Abstract
A rapid, simple, inexpensive, and reliable vortex-assisted dispersive liquid-liquid microextraction (VA-DLLME) method was developed for the determination of fipronil and its major metabolite, fipronil sulfone, in tomato fruits using liquid chromatography tandem mass spectrometry (LC-MS/MS). No salting-out or cleanup steps were required after extraction. Average recoveries ranged from 89.8 to 96.3% with relative standard deviation (RSD) values below 11.6%. The limit of quantitation (LOQ) of the method was 0.25 μg/kg, which is 20 times below the European Union-defined maximum residue limit (MRL) of 5 μg/kg. Good linearity was achieved, with a correlation coefficient ≥ 0.996 based on matrix-matched calibration. We investigated the dissipation and residue distribution of fipronil in tomato fruits after one application of suspension concentrate (SC) or emulsifiable concentrate (EC) formulations over 28 days under field conditions using the VA-DLLME method. The half-life (t1/2) of fipronil dissipation was 2.4 and 2.8 days, and the estimated pre-harvest interval (PHI) was 23 and 32 days for the SC and EC formulation, respectively. A risk assessment was conducted by evaluating the health risk index (HRI); the estimated PHIs values indicate it is safe to consume tomatoes after the recommended application of fipronil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tomato (Solanum lycopersicum L.), a plant belonging to the Solanaceae family, is one of the most important agricultural crops and is widely cultivated throughout the world (Dorais et al. 2008). Egypt is the third leading producer of tomatoes worldwide, producing approximately 7.3 million tonnes per year (Abd-Alrahman and Osama 2012; FAOSTAT 2018). Tomato fruits provide basic nutrition in the form of various biochemical compounds such as minerals, vitamins, carbohydrates, proteins, and antioxidant molecules. Several of these molecules have been shown to exert beneficial effects, such as preventing diabetes, maintaining blood pressure, and promoting cardiovascular health (Elbadrawy and Sello 2016). A number of factors may decrease the productivity and quality of tomato crops, including poor-quality seeds, pests, and climatic conditions (Oerke 2006).
Fipronil, 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-4-[(trifluoromethyl)sulfinyl]-pyrazole-3-carbonitrile (Fig. 1a), is a phenyl pyrazole insecticide (Tomlin 1994) that can be used on more than 60 crops to control piercing/sucking and chewing insect pests (Bobé et al. 1998). Fipronil prevents inhibition of gamma amino butyric acid (GABA) receptors (Sammelson et al. 2004). The major metabolites of fipronil include sulfone (Fig. 1b), sulfide, and desulfinyl, which are produced by oxidation, reduction, and photolysis, respectively (Bobé et al. 1998; Hainzl and Casida 1996; Ramesh and Balasubramanian 1999). These metabolites have toxicological significance (Tingle et al. 2003) and possess greater endocrine disrupting potential than the parent compound fipronil (Cravedi et al. 2013; Lu et al. 2015). Therefore, a number of countries, such as the USA, France, and Uruguay, have restricted or even prohibited the use of fipronil in specific applications (Li et al. 2015).
The formulation of a pesticide can affect the dissipation kinetics of pesticide residues, especially if the spray rate is increased (g active ingredient/ha). Increasing the spray rate will also increase the concentration of surfactant and other adjuvants in the spray solution, which may lead to the crop retaining the residue for a longer period of time (Angioni et al. 2011; Cabras et al. 1989; MacLachlan and Hamilton 2011). The efficacy of a pesticide and number of applications required to achieve adequate pest control are also affected by the formulation (Arthur 2004; Korunic 1998).
Various analytical procedures have been reported for the determination of fipronil and its metabolites in different crops. For instance, liquid-liquid extraction (LLE) using gas chromatography with an electron capture detector (GC-ECD) has been reported for pakchoi (Pei et al. 2004), and solid-phase microextraction using GC with tandem mass spectrometry MS/MS, for tea leaves (Zhou et al. 2011). Three steps of liquid-solid extraction, followed by liquid-liquid partitioning and solid-phase extraction (SPE) in combination with GC-ECD have been used for maize, grape leaves, berries, and cabbage (Bhardwaj et al. 2012; Mohapatra et al. 2010; Wang et al. 2014). At present, the QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) protocol is widely used as a conventional sample preparation method for liquid or gas chromatographic determination of fipronil in vegetables and fruits (Cheng et al. 2014; Duhan et al. 2015; Kaur et al. 2015; Li et al. 2015; Paramasivam and Chandrasekaran 2013). Dispersive liquid-liquid microextraction (DLLME) (Viñas et al. 2014) has also been successfully used, and offers several advantages including speed, simple operation, and lower cost due to the small volume of solvents required. However, there are few reports of the uses of QuEChERS in combination with DLLME for determination of fipronil in vegetables (Bai and LI MP 2014).
The Egyptian Agriculture Pesticide Committee (APC) has defined the goal of comparing pesticide residues with the maximum residue limits (MRLs) developed by the Codex Alimentarius Commission (CAC) (Codex-Alimentarius n.d.) or, if unavailable, the European Union MRL (EU-MRL-Database 2018a). The EU-MRL for fipronil in tomatoes is 0.005 mg/kg; this residue limit includes fipronil plus the sulfone metabolite (EU-MRL-Database 2018b). Thus, the low fipronil MRL necessitates the development of a sensitive and robust quantitative analytical method with acceptable precision and accuracy, as defined by the SANTE/11813/2017 guidelines (SANTE/11813/2017).
