimide (NHS) for the adsorption of Conge red. Hung et al. (2014) used pressure-assisted self-assembly technique to prepare a series of graphene oxide framework membranes with different diamine monomers (ethylenediamine, butylenediamine, and p-phenylenediamine). Fang et al. (2014) have applied diazonium salt reaction to synthesize a GO-NH2 for the removal of cobalt ions from aqueous solution.
In this work, an aminated GO composite nanomaterial (DABP-GO) was prepared by surface chemical modification of the graphene oxide with 4,4′-diaminobiphenyl (DABP) as the amination reagent. The DABP-GO was then explored as the SPE adsorbent to enrich some carbamates from benincasahispida and pakchoi samples. The main factors affecting the extraction, including sample pH, sample loading rate, adsorption capacity, and the type and dosage of eluent, were investigated. Finally, a sensitive and effective method for the determination of carbamates in vegetables was established.
Experimental
Reagents and Materials
Graphene oxide powder was purchased from Tangshan Jianhua Technology Development Co., Ltd. (Tangshan, China). 4,4′-Diaminobiphenyl, sodium nitrite, and HPLC-grade methanol were provided by Boaixin Co. (Baoding, China). The standards of metolcarb, arprocarb, carbaryl, isoprocarb, fenobcarb, and diethofencarb were purchased from Aladdin Industrial Corporation (Shanghai, China) and used without additional purification. The individual stock solutions for each of the carbamates were prepared in methanol at the concentration of 1 mg mL−1. The standard mixture solution at each concentration of 40 μg mL−1 was prepared by the dilution of the individual stock solutions with methanol and stored at 4 °C. The water used throughout the work was ultra-pure water prepared with a Molelement 1820D ultra-pure water machine purchased from Chongqing Molecular Water System Co., Ltd. (Chongqing, China).
Preparation of DABP-GO Composite
The preparation of the DABP-GO is divided into two steps, i.e., the diazotization and coupling ones. For the diazotization, 300 mg 4,4′-diaminobiphenyl was dissolved completely in the solution prepared by mixing 95 mL of water and 5 mL of concentrated hydrochloric acid together. Then, the solution was placed in an ice bath at the temperature below 5 °C. Sodium nitrite of 120 mg was first dissolved in 10 mL water and then was added dropwise into the solution. After the solution was stirred for 40 min in the ice bath, a uniform bright yellow diazonium salt solution was obtained.
For the coupling reaction, 50 mg GO powder was dissolved in 50 mL water via ultrasonication to form a clear and well-dispersed solution. Then, the resulting GO solution was added in drops into the diazonium salt solution. Then, the pH of the mixture solution was adjusted to 8~9. After the reaction proceeded at the temperature below 5 °C for 2 h, a brown solid product was produced. After the product was filtered, washed with water and methanol, and freeze-dried, the final DABP-GO absorbent was obtained.
Characterization
The surface morphology and pore structure of the DABP-GO composite were characterized by scanning electron microscope (SEM) with a Hitachi Model S-4800 instrument (Hitachi High Technologies, Tokyo, Japan). The Brunauer-Emmett-Teller (BET) surface areas were measured from the N2 adsorption at 77 K using V-Sorb 2800P specific surface analyzer (Gold APP Instrument Corporation, Beijing, China).The Fourier transform infrared spectra (FT-IR) were recorded on a WQF-510A FT-IR spectrometer (Ruili Analytical Instrument, Beijing, China). For the FT-IR measurements of the GO and DABP-GO, they were pressed into pellet using spectroscopic grade KBr.
Sample Preparation
The vegetable samples (Benincasahispida and pakchoi) were purchased from the local market (Baoding, China). The samples were first washed and homogenized with a blender. Then, 50.0 g of the homogenate was put into a 100-mL centrifuge tube and centrifuged at 10,000 rpm (9917×g) for 10 min. After that, the supernatant was poured into an Erlenmeyer. Then, 10 mL acetonitrile was added into the residue and the mixture was vortexed for 2 min. After the centrifugation at 10,000 rpm (9917×g) for 2 min, the acetonitrile extract was merged into the previous supernatant and made up to 100 mL with water. At last, the extract was filtered through 0.22-μm nylon filter for the following SPE procedures.
