Abstract
The dual purpose of this research was to investigate the presence of limonoid aglycones in Citrus essential oils and to develop a rapid and reliable SFC-APCI-QqQ MS method for their characterization. In this study, 12 limonoid aglycones were tentatively identified in 11 different citrus essential oils. The developed method allowed a very fast separation in less then 7 min with a very low amount of organic solvent. Calibration curve of limonin was constructed by using a triple quad MS detector in order to quantify the limonin content in the analyzed samples. The content of limonin ranged from 0.5 (clementine essential oil) to 21.2 mg L−1 (bergamot essential oil) in the cold-pressed Citrus essential oils analyzed. Moreover, product ion scan (PIS) acquisition mode was also used for the structure elucidation of isobaric compounds. To the best of our knowledge, this is the first investigation on limonoid aglycones in Citrus essential oils.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cold-pressed Citrus essential oils are industrially obtained from the peels of Citrus fruits like bergamot, lemon, mandarin, sweet orange, grapefruit, lime, and bitter orange using mechanical systems (Dugo and Di Giacomo 2002). Citrus essential oils have a large food industrial profile in beverages, gelatines, candy, and pastry products. These oils are added as ingredients in food stuffs for their organoleptic properties, and also for their antimicrobial, anticancer, anti-inflammatory, antiviral, and antioxidant properties (Adorjan and Buchbauer 2010; Mustafa 2015). Mono- and sesquiterpene hydrocarbons and their oxygenated derivatives, present in the volatile fraction of Citrus essential oils, give a natural flavor to beverages and sweet foods. The volatile fraction of Citrus essential oils has been extensively characterized (Tranchida et al. 2013; Zoccali et al. 2015).
Most of the above mentioned properties come from the volatile fraction of Citrus essential oils, but the cold-pressed contain also a non-volatile fraction that ranges from 1 to 15%. The non-volatile fraction of Citrus essential oils is composed of long chain hydrocarbons, fatty acids, sterols, wax, carotenoids, and oxygen heterocyclic components, mainly represented by coumarins, psoralens, and polymethoxylated flavones (Dugo and Mondello 2010).
All these molecules are present in peel of Citrus fruits, but according to literature data (Manners and Breska III 2004; Breksa III et al. 2009; Bilal et al. 2013; Russo et al. 2014, 2016; Kikuchi et al. 2017), the peel of Citrus fruits is characterized also by the presence of limonoids. Limonoids are higly oxygenated modified triterpenes derived from a precursor with 4,4,8-trimethyl-17-furanylsteroid skeleton biosynthesized from acetate-mevalonate pathway in Citrus (Vikram et al. 2007). These molecules have been investigated intensively due to their wide range of biological activities. Some research has shown the activity of limonoids as inhibitors of oral tumors (Miller et al. 2004), as inductor of glutathione S-transferase enzyme in liver and intestine mucosa of mice (Lam et al. 1989). Moreover, they have shown good ability on the inhibition of human immunodeficiency virus-1 (HIV-1) in culture of human peripheral blood mononuclear cells (PBMC) and on monocytes/macrophages (M/M) (Battinelli et al. 2003). Some limonoids have antimicrobial activity, offer some protection capacity against low-density lipoprotein (LDL) oxidation, and possess a strong antioxidant action (Govindachari et al. 1999; Yu et al. 2005).
There are many research articles regarding the composition of the non-volatile fraction in different cold-pressed Citrus essential oils (Frérot and Decorzant 2004; Russo et al. 2012, 2015a; Dugrand et al. 2013), but there is a lack of investigation on the content of limonoids. These molecules are present in the peel of Citrus fruits both in glucosidic and aglyconic form (Manners and Breska III 2004). Limonoid glucosides are water-soluble, so is highly unlikely to find this type of molecules in the cold-pressed essential oil. Vice versa, limonoid aglycones are water-insoluble, so is possible that limonoids in this form can pass in to the cold-pressed Citrus essential oils. The principal organoleptic property of limonoid aglycones is to confer a bitter taste to the Citrus fruits. The development of an analytical method for the characterization of limonoids in cold-pressed Citrus essential oils can be useful for food industry.