The objective of the present study was to develop a rapid, simple, inexpensive, and reliable VA-DLLME method for LC-MS/MS determination of fipronil and its major metabolite fipronil sulfone in tomatoes. Several experimental parameters affecting the performance of the proposed method were initially controlled and optimized, then compared with the conventional reported QuEChERS procedure. The VA-DLLME method was validated, then used to assess the levels of fipronil and fipronil sulfone, determine the pattern of dissipation and estimate the pre-harvest interval (PHI) and half-life (t1/2) values for tomato crops sprayed with two different commercial formulations of fipronil. The data generated was then used to conduct a risk assessment based on the dietary intake of tomatoes. To the best of our knowledge, the risk of exposure to fipronil based on tomato consumption has not been previously reported for the Egyptian population.
Materials and Methods
Chemicals, Reagents, and Apparatus
Analytical standard fipronil (98% purity) and fipronil sulfone MB46136 (99.7% purity) were purchased from Chem Service Inc. (West Chester, PA, USA). HPLC-grade acetonitrile, methanol, acetone, and chloroform and LC/MS-grade formic acid and ammonium formate were purchased from Fisher Scientific (Loughborough, UK). The ceramic homogenizer was purchased from Agilent Technologies Inc. (Wilmington, DE, USA). Two commercial formulations of fipronil, 20% suspension concentrate (SC) and 2.5% emulsifiable concentrate (EC), were purchased locally.
Preparation of Standard Solutions
Standard stock solutions of fipronil and fipronil sulfone were prepared separately by dissolving 0.0510 or 0.0502 g of the respective reference standard in 50 mL of acetonitrile in a 50-mL calibrated glass volumetric flask, to obtain final concentrations of 1000 μg/mL. The solutions were stored at − 20 °C; the analytes are stable for more than 15 months when stored in acetonitrile at − 20 °C (JMPR 2011).
The standard working solutions (10 μg/mL) were prepared by further dilution in acetonitrile and used to prepare calibration curves (0.5, 1, 2.5, 5, 10, 25, 50, 100, and 250 ng/mL).
Matrix-matched calibration curves were constructed to compensate for matrix effects, and were prepared by adding standard solutions (0.5, 1, 2.5, 5, 10, 25, 50, 100, or 250 ng/g) of fipronil or fipronil sulfone to blank tomato sample extracts prepared using the same sample preparation procedures. The resulting calibration curves were used to calculate the concentrations of analyte(s) in the unknown samples.
Field Trial Design
A field experiment was carried out from 2nd May to 1st June 2018 in Menof, Menofia governorate, Egypt. The commercial 2.5% emulsifiable concentrate (EC) or 20% suspension concentrate (SC) fipronil formulations were applied once at a dosage of 37.5 g a.i./ha and 50 g a.i./ha, respectively, using a knapsack sprayer to simulate commercial application using 1000 L water per hectare. The pesticides were applied at the start of the fruit formation stage (green stage), and samples were collected during this stage and up to the ripe stage (harvesting stage). Three replicate plots were prepared, and buffer areas were defined to separate each plot. There was no rain during the field trial. The daily average temperature ranged from 27 to 35 °C. Tomato samples were collected 0 (2 h), 1, 3, 7, 10, 15, 21, and 28 days after the application of fipronil. Approximately 1–2 kg of tomatoes were collected from each replicate plot and pooled, diced into small pieces (2–3 cm), frozen overnight, and then ground and homogenized using a HOBART food chopper. The homogenized subsamples were stored in polyethylene containers at − 20 °C until analysis.
VA-DLLME Procedure
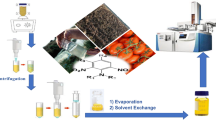
Pre-homogenized tomato samples (10 ± 0.1 g) were weighed into 50-mL polypropylene centrifuge tubes, 6 mL acetonitrile (dispersive solvent) was added, vortexed for 1 min after adding a piece of ceramic homogenizer, centrifuged at 5000 rpm for 5 min, and 4 mL of the supernatant (equivalent to 2.5 g sample) was filtered through a 0.45-μm syringe filter into a 15-mL centrifuge tube. Finally, 3 mL cold deionized water (4 °C) and 80 μL chloroform (extraction solvent) were immediately added, the mixture was shaken for few seconds, and the solution turned cloudy (i.e., dispersive liquid-liquid microextraction, DLLME). After centrifugation at 5000 rpm for 3 min, the sedimented chloroform phase (about 75 ± 8 μL, equivalent to 2.5 g sample) was quantitatively removed into a new vial and evaporated to dryness by centrifugal evaporation (Concentrator plus®; Eppendorf, Hamburg, Germany). The residue was reconstituted in 250-μL acetonitrile (10-fold higher enrichment factor), and 10.0 μL aliquots were injected into the LC-MS/MS system for analysis. Untreated tomato fruits were subjected to the VA-DLLME procedure to prepare the blank tomato extracts used to dilute the test samples, when necessary.
