Abstract
A sensitive and high-efficiency method for simultaneous detection of pyraclostrobin, prochloraz, and its metabolite in apple and soil based on QuEChERS pretreatment combined with rapid resolution liquid chromatography tandem mass spectrometry was established and validated. The limits of quantification of three compounds in two matrixes were 0.005 mg kg−1. The average recoveries of pyraclostrobin, prochloraz, and 2,4,6-trichlorophenol in soil matrix were in the ranges of 87–105%, 86–95%, and 90–96%, respectively, with all the relative standard deviations (RSDs) of < 9.6%, while recoveries were 89–93%, 83–97%, and 89–101% in apple with the RSDs of < 6.5% at three spiking levels. For verify the applicability of this method, the real samples from three representative locations were detected. The degradation behaviors and residue distributions of pyraclostrobin and prochloraz and its metabolite in apple ecosystem were investigated. The field experiment data showed that the dissipation of pyraclostrobin and prochloraz in apple and soil followed pseudo-first-order kinetic models. The half-lives of pyraclostrobin in soil and apple were 8.6–19.8 and 7.9–15.1 days, while prochloraz were 8.9–21 and 5.8–12.4 days. The highest terminal residue of total prochloraz and pyraclostrobin in apple, after spraying three to four times with the interval of 28 days, was far below the maximum residue limits recommended by China. This research could provide guidance on a reasonable usage of prochloraz and pyraclostrobin in apple orchard.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Apple, as one of the most popular fruits, is rich in polyphenols, vitamins, flavonoids, etc., which can reduce the risk of chronic diseases and cancer (Feskanich et al. 2000; Arts et al. 2001 Boyer and Liu 2004; Davis et al. 2006; Yoon and Liu 2007). It was reported that apple yield in China reached 43.8 million tons in 2016. However, many fungi could induce terrible diseases which result in the production of apple to sharply decrease and cause large economic loss. For example, the apple anthracnose, caused by Colletotrichum gloeosporioides and C. acutatum, is one of the most serious diseases of apple in the world (Lee et al. 2007; Bajpai et al. 2009). To reduce economic loss, various pesticides and their mixed formulation were developed (Patyal et al. 2013) in which pyraclostrobin and prochloraz are the most commonly used fungicides.
Pyraclostrobin, methyl 2-[1-(4-chlorophenyl)pyrazol-3-yloxymethyl]-N-methoxycarbanilate, is a fungicide belonging to a class of compounds called strobilurins (Fulcher et al. 2014). The strobilurin fungicides are synthetic active ingredients with similar action to the natural strobilurin A, produced by the fungus Strobilurus tenacellus (Bartlett et al. 2002), and their main effect is inhibiting the mitochondrial respiration of the fungus. Pyraclostrobin, with the advantage of high efficiency, low toxicity, broad spectrum, and environmentally friendly, has wonderful potential application in China (Li et al. 2016; Guo et al. 2017).
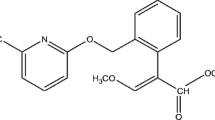
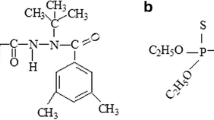
Prochloraz [N-propyl-N-[2-(2,4,6-trichlorophenoxy)-ethyl]imidazole-1-carboxamide PCZ], is a broad-spectrum and high-efficiency fungicide that classifies as imidazole and often be used on fruits, vegetables, and mushrooms as a post-harvest treatment and on cereals as a seed treatment (Cengiz et al. 2014). The mechanism of prochloraz to resist various diseases was anti-androgenic activity which can disrupt reproductive development and function of the target effected cells by several modes of action (Vinggaard et al. 2002). The prochloraz undergoes specific transformations in plants and the primary metabolic step is the breaking of the imidazole ring with the formation of its primary metabolites, namely N-formyl-N-propyl-N-[2-(2,4,6-trichlorophenoxy)ethyl] urea and N-propyl-N-[2-(2,4,6-trichlorophenoxy)ethyl] urea which contain formyl group and amidogen, respectively. Both of them disconnected the carbon–oxygen bond and degraded to 2,4,6-trichlorophenol, present as free and conjugated metabolites, together with trace of 2-(2,4,6-trichlorophenoxy)-acetic acid (Polese et al. 2006; Navickiene and Ribeiro 2005). It has been reported that 2,4,6-trichlorophenol could cause adverse effects on the human nervous system and respiratory problems such as chronic bronchitis, cough, and altered pulmonary function (Hameed 2007; Li et al. 2010). Due to its high toxicity, carcinogenic properties, structural stabilization, and persistence in the environment, 2,4,6-trichlorophenol was paid more attention in this paper (Hameed et al. 2008). The chemical structures of pyraclostrobin, prochloraz, and 2,4,6-trichlorophenol are shown in Fig. 1a–c.
The commercial formulation of 40% emulsion in water (EW), including pyraclostrobin (10%) and prochloraz (30%), was firstly introduced in open-field conditions to protect crops against anthracnose as well as other morbigenous fungi. However, food contaminant issues and potential environment risk have received popular attention. Hence, it is significantly important to establish an analytical method to detect the real samples and study the degradation behavior and residue distribution of these pesticides in the apple ecosystem.