SPE Procedures
The 3-mL empty polyethylene SPE cartridges were purchased from Agela Technologies (USA). Twenty milligrams of the DABP-GO composite was packed into the SPE cartridge in between two sieve plates. After the packed cartridge was conditioned in sequence with 2 mL methanol, 2 mL acetonitrile, and 5 mL water, 100-mL sample solution was passed through the cartridge at the flow rate of 3 mL min−1. Then, after the cartridge was washed with 5 mL of 10% (v/v) methanol water solution, 600 μL acetonitrile was added into the cartridge at a flow rate of about 1 mL min−1 for the elution. After the eluate was filtered through a 0.45-μm nylon filter, 20 μL of the resulting filtrate was injected into the HPLC-UV system for analysis.
HPLC Analysis
The Agilent 1260 Infinity LC instrument used in this work consists of an Agilent 1260 VWD detector, an Agilent 1260 pump, and a six-port valve with a 20-μL sample loop. A CenturySIL C18 column (200 mm × 4.6 mm, with 5 μm particle size) from Jiang Shen Separation Science Co., Ltd. (Dalian, China) was used for separations. The mobile phase was a mixture of acetonitrile and water (48:52, v/v) at the flow rate of 1 mL min−1. The common UV detection wavelength for the analytes was set at 208 nm.
Results and Discussions
Characterization of the DABP-GO Composite
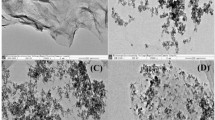
The typical SEM graphs for both the GO and DABP-GO are shown in Fig. 1. As shown in Fig. 1a, the GO sheet looks like a layer of silk with wrinkles. Figure 1b illustrates that 4,4′-diaminobiphenyl was incorporated onto the GO surface and the surface of the DABP-GO is rougher than GO, which should be more beneficial to the adsorption.
The FT-IR spectra for both GO and DABP-GO are shown in Fig. 1c. The characteristic absorption peaks of the GO at 3126 cm−1, 1678 cm−1, 1554 cm−1, and 1012 cm−1 from the hydrogen bonded O–H stretching, carbonyl (C=O) stretching, C=C stretching, and the C–O stretching of the epoxides (C–O–C) can be observed (Fang et al. 2014; Hung et al. 2014; Wu et al. 2016). Compared with the IR spectrum of the GO, the spectrum of the DABP-GO gives the additional absorption peaks at 1437 cm−1 and 830 cm−1, which can be attributed to the characteristic deformation vibration peak of amine N–H at 1437 cm−1 and the bending vibration peak of N–H at 830 cm−1, suggesting that amine groups are successfully compounded on the GO surface (Fang et al. 2014).
The N2 adsorption-desorption isotherms of the DABP-GO are shown in Fig. 1d. As can be seen from the Fig. 1d, the isotherms of the DABP-GO belong to type IV with H3, revealing a lamellar structure for the DABP-GO. The BET surface area for the DABP-GO was calculated to be 138.1 m2 g−1.
Optimization of SPE Conditions
In order to achieve the highest extraction recovery for the analytes, the main influencing factors for the SPE were optimized as follows.
Effect of Sample Loading Rate
Sample loading rate not only influences the analysis time of the whole analytical process but also affects the extraction recovery for the analytes. A too slow flow rate will result in an unnecessary prolonged time for the entire experiment. On the other hand, a too fast flow rate may cause the analytes to flow through the adsorbent without being adsorbed, resulting in a decrease in recovery. In this work, the effect of sample loading rate was optimized in the range of 1~5 mL min−1. Figure 2a shows that the extraction recoveries for the analytes are almost unchanged when the flow rate is less than 3 mL min−1, and when the flow rate is higher than 3 mL min−1, the extraction recoveries decrease slowly. Therefore, the optimal sample loading rate was chosen as 3 mL min−1.