LC-MS/MS
The LC-MS/MS system comprised of a Waters Alliance e2695 LC separation module coupled to a Micro mass Quattro Micro triple quadrupole tandem mass spectrometer (Waters Corp., Milford, MA, USA). A C18 Ultra BiPhenyl column (150 mm × 4.6 mm i.d., 5 μm; Restek®, Bellefonte, PA, USA) was used for separation at 40 °C. The mobile phase consisted of water (A) and acetonitrile (B); the initial gradient was 90% (A) and 10% (B). The (B) phase was held for 1 min, increased to 100% by 7.0 min, and held at 100% until 13 min. Finally, the (B) phase was returned to 10% over 0.1 min and then held for 5 min for column equilibration before the next sample injection. The flow rate was 0.4 mL/min; the injection volume was 10 μL. The retention times for fipronil and fipronil sulfone were 12.51 and 12.70 min, respectively. Multiple reaction monitoring (MRM) was conducted in negative electrospray ionization mode (ESI−). The drying gas temperature was set at 450 °C, and the nebulizer gas (N2) pressure was 50 psi; capillary voltage was 3500 V with a MS1 and MS2 heater temperature of 120 °C. The MS parameters are listed in Table 1.
Validation Study
A validation study was carried out based on the SANTE guidelines (SANTE/11813/2017); the SANTE criteria include specificity, linearity range, recovery and limits of detection (LOD), and quantitation (LOQ). Matrix-matched calibration was performed at nine concentrations in triplicate over the range of 0.5 to 500 ng/mL. Accuracy was evaluated by spiking control samples at four concentrations (0.25, 5, 50, and 500 μg/kg), with five replicates for each concentration.
Precision was evaluated by determining the coefficient values in terms of inter-day repeatability (RSDr; six replicates, 18 injections on the same day) and intra-day repeatability (RSDR; 18 replicates, 54 injections on three different days at 7-day intervals) at a spiking level of 0.25 μg/kg (equal to the LOQ value).
The LOD and LOQ were calculated based on the ratio between the standard deviation of the response (Sy) and the slope (S) of the matrix-matched calibration curve, assuming LOD and LOQ values of 3.3 and 10 Sy/S, respectively (Abdallah et al. 2017).
Statistical Analysis
The dissipation of total fipronil and sulfone metabolites in tomatoes was fitted to a first-order curve. The selected kinetic model was graphically confirmed using the equation Ct = C0 e −kt, in which Ct represents the concentration of total fipronil residues at time t, C0 represents the initial deposit after application, and k is the degradation rate constant (day−1). Half-lives (t1/2) were calculated from the k values using t1/2 = ln 2/k (Malhat et al. 2017).
Result and Discussion
Optimization of Instrumentation Conditions
The MS/MS parameters were manually optimized in negative electrospray ionization mode (ESI−) for each compound. The ions with m/z 434.7 and m/z 450.8 were selected as the precursor ions for fipronil and fipronil sulfone, respectively. According to the SANTE guidelines, confirmation of each analyte in multiple reaction monitoring (MRM) mode was achieved through selection of two products with high sensitivity and selectivity; one of the ions was used as a quantifier (with high response), the other was used as a qualifier. The optimized MS/MS parameters are shown in Table 1.
The mobile phase composition used for chromatographic separation was optimized to achieve sufficient peak separation with high sensitivity. Gradient elution using methanol-water or acetonitrile-water in the presence and absence of formic acid or ammonium formate as modifiers was evaluated. We evaluated 10 replicate injections (n = 10) at a concentration of 10 μg/L for each of the different mobile phase compositions (Fig. 2). Gradient elution of acetonitrile-water (as described in the experimental section) without formic acid or ammonium formate provided high sensitivity and a symmetrical peak shape. A flow rate of 0.4 mL/min, injection volume of 10 μL, and column temperature of 40 °C were selected as the optimal parameters. Figure 6b, c presents typical chromatograms for fipronil and fipronil sulfone.
Optimization of the Method
Preliminary Test
Type of Solvent and its Volume/Sample Weight Ratio
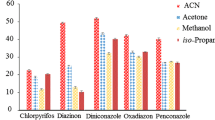
The choice of disperser solvent is mainly based on its miscibility with the water content of the sample, the polarity of the solvent, and the ability of the high-density solvent (extractant) to form a cloudy state when injected into the sample in the DLLME step. Extraction efficiency was evaluated using acetone, acetonitrile, and methanol on the basis of percentage recovery and RSD. The only parameter changed was the type of solvent; volume and sample weight were kept constant at 10 mL and 10 g, respectively, and the vortex time was 5 min. The DLLME conditions were as follows: exactly 2 mL of filtered supernatant (equivalent to 1 g sample) was diluted to 6 mL in 15-mL centrifuge tubes by adding 4 mL of cold water (4 °C) and 100 μL of chloroform as the extractant solvent. As shown in Fig. 3, highest recovery was achieved when acetonitrile or acetone were used. Compared to acetone, acetonitrile extracts less lipophilic material, e.g., waxes, fat, and lipophilic pigments (Cunha et al. 2007); consequently, acetonitrile was selected as the optimal extractant.
The effect of the acetonitrile volume/sample weight ratio on extraction efficiency was investigated by varying the volume of acetonitrile from 2 to 10 mL at 2-mL intervals, while keeping the sample weight (10 g) and DLLME conditions constant. Percentage recovery of the target analytes proportionally increased with the volume of acetonitrile from 2 to 6 mL, then remained almost constant at higher volumes (Fig. 4). Therefore, the lowest effective solvent volume of 6 mL was selected to minimize waste and ecological impact.