Since 1966, several analytical techniques were employed for the identification and quantification of limonoids: thin-layer chromatography (TLC) with silica gel plates, micellar electrokinetic capillary chromatography (MECC), normal phase liquid chromatography coupled with a UV–Vis detector (NP-HPLC/UV) (Rouseff 1982; Rouseff and Nagy 1982; Moodley et al. 1995; Dreyer 1996). But starting from the 1990s, the most employed analytical technique to analyze limonoids in Citrus fruits was reversed phase liquid chromatography coupled to a UV–Vis detector or in many cases with a mass spectrometer detector. Generally, atmospheric pressure chemical ionization (APCI) is used for identification of limonoid aglycones, whereas electrospray mass ionization (ESI) is used for identification of limonoid glucosides (Manners and Breska III 2004; Breksa III et al. 2009; Balestrieri et al. 2011; Russo et al. 2014, 2016). In the last years, due to significant technological instrumental advancements, supercritical fluid chromatography (SFC) coupled to mass spectrometry system has gained attention as a green, fast, and useful technology (Tarafder 2016; Giuffrida et al. 2017). According to our knowledge, only an early attempt is reported in the literature focused on the analysis of limonoids. Specifically, in 1993, Raynor et al. reported the analysis of limonoids extracted from different plants by using capillary supercritical fluid chromatography (Raynor et al. 1993).
In the present investigation, an SFC system, equipped with a C18 column, coupled with a triple quadrupole mass spectrometer was employed to investigate the content of limonoids aglycones in different cold-pressed Citrus essential oils (green, red, and yellow mandarin; bergamot; lemon (two); sweet, bitter and blood orange, pink grapefruit, and clementine) by using a multiple reaction monitoring (MRM) mode.
This work provides for the first time, according to the authors’ knowledge, a qualitative profile of limonoid aglycones and quali/quantitative for limonin in different Citrus essential oils, by means of an innovative SFC-QqQ MS system, in a fast, efficient, and environmental friendly way. Among the samples analyzed, differences in the composition of limonoids were observed, adding further data to the characterization of Citrus species.
Material and Methods
Samples and Sample Preparation
This research was carried out on 11 genuine cold-pressed Citrus essential oil: lemon (two), bergamot, sweet orange, clementine, bitter orange, blood orange, mandarin (green, yellow, red), pink grapefruit oils were provided by Simone Gatto s.r.l. (San Pier Niceto, Messina, Italy). All samples were analyzed without any pre-treatment and injected into the SFC system for three consecutive times. Limonoid, contained in lemon seeds, were extracted three times starting from 5 g with 40 mL of methanol (MeOH), 40 mL of acetone, and 40 mL of ethyl acetate. The extracts were washed with 50 mL of n-hexane, gathered, dried with anhydrous sodium sulfate, filtered on filter paper, and brought to dryness in a rotary evaporator; the extract thus obtained was dissolved in 10 mL of ethyl acetate, filtered on Acrodisc filter 0.45-μm Merck KGaA (Darmstadt, Germany), and injected into SFC. Methanol HPLC-MS grade, acetone and ethyl acetate, and n-hexane were purchased from Merck KGaA. Standard of limonin was previously isolated in our laboratory from bergamot seeds (Russo et al. 2016).
SFC-APCI-QqQ MS Analysis
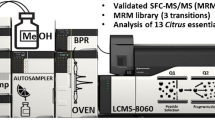
The SFC-QqQ MS analyses were performed on a Shimadzu Nexera-UC system (Shimadzu, Japan), consisting of a CBM-20A controller, two LC-20ADXR dual-plunger parallel-flow pumps, an LC-30ADSF CO2 pump, two SFC-30A back pressure regulator, a DGU degasser, a CTO-20AC column oven, a SIL-30AC autosampler, an LCMS-8050 triple quad mass spectrometer equipped with an APCI source. The entire system was controlled by the LabSolution ver. 5.8.
Separations were performed on an Ascentis™ C18, 250 × 4.6 mm, 5 μm d.p. (Merck KGaA) column; the mobile phase consisted of the following: eluent A, CO2; eluent B MeOH; make-up solvent, MeOH. The following gradient was used: 0 min, 0% B; 20 min 12% B, and the flow rate was of 2 mL min−1 (total flow pump A and B) and 0.5 mL min−1 make-up pump. The column oven temperature was 35 °C and the back pressure regulator was 150 bar. The injection volume was 3 μL. The MS was set as follows: acquisition mode (+): SCAN; selective ion monitoring (SIM); multiple reaction monitoring (MRM). Interface temperature: 350 °C; DL temperature: 300 °C; block heater temperature: 300 °C; nebulizing gas flow (N2) 4 L min−1; drying gas flow (N2) 5 mL min−1; full scan range: 200–800 m/z; event time 0.03 s for each event; argon was used as collision gas with a pressure of 270 kPa. The different limonoids were first recognized by using the SIM of their radical ions generated in the positive ionization mode, and in the case of limonin also by comparison with the available standard. The transitions used in the MRM experiments were optimized starting from the product ion scan (PIS) of the relative radical ions, using various collision energies (CE) from 5 to 50 V in positive mode. The best daughter ions were selected in order to improve both sensitivity and selectivity of the method. Two transitions were selected for each limonoid in order to provide a more reliable identification. The ratio of quantifier (Q) and qualifier (q) ions (provided by the LabSolution ver. 5.8 software) for each compound was taken into account in order to provide a further identificative parameter (Giuffrida et al. 2017). Product ion scan acquisition mode was also used for the structure elucidation of isobaric compounds.