Effect of Sample pH
The pH of the sample solution can affect the existing forms and stability of certain analytes. In this experiment, the effect of sample pH on the extraction was examined in the pH range of 2–10. As shown in Fig. 2b, when the pH is changed between 2 and 7, the extraction recoveries of the carbamates change little, and when the pH is larger than 7, the extraction recoveries decline to varying degrees, depending on the compounds. The above result is consistent with the literature reports (Li et al. 2015). This may be caused by the decomposition of the carbamates under alkaline conditions since carbamate pesticides are esters, and their carbon-oxygen ester bonds are unstable and easily broken down under alkaline conditions, resulting in a decrease in extraction recovery (Santalad et al. 2010).
In respect of the adsorbent, its adsorption behavior for the analytes was evaluated after it was treated with HCl solution (pH = 2), NaOH solution (pH = 12), and deionized water (pH = 7) for 24 h. The results (Fig. S1) show that the extraction recoveries for the carbamates were not significantly changed, indicating that the DABP-GO is stable to pH changes. Since the pH of the sample solutions involved in this study is lower than 7, there is no need to adjust the sample pH.
Elution of the Analytes
In this work, the elution of the analytes was examined by using the three commonly used organic solvents methanol, acetonitrile, and acetone. As can be seen from Fig. 2c, the elution capability of the three organic solvents for the carbamates is similar. Considering that the HPLC mobile phase contained acetonitrile, acetonitrile was chosen as the eluent.
Effect of Eluent Volume
The eluent volume is also an important parameter in SPE. If the eluent volume is too small, the analytes may not be completely eluted. On the other hand, an excessive amount of the eluent may decrease the sensitivity due to dilution effect. As can be seen from Fig. 2d, a complete elution can be achieved when the eluent volume is 0.6 mL or larger. Therefore, 0.6 mL acetonitrile was selected.
Adsorption Capacity
In order to explore the adsorption capacity of the DABP-GO composite, the saturated adsorption study of the DABP-GO composite was performed by increasing the analyte concentration without changing the volume of the sample solution.
As can be seen from Fig. 2e, the adsorption capability of the DABP-GO for the compounds is different. According to the figure, the adsorption capacity of the DABP for the analytes was calculated to be 3.5, 3.5, 3.0, 4.0, 4.0, and 4.5 mg g−1 for metolcarb, arprocarb, carbaryl, isoprocarb, fenobcarb, and diethofencarb, respectively.
Effect of Washing Prior to Elution
Due to the complexity of the real sample matrix, the co-existence of other matrix substances may interfere with the detection of the analytes of interest. Therefore, a washing process prior to elution is often required to alleviate the matrix effect. When the proportion of an organic solvent in the washing solution is too small, the interferences cannot be washed away. On the other hand, when the proportion of the organic solvent is too large, the target analytes may be washed away together with the interferences. Therefore, the ratio of the organic solvent methanol in the washing solution was optimized in the experiment.
Figure 2 f shows that with increased proportion of methanol, the extraction recoveries for the analytes decrease slowly. Therefore, the lowest proportion of methanol in the washing solution should be used for the elimination of the interferences. Figure S2 in Supporting Information shows that at the methanol content of 10%, the extraction recoveries for the analytes were not much influenced but most of the interferences were eliminated. Therefore, 5 mL of 10% methanol aqueous solution was selected for washing.