Optimization of the Vortex Time
The effect of varying the duration of vortexing of acetonitrile/sample on the efficiency of the recovery of fipronil and fipronil sulfone was examined; the duration of vortexing was varied from 1 to 5 min (at 1-min intervals), while keeping other parameters constant. The peak area of both analytes slightly decreased as the duration of vortexing increased up to 3 min, then the peak areas increased at 4 min and were similar at 5 min. This indicated that extraction equilibrium could be reached with 1 min, while achieving high extraction recovery of the target analytes. There were no observable differences in the resulting peak areas over the range of times tested. Therefore, 1-min vortexing was deemed sufficient for extraction; this short time is one of the most important features of the DLLME step.
Dispersive Liquid-Liquid Microextraction
Optimization of Disperser Solvent Volume/Total Volume Ratio
The volume of acetonitrile disperser in the DLLME step was optimized by adding different volumes of cold water (4 °C) to 4 mL of filtered supernatant (equivalent to 2.5 g sample) that already contained 1.5 mL of acetonitrile. The volume of water was varied from 1 to 6 mL at 1-mL intervals to achieve acetonitrile disperser ratios of 30 to 15%, respectively. A chloroform sediment layer did not form when 0, 1, or 2 mL of water were added; adding 3 mL of water resulted in approximately 155 μL of sedimented chloroform. Thereafter, the sediment chloroform layer decreased as the water volume increased, to about 85 μL of chloroform when 6 mL of water was added to 4 mL of extract supernatant. Accurate measurement of the volume of the sediment layer is not crucial, as the sediment layer will evaporate and a change in solvent to 250 μL of acetonitrile (equivalent to 2.5 g sample, with 10-fold enrichment factor) occurs prior to LC-MS/MS analysis, so the lowest volume of water that achieved sufficiently high recovery was selected. When 3 mL of water was added (resulting a total volume of 7 mL and of 21.4% of acetonitrile as a disperser), the peak areas for both analytes were non-significantly different to the peaks resulting from subsequent addition of water. Therefore, 3 mL of water was added to 4 mL of supernatant extract.
Effect of Extractant Solvent Volume
The effect of varying the volume of chloroform (CHCl3) from 40 to 160 μL at 20-μL intervals on extraction efficiency was investigated, keeping all other parameters constant. Figure 5 shows the variations in peak area against the volume of chloroform. The peak areas of both analytes increased with the volume of chloroform up to 80 μL; there were no significant difference in the peak area when the volume added increased to 100 μl, and then slightly decreased as the volume of chloroform increased further. Therefore, 80 μl of chloroform was selected as the optimal extraction volume.
Effect of Shaking Time
The effect of the duration of the hand shaking step during DLLME on the peak areas of both analytes was investigated over the range from 5 to 60 s. The duration of hand shaking had no significant effect on extraction efficiency. After the cloudy solution forms, a very large area of the chloroform solution is exposed to the aqueous phase and equilibrium between the phases is achieved very quickly. Therefore, a very short extraction time is sufficient, which is a remarkable advantage of the DLLME technique.
Method Validation
Specificity
The specificity of the method was assessed by comparing the chromatograms of the matrix-free analytes (blank sample) with the chromatograms of the matrix fortified at 2.5 μg/kg for each analyte. Fipronil and fipronil sulfone showed retention times of 12.51 and 12.70 min, respectively. The absence of interfering peaks at or close to the retention time of fipronil and fipronil sulfone indicated an absence of interfering compounds that could significantly influence the consistency of the resulting peaks (Fig. 6). This indicates the specificity of the method is acceptable for determination of fipronil and fipronil sulfone in tomato samples.
Linearity and Matrix Effects
The linearity of the instrument response was achieved by evaluating the correlation coefficient (R2) of the matrix-matched calibration curves at 10 concentrations ranging from 0.5 to 500 μg/L, which correspond to 0.05 to 50 μg/kg in samples. The curves were constructed by plotting the areas of the analytes versus the concentrations. The calibration curves for fipronil and fipronil sulfone showed good linearity with strong correlations between the concentrations and peak areas in the range of 1–250 and 0.5–250 μg/L; the curves had correlation coefficients of 0.999 and 0.996, respectively. The high correlation coefficients demonstrated good agreement between the back-calculated concentrations and the true concentrations of the analytes, as required in the SANTE guidelines (SANTE/11813/2017). The equations y = 222.5x + 52.8 and y = 134.8x + 19.5 were derived from the constructed matrix-matched calibration curves and used to calculate the concentrations of fipronil and fipronil sulfone, respectively, in the unknown samples (where x = unknown concentration and y = peak area).
To evaluate matrix effects (ME), the slopes of the matrix-matched calibration curves (MMCC) were compared with the slopes of the standard calibration curves (CCS). Equation (1) was used to calculate the percentage signal enhancement or suppression to investigate MEs:
If ME% is ≤ + 20% and ≥ − 20%, then no ME are present; values ˃ + 20% and ˂ − 20% indicate signal enhancement and suppression, respectively (Ferrer et al. 2011; Kaczyński and Łozowicka 2017). The MEs for fipronil and fipronil sulfone were − 16.4% and − 13.7%, respectively, which indicated ion suppression occurred for both analytes (Table 2). Therefore, the matrix-matched calibration curves were used to compensate for these matrix effects and achieve accurate quantitation.