SFC-APCI-QqQ MS Method Validation and Statistical Analysis
To quantify limonin content in the various samples tested, calibration curve has been constructed by using limonin standard. Five different concentrations of limonin, in the range between 0.025 and 50 mg L−1, prepared by diluting a stock solution of 1000 mg L−1, using MeOH as solvent, were analyzed for five consecutive times by SFC-APCI-QqQ MS under the same chromatographic conditions optimized for the samples. Calibration curve was constructed using the least square method to estimate the regression line; the linearity and the goodness of the curve used were confirmed using Mandel’s fitting tests. The significance of the intercept was established running a t test. All the statistical tests were carried out at the 5% significance level. Limits of detection (LoD) and quantification (LoQ) were calculated by multiplying the standard deviation of the limonin area at the lowest concentration level (n = 5), three and ten times, respectively, and then by dividing the result by the slope of the calibration curve. The instrumental intra-day precision and the recovery were calculated for limonin by using six replicated injections at the lowest concentration level (0.05 mg L−1). The instrumental inter-day precision was calculated by analyzing the same solution of limonin, used for the intra-day precision calculation, three times, on five consecutive days.
Results and Discussion
General Considerations
Non-volatile fraction of Citrus essential oils has been mainly investigated by using HPLC-PDA-MS (Dugo and Di Giacomo 2002; Dugo and Mondello 2010) instrumentation. Due to the above considerations, the logical consequence is to apply the same technique for the analysis of limonoids in Citrus essential oils. However, this technique is often time and organic solvent consuming, and sometimes for the analysis of limonoids, the addition of acids (formic or acetic) in the mobile phase is required in order to improve the chromatographic separation. In such a case, the MS identification can be more challenging due to the formation of adducts (Donato et al. 2009).
Due to the lipophilic nature of the Citrus essential oils and the molecules investigated in the present research (limonoid aglycones), the use of supercritical fluid chromatography represents a valid alternative to the conventional liquid chromatography. SFC technique allows for short run time without losing of separation power, by using low amount of organic solvent (Lesellier and West 2015; Tarafder 2016; Giuffrida et al. 2017; Zoccali et al. 2017).
The use of a photodiode array as detector in the UV range after the chromatographic separation, for the untargeted analysis of limonoids is not sufficient for a deep characterization of unknown samples due to the low molecular absorptivity of these molecules. Instead, the use of a mass spectrometer detector, due to its great selectivity and sensitivity, allows the identification and structural analysis of targeted and untargeted compounds. The use of a triple quadrupole or a high-resolution MS detectors allows compound structural elucidation. The pairs of the molecule here analyzed are isobaric species with the same structural formula and with the same molecular weight. For such a reason, the use of a high-resolution MS system is not able to discriminate them [limonexin and limonexic acid: molecular weight 502.516 (C26H30O10); deoxylimonin and obacunone: molecular weight 454.519 (C26H30O7); nomilin and 7-α-limonyl acetate: molecular weight 514.56 (C28H34O9)]. In our opinion, a triple quadrupole MS detector represents one of the best choice for the identification of limonoid aglycones in cold-pressed Citrus essential oils.
This research presents the characterization of 11 cold-pressed Citrus essential oils [lemon, bergamot, sweet orange, clementine, bitter orange, blood orange, mandarin (green, yellow, red), and pink grapefruit oils] by using SFC-APCI-QqQ MS instrumentation equipped with an APCI interface. For the chromatographic separation, a C18 (250 × 4.6 mm, 5 μm d.p.) column was used with MeOH as modifier in order to increase the strength of the mobile phase.