Method Validation
The method was evaluated from the perspectives of linearity, limits of detection (LODs), and limits of quantification (LOQs). In this study, matrix-matched calibration samples for pakchoi and benincasahispida were prepared by spiking the standards in the analyte-free sample homogenate from 1.0 to 40 ng g−1 at eight concentration levels (0.5, 1.0, 1.5, 2.0, 5.0, 10.0, 20.0, and 40.0 ng g−1), respectively. As shown in Table 1, the linear correlation coefficients (r) for the calibrations for both samples were greater than 0.9900, showing a good linearity. The measured LODs (S/N = 3)and LOQs (S/N = 9) for the analytes were in the range from 0.5 to 1.0 ng g−1 and from 1.5 to 3.0 ng g−1 for pakchoi sample, and in the range of 0.3–0.5 ng g−1 and 0.9–1.5 ng g−1 for benincasahispida sample, respectively. The LOQs are lower than the MRLs set by EU, meaning that the current method is sensitive enough for the detection of the carbamates.
The intra- and inter-day precisions of the method were studied by analyzing spiked samples at 40 ng mg−1. The intra-day precision was investigated by analyzing the spiked sample five times in a day and the inter-day precision was obtained by analyzing the spiked sample for five times on five continuous days. The intra-day RSDs for the pakchoi and benincasahispida samples ranged from 2.3 to 8.2% and the inter-day RSDs ranged from 4.5 to 9.2%, indicating that the method has a quite good repeatability.
Analysis of Real Samples
In order to verify the applicability of the established method, real pakchoi and benincasahispida samples were analyzed. The results are listed in Table 2. Carbaryl was detected out in the pakchoi sample and metolcrb was found in benincasahispida sample. Both their concentrations were below their LOQs. The method recoveries for the benincasahispida samples spiked at two different levels (2.0 and 10.0 ng g−1) were measured to be between 90 and 115%. For the pakchoi samples, the method recoveries at the spiked concentrations of 5.0 ng mg−1 and 10.0 ng mg−1 were in the range from 94 to 116%. The typical chromatograms for the pakchoi sample at 5.0 ng mg−1 of the carbamates and the benincasahispida sample at 2.0 ng mg−1 of the carbamates are shown in Fig. 3.
Typical chromatograms of a benincasahispida sample, b the Benincasahispida sample spiked with the carbamate pesticides at the concentration of 5.0 ng mg−1, c pakchoisampl, and d the pakchoi sample spiked with the carbamates at the concentration of 2.0 ng mg−1; peak identification: (1) metolcarb, (2) arprocarb, (3) carbaryl, (4) isoprocarb, (5) fenobcarb, and (6) diethofencarb
Reusability of the Composite
The reusability of the DABP-GO composite was evaluated by its repeated use for more extractions. As shown in Fig. 4a, after 25 extractions, the extraction recoveries for the carbamates declined about 10%. This indicated that the DABP-GO has a quite good reusability.
Comparison with Other Adsorbents
The DABP-GO was compared with other commercial adsorbents for the extraction of the carbamates. In the study, same amount of the adsorbents (20 mg each) was used. After being activated with 3 mL of methanol and washed with 3 mL water, 100 mL of 40 ng mL−1 carbamate solution was loaded. Then, 1 mL acetonitrile was used for desorption with the flow rate of about 1 mL min−1. Figure 4 b shows that the extraction recoveries of the DABP-GO for the analytes are similar to that of the commercial adsorbent HLB but are superior to other adsorbents. It is observed that the extraction recoveries for the analytes by GO alone are much lower than that by the DABP-GO, suggesting that the GO modified by DABP is more suitable for the adsorption of the carbamates.
Comparison with Other Reported Methods
The established method was compared with some relevant reported methods for the determination of carbamates in vegetables. The relevant data are shown in Table 3. The methods reported by Saraji and Esteki (2008) and Chen et al. (2010) provided lower LODs, but they only dealt with simple water samples. For the analysis of fruit and vegetable samples, the current method gives lower LODs than that of the SPEs either with CTAB-modified zeolite NaY (Salisaeng et al. 2016) or with PAG (Wu et al. 2017) as the adsorbent. The LODs of the current method are also lower than those by the MSPE with G-MNPs as the magnetic adsorbent (Santalad et al. 2009). The current method provides the LODs much lower than the MRLs set by many countries, and therefore, it can serve as an alternative method of choice for the determination of carbamates in real vegetable samples.