LOD and LOQ
The calculated LOD and LOQ values were 0.07 and 0.21 μg/kg for fipronil and 0.04 and 0.12 μg/kg for fipronil sulfone, respectively. According to the SANTE guidelines, the LOQ is defined as the lowest spiked level in the validation experiments that results in an acceptable trueness of 70 to 120% and precision (RSD) lower than 20%. The validity of the LOQ value was successfully established at a concentration of 0.25 μg/kg for each analyte (n = 6), with 94.4 and 96.3% percentage recovery and relative standard deviation (RSD) values of 4.9 and 6.9% for fipronil and fipronil sulfone, respectively. The quantification limit value of the method is 20 times lower than the prescribed MRL value of 5 μg/kg for total fipronil residue in tomatoes (EU-MRL-Database 2018b).
Accuracy and Precision
Inter-day repeatability (RSDr) was below 6.9% and intra-day repeatability (RSDR) was below 11.6%. The resulting recoveries ranged from 89.9 to 94.4% and 90.5 to 96.3% for fipronil and fipronil sulfone, respectively, with RSD below 7.1%. These results are consistent with the SANTE guidelines (SANTE/11813/2017) and indicate the proposed method provides good accuracy and precision.
Comparison of DLLME with Other Sample Preparation Techniques
The LOQ, RSD, and recovery values of the validated VA-DLLME procedure and the conventional QuEChERS procedures were compared (Table 3). The VA-DLLME method offers several advantages, such as high recovery, lower LOQ, high sample throughput, low consumption of organic solvents and salts, and simple laboratory processing for sample treatment. In addition, there is no need to perform a cleanup step during sample extraction before chromatographic analysis. Therefore, the VA-DLLME method is simple, rapid, easy-to-use, and environmentally friendly.
Duhan et al. reported QuEChERS and GC-MS/MS provided a LOQ of 0.003 mg/kg and ˃ 80% recovery for fipronil in cauliflower (Duhan et al. 2015). Li et al. reported a lower LOQ (0.001 mg/kg) for QuEChERS and LC-MS/MS analysis of fipronil in peanut, with a higher RSD (˂ 19%) and 86–112% recovery (Li et al. 2015). Biswas et al. reported a LOQ of 0.005 mg/kg, RSD of ˂ 16.5 and 80.7–98.5% recovery for analysis of fipronil in sugarcane using QuEChERS and a GC-electron capture detector (ECD) (Biswas et al. 2019). Paramasivam and Chandrasekaran reported LOQ of 0.01 mg/kg, with an RSD of ˂ 10.15% and 86–112% recovery for QuEChERS and GC-MS analysis of fipronil in vegetables (Paramasivam and Chandrasekaran 2013). The VA-DLLME method developed in this study provides adequate performance for analysis of fipronil and fipronil sulfone in tomato fruits, including lower LOQs of 0.00025 mg/kg, RSD of ˂ 11.6, and 89.8–96.3% recovery. The most important advantage of VA-DLLME is the fact the salting-out step in the liquid-liquid partition step of QuEChERS (CEN 2008; Lehotay 2007) can be omitted.
Dissipation Kinetics of Fipronil in/on Tomatoes
The VA-DLLME method was applied to analyze the field tomato samples. Figure 7 shows the dissipation curves for fipronil and its metabolite fipronil sulfone in tomato fruits after a single application of the 2.5% EC formulation or 20% SC formulation at the manufacturer’s recommended doses of 37.5 g a.i./ha or 50 g a.i/.ha, respectively, under open-field conditions. The initial concentrations of total fipronil in tomatoes after a single application of the EC and SC formulations were 0.225 and 0.296 mg/kg, respectively. The initial deposits immediately declined by 32.03 and 20.9%, respectively, within the first 24 h. Thereafter, the residues gradually declined and reached 0.007 and 0.002 mg/kg, with a percentage loss of 96.9 and 99.3%, respectively, on day 28. The fipronil declined rapidly after application to fipronil sulfone metabolite. The initial concentrations of fipronil sulfone were 0.01 and 0.023 mg/kg after application of the EC and SC formulations reached 0.035 and 0.026 mg/kg at 7 and 10 days, and then continuously decreased to below the limit of quantitation at day 28 (Table 4). The sulfone metabolite is formed by oxidation under aerobic conditions; tomato plants utilize oxygen for making food and for plant defense mechanisms. Fipronil exerts unique insecticidal activity and provides long-term crop protection; this may be due in part to the combined action of the parent compound and the sulfone and sulfide derivatives, which have a similar insecticidal activity as fipronil, consistent with their composition as major metabolites (Hainzl and Casida 1996).
The dissipation kinetics of fipronil along with its metabolite fipronil sulfone (Table 4) could be mathematically described using Ct = 0.225e- 0.12t (R2 = 0.981) for the 2.5% EC formulation and Ct = 0.32e- 0.178t (R2 = 0.992) for the 20% SC formulation. The correlation coefficient, R2, indicates the strength of the relationship to first-order kinetics. The half-life (t1/2) of fipronil, calculated as described by Hoskins (1961) (Hoskins 1961), was 2.8 and 2.4 days after application of the manufacturer’s recommended doses of the 2.5% EC and 20% SC formulations, respectively (Table 3). These half-lives are consistent with the values reported by Gupta et al. (2007) (Gupta et al. 2007) who determined t1/2 of fipronil of 2.50 and 3.48 days in eggplant/brinjal following application at 75 and 150 g a.i./ha, respectively, and 0.65 and 1.12 days in okra following application at 50 and 100 g a.i./ha, respectively (Gupta et al. 2009).