Essential Oil Screening
Initially, a pure standard of limonin, previously isolated and purified in our lab [Russo et al. 2016], and limonoid aglycones extracted from lemon seeds, as reported in paragraph 2.1, were used for the optimization of both SFC separation and MS parameters. Limonin standard was used to confirm the identification and for quantitative analysis as reported in paragraph 2.3, while lemon seeds extract was used as reference material containing the following: nomilin, limonin, ichangin, deacethylnomilin, and obacunone (Russo et al. 2014). Only three compounds are present as standards on the market namely limonin, nomilin, and obacunone (on the best of our knowledge). The other compounds are present in very small amounts. Considering the literature, for example, limonexic acid has been previously isolated starting from 5 kg of mandarin peels (Kikuchi et al. 2017).
The first step was the optimization of the SFC conditions according to injection volume, elution gradient, and back pressure. Two different columns were tested, a C18 fused core Ascentis Express (150 × 4.6 mm, 2.7 μm d.p) and a conventional Ascentis™ (250 × 4.6 mm, 5 μm d.p). In both cases, the best gradient was from 0 to 20 min increasing up to 12% of MeOH, with an injection volume of 3 μL and a back pressure of 150 bar. However, despite the higher efficiency of the fused core one, the best results were obtained by using a conventional C18 5 μm d.p. As you can observe in Supplementary Material S1, the targeted compounds, namely obacunone, nomilin, and limonin, were partially co-eluted. It must be highlighted that by using the fused core column, the analysis is faster. Considering the main purpose of the research, the conventional C18 column was chosen in order to achieve a better separation instead of a faster analysis.
The SFC method here developed, despite the use of a conventional 5 μm column, provided a fast separation of the target analytes in less than 7 min (without considering the reconditioning time) with a total consumption of ca. 170 μL of organic solvent (MeOH), representing an attractive alternative to the conventional LC methods.
For such a reason, we decided to apply the developed method for the analysis of the 11 Citrus essential oils. The first step was devoted to a targeted screening, by using both full scan and SIM mode, of the essential oil samples, of 19 limonoid aglycones most commonly found in seeds, peels, and juices of Citrus species, as reported in available literature (Manners and Breska III 2004; Breksa III et al. 2009; Balestrieri et al. 2011; Russo et al. 2016).
The structure of the 19 target limonoid aglycones, selected for the initial essential oil screening, are reported in Fig. 1. As can be seen from Fig. 1, some limonoid aglycones have the same molecular weight: nomilin and 7-α-limonyl acetate (514); obacunone and deoxylimonin (454); deacetyl nomilin, isoobacunoic acid, and 7-α-limonol (472); limonexic acid, limonexin, and cyclocalamin (502); 7-α-obacunol and deoxylimonol (456).
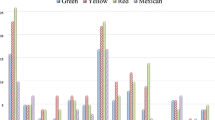
The results of the initial screening, showed the presence of 12 limonoid aglycones (as shown in Fig. 2) with respect to the 19 originally selected. Particularly, for all cases of isobaric molecules, only one signal (with the same retention time) was detected in each oil samples. For such a reason, further investigation was needed to exploit the correct identification of the isobaric compounds.
Compound Elucidation and MRM Optimization
As mentioned in the previous paragraph, due to the lack of available standards of limonoids and the presence of isobaric molecules, the use of SIM mode is not sufficient for the correct compound identification.
In order to tentatively identify the correct limonoid aglycones, the PIS mode was used to obtain a fragmentation pattern correlated to the characteristic structures of the selected [M + H]+. However, lack of high-resolution mass spectrometer precludes the definitive elucidation of fragmentation pathways; therefore, discussion of the group assignments of the limonoid aglycones is based on general mass fragmentation pattern comparisons, and comments about the origins of fragment ions are tentative. PIS and MRM analyses of each compound were carried out injecting samples, which show the most abundant content. Only in one case, namely limonin, the MRM transitions were optimized by injecting a pure standard. For the other limonoid aglycones, the following samples were used: lemon seeds extract for [M + H]+ 515, 489, and 455; lemon essential oil for [M + H]+ 445; bitter orange essential oil for [M + H]+ 505 and 503; pink grapefruit for [M + H]+ 473, 521, 463, and 457; sweet orange essential oil for [M + H]+ 487 as reported in Table 1.
As can be seen in Fig. 1, nomilin and 7-α-limonyl acetate present a different A ring: a seven-membered oxepine ring for the first one, while 7-α-limonyl acetate belong to the A-seco-limonoids. After a careful interpretation of the PIS, carried out injecting the lemon oil with different collision energy values, two characteristic fragments at m/z 369 and 351 were found as reported in Fig. 3.