Conclusions
In this work, a graphene oxide-based composite DABP-GO composite was prepared for the enrichment of some carbamate pesticide residues from vegetable samples. The DABP-GO showed to be a good SPE adsorbent for the extraction of some carbamates from benincasahispida and pakchoi samples. The current method provides a new alternative of choice for the determination of carbamate pesticides in complex vegetable samples. However, the adsorption mechanism of the DABP-GO towards the analytes needs to be further elucidated and its more applications for different analytes and different samples have yet to be explored.
References
Casale GP, Vennerstrom JL, Bavari S, Wang TL (1993) Inhibition of interleukin 2 driven proliferation of mouse CTLL2 cells, by selected carbamate and organophosphate insecticides and congeners of carbaryl. J Immunopharmacol 15:199–215
Cavaliere B, Monteleone M, Naccarato A, Sindona G, Tagarelli A (2012) A solid-phase microextraction-gas chromatographic approach combined with triple quadrupole mass spectrometry for the assay of carbamate pesticides in water samples. J Chromatogr A 1257:149–157
Chen H, Chen R, Li S (2010) Low-density extraction solvent-based solvent terminated dispersive liquid-liquid microextraction combined with gas chromatography-tandem mass spectrometry for the determination of carbamate pesticides in water samples. J Chromatogr A 1217:1244–1248
Damalas CA, Eleftherohorinos IG (2011) Pesticide exposure, safety issues, and risk assessment indicators. Int J Env Res Pub He 8:1402–1419
David MG, Laura GG, García-Campaa AM, Bosque-Sendra JM (2011) Use of dispersive liquid-liquid microextraction for the determination of carbamates in juice samples by sweeping-micellarelectrokinetic chromatography. AnalBioanalChem 400:1329–1338
Dreyer DR, Park S, Bielawski CW, Ruoff RS (2010) The chemistry of graphene oxide. ChemSoc Rev 39:228–240
Fang F, Kong L, Huang J, Wu S, Zhang K, Wang X, Sun B, Jin Z, Wang J, Huang XJ, Liu J (2014) Removal of cobalt ions from aqueous solution by an aminationgraphene oxide nanocomposite. J Hazard Mater 270:1–10
Hung WS, Tsou CH, De Guzman M, An QF, Liu YL, Zhang YM, Hu CC, Lee KR, Lai JY (2014) Cross-linking with diaminemonomers to prepare composite grapheneoxide-framework membranes with varying d-spacing. Chem Mater 26:2983–2990
Ibrahim WA, Nodeh HR, Sanagi MM (2016) Graphene-based materials as solid phase extraction sorbent for trace metal ions, organic compounds, and biological sample preparation. Crit Rev Anal Chem 46:267–283
Kyzas GZ, Deliyanni EA, Bikiaris DN, Mitropoulos AC (2018) Graphene composites as dye adsorbents: review. ChemEng Res Des 129:75–88
Lambropoulou DA, Albanis TA (2007) Methods of sample preparation for determination of pesticide residues in food matrices by chromatography-mass spectrometry-based techniques: a review. Anal Bioanal Chem 389:1663–1683
Li N, Chen J, Shi YP (2015) Magnetic graphene solid-phase extraction for the determination of carbamate pesticides in tomatoes coupled with high performance liquid chromatography. Talanta 141:212–219
Li N, Jiang HL, Wang X, Wang X, Xu G, Zhang B, Wang L, Zhao RS, Lin JM (2018) Recent advances in graphene-based magnetic composites for magnetic solid-phase extraction. TrAC-Trend Anal Chem 102:60–74
Papageorgiou DG, Kinloch IA, Young RJ (2017) Mechanical properties of graphene and graphene-based nanocomposites. Prog MaterSci 90:75–127
Rahmani T, Bagheri H, Behbahani M, Hajian A, Afkhami A (2018) Modified 3D Graphene-au as a novel sensing layer for direct and sensitive electrochemical determination of Carbaryl pesticide in fruit, vegetable, and water samples. Food Anal Methods 11:3005–3014