The rate of dissipation was higher for the SC fipronil formulation than the EC formulation. Almost 90% of fipronil SC had degraded within 15 days of application, compared to 80% of fipronil EC (Fig. 7). Different adjuvants are used in the SC and EC formulations and there is more surfactant in the SC formulation. Moreover, the application dose is not the same for each formulation (Liu et al. 2015), which leads to a different initial concentration in tomatoes, resulting in varied residue levels and in turn influencing dissipation kinetics.
Since Egypt and most Arab countries have not defined MRL for fipronil residues, the results of this study will potentially help the government to establish an MRL for fipronil in tomato fruits. Egypt is fully committed to meeting the MRLs defined by the Codex Alimentarius MRLS. If there is no MRL in the Codex, then Egypt follows the EU-MRL (excluding the default limits of 0.01 ppm), or as a last resort, the MRL of the United States Environmental Protection Agency (EPA).
The combined MRL for fipronil and fipronil sulfone in the EU-MRL database is 0.005 mg/kg for tomato fruits (EU-MRL-Database 2018b). The results of this study suggest that the pre-harvest interval period (PHI) after application of the recommended doses of fipronil 2.5% EC or fipronil 20% SC to open-field tomatoes should be 32 and 23 days, respectively.
Risk Assessment
The use of pesticides on food crops may lead to undesirable high levels of residues at harvest, which may constitute barriers to exporting and domestic consumption if the crops exceed the MRL. The risk to human health varies for each specific pesticide. If the hazard is small and fixed, then the risk (risk = hazard × exposure) will be proportional to exposure; thus, the risk can be reduced by consuming crops with low levels of residues (Bates 2002). A theoretical maximum residue contribution (TMRC) for total fipronil was previously calculated by multiplying the average residue levels from the field trials (Table 4) by the average daily vegetable consumption estimates for adults in the Middle East of 0.0815 or 0.233 kg/person according to the GEMS/Food regional diet, assuming that 100% of vegetables consumed will contain residues (WHO 2003). The TMRC was compared with the maximum permissible intake (MPI), obtained by multiplying the acceptable daily intake (ADI) by the weight of the average adult (60 kg) (FAO 2009), to evaluate the potential health risk index (HRI) to the consumer for fipronil residues on tomato fruits. Pesticide residues could cause a health risk over lifetime consumption if the HRI is > 1 (Abdallah et al. 2018; Wang et al. 2011). The ADI of fipronil is 0.0002 mg/kg body weight/day (JMPR 2011). The MPI was calculated to be 12 μg/person/day. The TMRC values for fipronil residues on 0 day after application of the EC and SC formulations were calculated to be 18.33 and 24.12 μg/person/day, respectively (Table 5). Although the TMRC values are below the maximum permissible intake on days 3 and 7 (based on dietary daily consumption of the treated tomato fruit samples at a given application dosages) and below the MPI after day 15 (based on the combined average total vegetable daily consumption), residues persisted up to day 28 after application. MRLs are used to ensure that pesticide residues in crops at harvest do not exceed the acceptable daily intake of pesticide residues. The presence of higher levels of pesticide residues above the MRL on a given crop may result in bio-accumulation and pose a risk to consumers’ health (Abdallah et al. 2018). Thus, the pre-harvest period indicates safe pesticide use and this information should be included within the instructions on the label. Hence, a waiting period of 32 and 23 days before consuming tomato fruit sprayed with fipronil is suggested to further reduce the risk to consumers. Therefore, this study suggests consumption of tomato fruits treated with formulations of fipronil after the suggested waiting time confers no risk to human health.
Conclusion
Fipronil and fipronil sulfone were successfully quantified in tomato fruits using the vortex-assisted dispersive liquid-liquid microextraction (VA-DLLME) procedure and liquid chromatography tandem mass spectrometry (LC-MS/MS) technique developed in this study. Good recoveries with low RSD values were observed at low spiking levels, and the low LOQ values demonstrate the VA-DLLME method holds potential as a satisfactory analytical technique for investigating target analytes in tomato fruits. The proposed method achieved an excellent quantitation limit of up to 20 times lower than the EU-MRL for fipronil in tomatoes. The VA-DLLME method offers the advantages of direct extraction of fipronil and its metabolite sulfone from tomatoes without salting-out and cleanup steps. The method is simple, rapid, inexpensive, and can be used for various vegetable crops. We investigated the dynamics and residues of fipronil after a single application of the EC and SC formulations to tomato crops under open-field conditions. Fipronil dissipated more rapidly after application of the SC formulation than the EC formulation, with respective half-lives of 2.4 and 2.8 days. Our results suggest the use of the fipronil SC and EC formulations at the recommended doses is safe if withdrawal periods of 23 and 32 days are allowed. The long-term stability of fipronil in tomatoes may lead to a high risk of exposure to fipronil residues; therefore, further studies are required to further evaluate the dissipation behavior and risk of exposure to fipronil residues.