Evaluating the different fragmentation ways of the [M + H]+ 515, the most likely fragmentation pathway is the one leading to the formation of ions characterized by the seven-membered oxepine A ring structure intact as confirmed by Ren et al. (2015). As a consequence, the [M + H]+ 515 was tentatively identified as nomilin. During the MRM optimization, the most abundant daughter ions at m/z 161 (CE − 25 V) and 411 (CE − 15 V) were selected as quantifier and qualifier in order to obtain a more sensitive method.
As can be observed in Fig. 1, the molecular structure of obacunone and deoxylimonin differs for the A ring as in the case of nomilin and 7-α-limonyl acetate, and for the D ring. In the D ring, carbons 14 and 15 show a double bond in the case of deoxylimonin, while obacunone presents an epoxy-ring. PIS for the [M + H]+ 455 show two characteristic fragments with m/z value of 391 and 349 that can be associated, according to the fragmentation pathway, to the obacunone molecule tentatively identified in such a way, as can be seen in Fig. 3. For the MS/MS experiments, the daughter ions at m/z 161 (CE − 30 V) and 409 (CE − 15 V) were selected as quantifier and qualifier.
The same considerations can be made for 7-α-obacunol and deoxylimonol couple (Fig. 1). These two molecules show the same molecular structures of obacunone and deoxylimonin, respectively, but with a hydroxyl group in position 7 of the B ring, instead of the carbonyl group. The fragment with m/z 187 allows the tentative identification of the 7-α-obacunol considering the fragmentation pathway as reported in Fig. 3. In this case for the MRM experiments, the daughter ions at m/z 161 (CE − 25 V) and 187 (CE − 20 V) were selected as quantifier and qualifier deriving from the [M + H]+ 457.
As previously mentioned, and also for the following molecules: deacetyl nomilin, isoobacunoic acid, and 7-α-limonol, the major structural differences come from the A ring (Fig. 1). But, after a careful investigation of the fragments derived from the PIS, the most characteristic part of the molecule seems to be the presence of the hydroxyl group in the B ring. In fact, only the presence of the hydroxyl group can justify the formation of the ions with m/z value of 352 and 337 from the [M + H]+ 473. Only 7-α-limonol presents a hydroxyl group in the B ring, while deacetyl nomilin and isoobacunoic acid show a carbonyl group as reported in Fig. 1. For MRM analysis, the following transitions were chosen: quantifier 473 > 215 (− 25 V) and qualifier 473 > 187 (− 45 V).
The isobaric compunds limonexic acid, limonexin, and cyclocalamin, reported in Fig. 1, with a [M + H]+ 503 differ each other for the substituent in position 17 of the D ring. Characteristic, for the tentative identification, is the furanic ring presents only in the cyclocalamin, thus generating the characteristic ions with m/z 243 and 189 during the PIS experiments. In fact, limonexic and limonexin show in position 17 of the D ring a hydroxy butenolide molecule as reported in Fig. 4.
In this case, the fragments used for the tentative identification were also used for the MRM experiments with a collision energy of − 25 and − 20 V, respectively. All the selected MRM transitions are reported in Table 1. Moreover, in Supplementary Material S2, the possible fragmentation pattern for each molecule has been reported.
Essential Oil Characterization
The SFC-QqQ MS developed was used for the characterization of limonoid aglycones in the 11 cold-pressed Citrus essential oils. Calibration curve of limonin was constructed under the same chromatographic conditions optimized for the sample analysis to quantify the limonin content. The validation process provided the following LoD and LoQ values: 0.006 and 0.021 mg L−1, respectively. Excellent linearity was obtained for limonin as confirmed by the correlation coefficient R2 0.9998. Concerning the intra-day precision, percentage coefficient of variation (%CV) value of 3.99% demonstrated very good precision at the concentration level tested. Concerning recovery, value of 98% was obtained thus demonstrating an excellent accuracy. Accuracy was measured by spiking a bergamot sample with 0.05 mg L−1 of limonin, a value close to the LoQ, bergamot sample was chosen because in this sample, limonin was not detected.
Finally, an inter-day precision value of 6.54% was obtained. For the tentative identification, the MRM optimized transitions among with quantifier and qualifier ion ratio (Q/q) and retention times were used.