Ren X, Chen C, Nagatsu M, Wang X (2011) Carbon nanotubes as adsorbents in environmental pollution management: a review. ChemEng J 170:395–410
Salisaeng P, Arnnok P, Patdhanagul N, Burakham R (2016) Vortex-assisted dispersive micro-solid phase extraction using CTAB-modified zeolite NaYsorbent coupled with HPLC for the determination of carbamateinsecticides. J Agric Food Chem 64:2145–2152
Santalad A, Srijaranai S, Burakham R, Glennon JD, Deming RL (2009) Cloud-point extraction and reversed-phase high-performance liquid chromatography for the determination of carbamate insecticide residues in fruits. Anal Bioanal Chem 394:1307–1317
Santalad A, Zhou L, Shang F, Fitzpatrick D, Burakham R, Srijaranai S, Glennon JD, Luong JH (2010) Micellarelectrokinetic chromatography with amperometric detection and off-line solid-phase extraction for analysis of carbamate insecticides. J Chromatogr A 1217:5288–5297
Saraji M, Esteki N (2008) Analysis of carbamate pesticides in water samples using single-drop microextraction and gas chromatography-mass spectrometry. Anal Bioanal Chem 391:1091–1100
Sitko R, Zawisza B, Malicka E (2013) Graphene as a new sorbent in analytical chemistry. TrAC-Trend Anal Chem 51:33–43
Song XY, Shi YP, Chen J (2013) Carbon nanotubes-reinforced hollow fibre solid-phase microextraction coupled with high performance liquid chromatography for the determination of carbamate pesticides in apples. Food Chem 139:246–252
Wang S, Li X, Liu Y, Zhang C, Tan X, Zeng G, Song B, Jiang L (2018) Nitrogen-containing amino compounds functionalized graphene oxide: synthesis, characterization and application for the removal of pollutants from wastewater: a review. J Hazard Mater 342:177–191
Wu J, Liang X, Hao L, Wang C, Wu Q, Wang Z (2017) Graphene oxide cross-linked with phytic acid: an efficient adsorbent for the extraction of carbamates. MicrochimActa 184:3773–3779
Wu ZL, Liu F, Li CK, Chen XQ, Yu JG (2016) A sandwich-structured graphene-based composite: preparation, characterization, and its adsorption behaviors for Congo red. Colloid Surface A 509:65–72
Ye ZQ, Cao BY, Yao WJ, Feng T, Ruan X (2015) Spectral phonon thermal properties in graphenenanoribbons. Carbon 93:915–923
Zhang BT, Zheng X, Li HF, Lin JM (2013) Application of carbon-based nanomaterials in sample preparation: a review. Anal ChimActa 784:1–17
Funding
Financial supports were from the National Natural Science Foundation of China (31471643, 31571925, 31671930), the Natural Science Foundation of Hebei Province (B2016204136, B2016204146, B2017204025), the Scientific and Technological Research Foundation of the Department of Education of Hebei Province (ZD2016085), the Natural Science Foundation of Hebei Agricultural University (LG201607), and the Innovation Ability Training Program of the Department of Education of Hebei Province for graduate students (1009050).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Tian Gao declares that he has no conflict of interest. Juntao Wang declares that he has no conflict of interest. Qiuhua Wu declares that he has no conflict of interest. Chun Wang declares that he has no conflict of interest. Zhi Wang declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1989 kb)
Rights and permissions
About this article
Cite this article
Gao, T., Wang, J., Wu, Q. et al. A Graphene Oxide–Based Composite for Solid-Phase Extraction of Carbamate Pesticides from Vegetables. Food Anal. Methods 13, 690–698 (2020). https://doi.org/10.1007/s12161-019-01685-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-019-01685-3