References
Abdallah OI, Hanafi A, Ghani SBA, Ghisoni S, Lucini L (2017) Pesticides contamination in Egyptian honey samples. J Verbrauch Lebensm 12:317–327
Abdallah OI, Alamer SS, Alrasheed AM (2018) Monitoring pesticide residues in dates marketed in Al-Qassim, Saudi Arabia using a QuEChERS methodology and liquid chromatography–tandem mass spectrometry. Biomed Chromatogr 32:e4199
Abd-Alrahman SH, Osama I (2012) Dissipation rate of different commercial formulations of malathion applied to tomatoes. Afr J Agric Res 7:332–5335
Angioni A, Dedola F, Garau A, Sarais G, Cabras P, Caboni P (2011) Chlorpyrifos residues levels in fruits and vegetables after field treatment. J Environ Sci Health B 46:544–549
Arthur FH (2004) Evaluation of a new insecticide formulation (F2) as a protectant of stored wheat, maize, and rice. J Stored Prod Res 40:317–330
Bai B, LI MP ZSW (2014) Determination of chlorfenapyr and fipronil residues in vegetables by QuEChERS combined with dispersive liquid⁃ liquid microextraction and high performance liquid chromatography. Food Sci 35:254–258
Bates R (2002) Pesticide residues and consumer risk assessments. Pestic Outlook 13:142–147
Bhardwaj U, Kumar R, Kaur S, Sahoo SK, Mandal K, Battu R, Singh B (2012) Persistence of fipronil and its risk assessment on cabbage, Brassica oleracea var capitata L. Ecotoxicol Environ Saf 79:301–308
Biswas S, Mondal R, Mukherjee A, Sarkar M, Kole RK (2019) Simultaneous determination and risk assessment of fipronil and its metabolites in sugarcane, using GC-ECD and confirmation by GC-MS/MS. Food Chem 272:559–567
Bobé A, Meallier P, Cooper J-F, Coste CM (1998) Kinetics and mechanisms of abiotic degradation of fipronil (hydrolysis and photolysis). J Agric Food Chem 46:2834–2839
Cabras P, Gennari M, Meloni M, Cabitza F, Cubeddu M (1989) Pesticide residues in lettuce. 2. Influence of formulations. J Agric Food Chem 37:1405–1407
CEN (2008) Foods of plant origin-determination of pesticide residues using GC-MS and/or LC-MS/MS following acetonitrile extraction/partitioning and clean-up by dispersive SPE QuEChERS-method EN 15662:2008
Cheng Y, Dong F, Liu X, Xu J, Meng W, Liu N, Chen Z, Tao Y, Zheng Y (2014) Simultaneous determination of fipronil and its major metabolites in corn and soil by ultra-performance liquid chromatography-tandem mass spectrometry. Anal Methods 6:1788–1795
Codex-Alimentarius (n.d.) Joint FAO/WHO Secretariat. Rome. [further information and database on Codex maximum residue limits (MRLs) at: http://www.fao.org/AG/AGP/AGPP/Pesticid/] Accessed 20.Jul 2018
Cravedi J, Delous G, Zalko D, Viguie C, Debrauwer L (2013) Disposition of fipronil in rats. Chemosphere 93:2276–2283
Cunha SC, Fernandes JO, Oliveira MBP (2007) Comparison of matrix solid-phase dispersion and liquid–liquid extraction for the chromatographic determination of fenthion and its metabolites in olives and olive oils. Food Addit Contam 24:156–164
Dorais M, Ehret DL, Papadopoulos AP (2008) Tomato (Solanum lycopersicum) health components: from the seed to the consumer. Phytochem Rev 7:231–250
Duhan A, Kumari B, Duhan S (2015) Determination of residues of fipronil and its metabolites in cauliflower by using gas chromatography-tandem mass spectrometry. Bull Environ Contam Toxicol 94:260–266
Elbadrawy E, Sello A (2016) Evaluation of nutritional value and antioxidant activity of tomato peel extracts. Arab J Chem 9:S1010–S1018
EU-MRL-Database (2018a) http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=pesticide.residue.CurrentMRL&language=EN Accessed 20 Jul 2018
EU-MRL-Database (2018b) MRL of fipronil in tomato samples. Retrieved from (http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=pesticideresidueCurrentMRL&language=EN) Accessed 25 Dec 2018
FAO (2009) Manual on the submission and evaluation of pesticide residues data. Food and Agriculture Organization, Rome
FAOSTAT (2018) Food and Agriculture Organization of the United Nations. Available at: http://www.fao.org/faostat/en/#data/QC. Accessed on 1 Apr 2019
Ferrer C, Lozano A, Agüera A, Girón AJ, Fernández-Alba A (2011) Overcoming matrix effects using the dilution approach in multiresidue methods for fruits and vegetables. J Chromatogr A 1218:7634–7639
Gupta S, Sharma R, Sinha S, Gupta R, Gajbhiye V (2007) Persistence of some new insecticides in brinjal and their efficacy against brinjal leafhopper and borer. Pestic Res J 19:205–209
Gupta S, Sharma R, Gupta R, Sinha S, Singh R, Gajbhiye V (2009) Persistence of new insecticides and their efficacy against insect pests of okra. Bull Environ Contam Toxicol 82:243–247
Hainzl D, Casida JE (1996) Fipronil insecticide: novel photochemical desulfinylation with retention of neurotoxicity. Proc Natl Acad Sci 93:12764–12767
Hoskins W (1961) Mathematical treatment of the rate of loss of pesticide residues FAO. Plant Prot Bull 9:214–215
JMPR (2011) Acceptable daily intakes, acute reference doses, short-term and long-term dietary intakes, recommended maximum residue limits and supervised trials median residue values recorded by the 2011 meeting. Summary report from the 2011 Joint FAO/WHO Meeting on Pesticide Residues (JMPR). Geneva