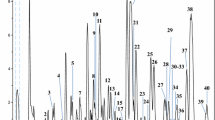
Totally, 12 different limonoid aglycones were tentatively identified in 11 Citrus essential oils. Table 1 reports the qualitative composition of all the samples analyzed and the amount of limonin expressed as mg L−1. The most complex sample, from a qualitative point of view, is pink grapefruit essential oil, with 10 tentatively identified limonoid aglycones. MRM chromatogram is shown in Fig. 5a. Only three limonoid aglycones were tentatively identified in all mandarin essential oil samples, in bergamot essential oil and in one of the two lemon essential oil samples. All the cold-pressed Citrus essential oils, except bitter orange (MRM chromatogram is shown in Fig. 5b), contain limonin and nomilin. Green mandarin essential oil is the only one sample that does not contain ichangensin. Green mandarin essential oil differs from yellow and red mandarin for the presence of obacunone and the absence of ichangesin present in the other two mandarin essential oil samples. 7-α-obacunol was tentatively identified only in pink grapefruit essential oil. Bitter orange and pink grapefruit essential oils represent the only two samples containing methyl deacetyl nomilinate, cyclocalamin, calamin, and retrocalamin. As previously discussed in the introduction paragraph, to the best of our knowledge, the first investigation based on the analysis of limonoid aglycones in cold-pressed Citrus essential oils is here reported. So, comparison with literature data has been done taking into account research papers focused on the analysis of the entire Citrus fruits (peels, juices, and seeds). Unfortunately, no data on limonoid composition in clementine fruits were found in literature. Qualitative data for the three samples of mandarin essential oil are in agreement with literature data; in fact, all the limonoid aglycones tentatively identified in our samples were previously reported, mainly in mandarin seeds (Manners and Breska III 2004; Vikram et al. 2007; Breksa III et al. 2011; Bilal et al. 2013; Kim et al. 2013). The same consideration can be made for bergamot essential oil; in fact, the three limonoid aglycones tentatively identified in our research were previously reported (Manners and Breska III 2004; Balestrieri et al. 2011; Russo et al. 2016). For blood and blond sweet orange essential oils, six limonoid aglycones were tentatively identified, and four of them were reported here for the first time: obacunone, ichangensin, 7-α-limonol, and methyl isobacunoate, as can be observed in Table 1 (Hasegawa et al. 1980; Rouseff and Nagy 1982; Manners et al. 2003; Bilal et al. 2013; Russo et al. 2015b).
Regarding bitter orange essential oil, five limonoid aglycones were reported for the first time: ichangensin, methyl deacetyl nomilinate, cyclocalamin, calamin, and retrocalamin (Hasegawa et al. 1980; Rouseff 1982; Vikram et al. 2007; Breksa III et al. 2011; Kikuchi et al. 2017). In the two samples of lemon essential oils, ichangensin was tentatively identified for the first time (Hasegawa et al. 1980; Rouseff and Nagy 1982; Kim et al. 2013; Russo et al. 2014). Finally, in pink grapefruit, most of the compounds were here tentatively identified for the first time: ichangensin, ichangin, 7-α-limonol, methyl deacetyl nomilinate, cyclocalamin, calamin, retrocalamin, and 7-α-obacunol (Hasegawa et al. 1980; Rouseff 1982; Rouseff and Nagy 1982; Manners and Breska III 2004; Vikram et al. 2007).
As can be seen in Table 1, bergamot essential oil is the richest sample in limonin (21.2 mg L−1), while clementine and blood orange essential oils present the lowest limonin content (0.5 and 0.9 mg L−1, respectively). The two lemon essential oil samples showed a limonin content slightly different (4.4 and 5.3 mg L−1). Considering the three mandarin samples, yellow and red present the same content of limonin ca. 1.1 mg L−1, while the green one reports a content four times higher (4.5 mg L−1).
Conclusions
The SFC-APCI-QqQ MS method developed in this work represents a valid tool for the rapid characterization (ca. 7 min) using a very low amount of organic solvent (MeOH, 170 μL) per analysis. The use of a triple quadrupole MS detector allowed to reach a very high sensitivity thus identifying 12 limoind aglicones in 11 cold-pressed Citrus essential oil, and to the best of our knowledge, most of them were tentatively identified for the first time. Moreover, the use of MRM acquisition mode allowed the discrimination of isobaric molecules. The validated method enabled the quantification of limonin with a low LoD and LoQ. The concentration range 0.5–21.2 mg L−1 of bitter taste limonin in the various samples analyzed should not represent a problem for the food industries, due to the low amount of essential oils added in the food and beverage products. To the best of our knowledge, this is the first investigation on limonoid aglycones in Citrus essential oils.