Kaczyński P, Łozowicka B (2017) One-step QuEChERS-based approach to extraction and cleanup in multiresidue analysis of sulfonylurea herbicides in cereals by liquid chromatography–tandem mass spectrometry. Food Anal Methods 10:147–160
Kaur R, Mandal K, Kumar R, Singh B (2015) Analytical method for determination of fipronil and its metabolites in vegetables using the QuEChERS method and gas chromatography/mass spectrometry. J AOAC Int 98:464–471
Korunic Z (1998) Review diatomaceous earths, a group of natural insecticides. J Stored Prod Res 34:87–97
Lehotay S (2007) AOAC official method 2007.01 pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate. J AOAC Int 90:485–520
Li M, Li P, Wang L, Feng M, Han L (2015) Determination and dissipation of fipronil and its metabolites in peanut and soil. J Agric Food Chem 63:4435–4443
Liu Y, Ni Z, Mo R, Shen D, Zhong D, Tang F (2015) Environmental behaviors of phoxim with two formulations in bamboo forest under soil surface mulching. J Environ Sci 35:91–100
Lu M, Du J, Zhou P, Chen H, Lu C, Zhang Q (2015) Endocrine disrupting potential of fipronil and its metabolite in reporter gene assays. Chemosphere 120:246–251
MacLachlan DJ, Hamilton D (2011) A review of the effect of different application rates on pesticide residue levels in supervised residue trials. Pest Manag Sci 67:609–615
Malhat F, Boulangé J, Abdelraheem E, Allah OA, El-Hamid RA, El-Salam SA (2017) Validation of QuEChERS based method for determination of fenitrothion residues in tomatoes by gas chromatography–flame photometric detector: decline pattern and risk assessment. Food Chem 229:814–819
Mohapatra S, Deepa M, Jagdish G, Rashmi N, Kumar S, Prakash G (2010) Fate of fipronil and its metabolites in/on grape leaves, berries and soil under semi arid tropical climatic conditions. Bull Environ Contam Toxicol 84:587–591
Oerke E-C (2006) Crop losses to pests. J Agric Sci 144:31–43
Paramasivam M, Chandrasekaran S (2013) Determination of fipronil and its major metabolites in vegetables, fruit and soil using QuEChERS and gas chromatography-mass spectrometry. Int J Environ Anal Chem 93:1203–1211
Pei Z, Yitong L, Baofeng L, Gan JJ (2004) Dynamics of fipronil residue in vegetable-field ecosystem. Chemosphere 57:1691–1696
Ramesh A, Balasubramanian M (1999) Kinetics and hydrolysis of fenamiphos, fipronil, and trifluralin in aqueous buffer solutions. J Agric Food Chem 47:3367–3371
Sammelson RE, Caboni P, Durkin KA, Casida JE (2004) GABA receptor antagonists and insecticides: common structural features of 4-alkyl-1-phenylpyrazoles and 4-alkyl-1-phenyltrioxabicyclooctanes. Bioorg Med Chem 12:3345–3355
SANTE/11813/2017 Guidance document on analytical quality control and method validation procedures for pesticides residues analysis in food and feed. https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_wrkdoc_2017-11813.pdf Accessed 11 Mar 2018
Tingle CC, Rother JA, Dewhurst CF, Lauer S, King WJ (2003) Fipronil: environmental fate, ecotoxicology, and human health concerns. In: Reviews of environmental contamination and toxicology. Springer, pp 1–66
Tomlin C (1994) The pesticide manual incorporating the agrochemical handbook British crop protection, Surrey, England
Viñas P, Campillo N, López-García I, Hernández-Córdoba M (2014) Dispersive liquid–liquid microextraction in food analysis. A critical review. Anal Bioanal Chem 406:2067–2099
Wang H-S, Sthiannopkao S, du J, Chen ZJ, Kim KW, Mohamed Yasin MS, Hashim JH, Wong CKC, Wong MH (2011) Daily intake and human risk assessment of organochlorine pesticides (OCPs) based on Cambodian market basket data. J Hazard Mater 192:1441–1449
Wang T, Hu J, Liu C (2014) Simultaneous determination of insecticide fipronil and its metabolites in maize and soil by gas chromatography with electron capture detection. Environ Monit Assess 186:2767–2774
WHO (2003) GEMS/food regional diets (regional per capita consumption of raw and semiprocessed agricultural commodities http://www.who.int/foodsafety/publications/chem/regional_diets/en Accessed 29 Dec 2018
Zhou Y, Xu D, Chen D, Zhang Z, Zheng X, Fang E (2011) Determination of fipronil and its metabolites in tea by solid-phase microextraction coupled with gas chromatography and gas chromatography-mass spectrometry Se pu=. Chin J Chromatogr 29:656–661
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Osama Abdallah declares that he has no conflict of interest. Nevein S. EL Deen declares that she has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
The authors named in the manuscript are entitled to the authorship and have approved the final version of the submitted manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdallah, O.I., Ahmed, N.S. Development of a Vortex-Assisted Dispersive Liquid-Liquid Microextraction (VA-DLLME) and LC-MS/MS Procedure for Simultaneous Determination of Fipronil and its Metabolite Fipronil Sulfone in Tomato Fruits. Food Anal. Methods 12, 2314–2325 (2019). https://doi.org/10.1007/s12161-019-01562-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-019-01562-z