References
Adorjan B, Buchbauer G (2010) Biological properties of essential oils: an updated review. Flavour Fragr J 25:407–426
Balestrieri E, Pizzimenti F, Ferlazzo A, Giofrè SV, Iannazzo D, Piperno A, Romeo R, Chiacchio MA, Mastino A, Macchi B (2011) Antiviral activity of seed extract from Citrus bergamia towards human retroviruses. Bioorg Med Chem 19:2084–2089
Battinelli L, Mengoni F, Lichtner M, Mazzanti G, Saija A, Mastroianni CM, Vullo V (2003) Effect of limonin and nomilin on HIV-1 replication on infected human mononuclear cells. Planta Med 69:910–913
Bilal H, Akram W, Hassan SA, Sahar S, Iqbal MM (2013) Determination of Limonoids and Nomilin contents in different Citrus cultivars using high performance liquid chromatography. Pak J Sci Ind Res 56:36–40
Breksa AP III, Hidalgo MB, Lee YM (2009) Liquid chromatography-electrospray ionization mass spectrometry method for the rapid identification of citrus limonoid glucosides in citrus juices and extracts. Food Chem 117:739–744
Breksa AP III, Kahn T, Zukas AA, Hidalgo MB, Yuen ML (2011) Limonoid content of sour orange varieties. J Sci Food Agric 91:1789–1794
Donato P, Dugo P, Cacciola F, Dugo G, Mondello L (2009) High peak capacity separation of peptides through the serial connection of LC columns under high temperature. J Sep Sci 32:1129–1136
Dreyer DL (1996) Citrus bitter principles–V. Botanical distribution and chemotaxonomy in the Rutaceae. Phytochemistry 5:367–378
Dugo G, Di Giacomo A (2002) Citrus: the genus Citrus. Taylor & Francis, London
Dugo G, Mondello L (2010) Citrus oils: composition, advanced analytical techniques, contaminants, and biological activity. Taylor & Francis, Boca Raton
Dugrand A, Orly A, Duval T, Hehn A, Froelicher Y, Bourgaud F (2013) Coumarin and furocoumarin quantitation in citrus peel via ultraperformance liquid chromatography coupled with mass spectrometry (UPLC-MS). J Agric Food Chem 61:10667–10684
Frérot E, Decorzant E (2004) Quantification of total furocoumarins in Citrus oils by HPLC coupled with UV, fluorescence, and mass detection. J Agric Food Chem 52:6879–6889
Giuffrida D, Zoccali M, Giofrè SV, Dugo P, Mondello L (2017) Apocarotenoids determination in Capsicum chinense Jacq. cv. Habanero, by supercritical fluid chromatography-triple-quadrupole/mass spectrometry. Food Chem 231:316–323
Govindachari TR, Suresh G, Banumathi B, Masilamani S, Gopalakrishnam G, Kumari GNK (1999) Antifungal activity of some B,D-Seco Limonoids from two Meliaceous plants. J Chem Ecol 25:923–933
Hasegawa S, Bennett RD, Verdon CP (1980) Limonoids in Citrus seeds: origin and relative concentration. J Agric Food Chem 28:922–925
Kikuchi T, Ueno Y, Hamada Y, Furukawa C, Fujimoto T, Yamada T, Tanaka R (2017) Five new Limonoids from peels of Satsuma Orange (Citrus reticulata). Molecules 22:907–916
Kim J, Jayaprakasha GK, Patil BS (2013) Limonoids and their anti-proliferative and antiaromatase properties in human breast cancer cells. Food Funct 4:258–265
Lam LKT, Li Y, Hasegawa S (1989) Effects of Citrus Limonoids on glutathione S-transferase activity in mice. J Agric Food Chem 37:878–880
Lesellier E, West C (2015) The many faces of packed column supercritical fluid chromatography - a critical review. J Chromatogr A 1382:2–46
Manners GD, Breska AP III (2004) Identifying Citrus Limonoid Aglycones by HPLC-EI/MS and HPLC-APCI/MS techniques. Phytochem Anal 15:372–381
Manners GD, Breska AP III, Schoch TK, Hidalgo MB (2003) Analysis of bitter Limonoids in Citrus juices by atmospheric pressure chemical ionization and electrospray ionization liquid chromatography-mass spectrometry. J Agric Food Chem 51:3709–3714
Miller EG, Porter JL, Binnie WH, Guo IY, Hasegawa S (2004) Further studies on the anticancer activity of Citrus Limonoids. J Agric Food Chem 52:4908–4912
Moodley VE, Mulholland DA, Raynor MW (1995) Micellar electrokinetic capillary chromatography of limonoid glucosides from citrus seeds. J Chromatogr A 718:187–193
Mustafa NEM (2015) Citrus essential oils: current and prospective uses in the food industry. Recent Pat Food Nutr Agric 7:115–127
Raynor MW, Roberts SL, Mulholland DA (1993) Capillary supercritical fluid chromatography of limonoids. J High Resolut Chromatogr 16:469–472
Ren W, Xin S, Han L, Zuo R, Li Y, Gong M, Wei X, Zhou Y, He J, Wang H, Si N, Zhao H, Yang J, Bian B (2015) Comparative metabolism of four limonoids in human liver microsomes using ultra-high-performance liquid chromatography coupled with high-resolution LTQ-Orbitrap mass spectrometry. Rapid Commun Mass Spectrom 29:2045–2056
Rouseff RL (1982) Nomilin, a new bitter component in grapefruit juice. J Agric Food Chem 30:504–507
Rouseff RL, Nagy S (1982) Distribution of limonoids in citrus seeds. Phytochemistry 21:85–90
Russo M, Torre G, Carnovale C, Bonaccorsi I, Mondello L, Dugo P (2012) A new HPLC method developed for the analysis of oxygen heterocyclic compounds in Citrus essential oils. J Essent Oil Res 24:119–129
Russo M, Bonaccorsi I, Torre G, Sarò M, Dugo P, Mondello L (2014) Underestimated sources of flavonoids, limonoids and dietary fibre: availability in lemon's by-products. J Funct Foods 9:18–26
Russo M, Bonaccorsi I, Costa R, Trozzi A, Dugo P, Mondello L (2015a) Reduced time HPLC analyses for fast quality control of citrus essential oils. J Essent Oil Res 27:307–315
Russo M, Bonaccorsi I, Inferrera V, Dugo P, Mondello L (2015b) Underestimated sources of flavonoids, limonoids and dietary fiber: availability in orange’s by-products. J Funct Foods 12:150–157
Russo M, Arigò A, Calabrò ML, Farnetti S, Mondello L, Dugo P (2016) Bergamot (Citrus bergamia Risso) as a source of nutraceuticals: limonoids and flavonoids. J Funct Foods 20:10–19
Tarafder A (2016) Metamorphosis of supercritical fluid chromatography to SFC: an overview. TrAC, Trend Anal Chem 81:3–10
Tranchida PQ, Zoccali M, Bonaccorsi I, Dugo P, Mondello L, Dugo G (2013) The off-line combination of high performance liquid chromatography and comprehensive two-dimensional gas chromatography–mass spectrometry: a powerful approach for highly detailed essential oil analysis. J Chromatogr A 1305:276–284
Vikram A, Jayaprakasha GK, Patil BS (2007) Simultaneous determination of citrus limonoid aglycones and glucosides by high performance liquid chromatography. Anal Chim Acta 590:180–186
Yu J, Wang L, Walzem RL, Miller EG, Pike LM, Patil BS (2005) Antioxidant activity of Citrus Limonoids. J Agric Food Chem 53:2009–2014
Zoccali M, Bonaccorsi IL, Tranchida PQ, Dugo P, Mondello L, Dugo G (2015) Analysis of the sesquiterpene fraction of citrus essential oils by using the off-line combination of high performance liquid chromatography and gas chromatography-based methods: a comparative study. Flavour Fragr J 30:411–422
Zoccali M, Giuffrida D, Dugo P, Mondello L (2017) Direct online extraction and determination by supercritical fluid extraction with chromatography and mass spectrometry of targeted carotenoids from red habanero peppers (Capsicum chinense Jacq.). J Sep Sci 40:3905–3913
Acknowledgments
We gratefully thank Shimadzu and Merck KGaA corporations for their continuous support. Adriana Arigò thanks the “Prof. Antonio Imbesi Foundation” of the University of Messina for the financial support. Fabio Salafia tanks the “Programma Operativo Fondo Sociale Europeo (FSE) Regione Sicilia 2014-2020-Asse 3 Ob. 10.5” for thefinancial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mariosimone Zoccali declares that he has no conflict of interest. Adriana Arigò declares that she has no conflict of interest. Marina Russo declares that she has no conflict of interest. Fabio Salafia declares that he has no conflict of interest. Paola Dugo declares that she has no conflict of interest. Luigi Mondello declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human or animal subjects.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Zoccali, M., Arigò, A., Russo, M. et al. Characterization of Limonoids in Citrus Essential Oils by Means of Supercritical Fluid Chromatography Tandem Mass Spectrometry. Food Anal. Methods 11, 3257–3266 (2018). https://doi.org/10.1007/s12161-018-1303-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-018-1303-1