Few analytical methods have been established for the determination of pyraclostrobin with some other pesticides in green coffees, green beans, spring onions, and grapes. Among them, the common instruments are high-performance liquid chromatography coupled with single or tandem mass spectrometry (HPLC-MS-MS; HPLC-ESI-MS) (de Oliveira et al. 2016; Hanafi et al. 2010; Shabeer et al. 2015). Besides, a modified QuEChERS pretreatment method coupled with LC-MS/MS detection for the analysis of pyraclostrobin residue in peanut and soil was established (Zhang et al. 2012). Many detection methods based on gas chromatography (GC) and liquid chromatography (LC) coupled with various detectors have been developed for the determination of prochloraz residues as its 2,4,6-trichlorophenol derivative. So far, several analytical methods have been reported for indirect detection of the total prochloraz residues by transforming the parent and primary metabolites into secondary metabolite, namely 2.4.6-trichlorophenol, in ginger, mango, papaya, and orange (Fuse et al. 2010; Navickiene and Ribeiro 2005; Polese et al. 2006). Meanwhile, some literatures described the analytical methods to detect only the parent of prochloraz in citrus fruit, mushroom, and water of different pH values by HPLC and GCE-CD instruments (Aktar et al. 2008; Cengiz et al. 2014; Lafuente and Tadeo 2015). To our knowledge, scarcely any literature reported about the direct detection of prochloraz and 2,4,6-trichlorophenol in apple and soil matrix by RRLC-MS/MS. Furthermore, the method as to stimulate multi-determination of pyraclostrobin, prochloraz, and its secondary toxicology metabolite has not been reported before.
This paper, thus, aims on the validation of an accurate and repeatable analytical method by using QuEChERS pretreatment combined with RRLC-MS/MS to simultaneously detect and quantify the pyraclostrobin, prochloraz, and 2,4,6-trichlorophenol in apple and soil. The advantages of the method are being time saving, easy operation, high sensitivity, and cost-effective without transforming the parent prochloraz into 2,4,6-trichlorophenol. Based on this method, the real samples from open fields were detected to investigate the dissipation dynamics and terminal residues of pyraclostrobin and total prochloraz in apple and soil. This method appears to be promising to quantify the pesticides and was suitable to dispose and determine a large amount of samples with each instrument run of 3.9 min. This current study has great importance for providing guidance on a reasonable application of prochloraz and pyraclostrobin under open-field apple production conditions.
Materials and Methods
Reagents and Standards
The standards of pyraclostrobin (> 98.5% purity), prochloraz (> 98.4% purity), and 2,4,6-trichlorophenol (> 98.7% purity) were provided by Rotam Crop Science Ltd. (Jiangsu province, China), National pesticide quality inspection center (China), and Alfa Aesar (USA), respectively. The formulation of 40% emulsion in water (EW) of pyraclostrobin (10%) and prochloraz (30%) that was used on an open field was supplied by Henan Beautiful Star Crop Protection Co., Ltd. (Henan province, China). HPLC-grade acetonitrile was purchased from Dikma Co., Ltd. (Beijing, China). HPLC-grade ammonium acetate was purchased from Thermo Fisher Scientific (USA) and was dissolved by pure water which was supplied by Wahaha group. The cleanup sorbents C18, PSA, GCB, and MWCNTs were obtained from Bonna-Agela Technologies Venusil Technology Co., Ltd. (Tianjin city, China). Anhydrous sodium chloride (NaCl) and magnesium (MgSO4) of analytical grade were provided by Sinopharm Chemical Reagent Co., Ltd. (Beijing, China).
Stock solutions of individual pyraclostrobin, prochloraz, and 2,4,6-trichlorophenol standards were prepared in HPLC-grade acetonitrile to reach 500 mg L−1 and blended with the same volume rate to obtain 100 mg L−1 pesticide mix. Stepwise dilution of pesticide mix obtained 10, 5, 1, 0.1, and 0.05 mg L−1 working solution, and overall solution was stored in the dark at 4 °C.
Instrument Parameters
The rapid resolution liquid chromatography tandem triple quadruple mass spectrometer (Agilent 6420, USA) equipped with an electrospray ionization (ESI) source and coupled with a reversed phase C18 column (50 mm × 3.0 mm ID, 2.7 μm) was applied to test and quantify the contents of pyraclostrobin, prochloraz, and 2,4,6-trichlorophenol.
The chromatographic separation and examination were carried out at 30 °C. About 5 μL of the sample was injected for detection, the flow rate was 0.4 mL min−1, and the acquisition time was 3.9 min. The appearance times of 2,4,6-trichloroplenol, prochloraz, and pyraclostrobin were about 1.4, 2.25, and 3 min respectively. The mobile phase was a mixture of A and B with a volume ratio of 70:30 (v/v) in which A represents HPLC-grade acetonitrile and B was 20 mM ammonium acetate dissolved by pure water.
As to the MS/MS analysis, ESI was performed in positive ionization mode for prochloraz and pyraclostrobin and negative ionization mode for 2,4,6-trichlorophenol with 10 and 8 L min−1 drying gas (N2) flow rates. The two modes were at a temperature of 350 °C. The precursor ions of prochloraz and pyraclostrobin were smashed and their fragment ions were chosen for quantitative and qualitative analysis, whereas the 2,4,6-trichlorophenol had not been smashed with zero collision energy, and the patent ions were used for quantification. Nebulizer pressure was 45 psi, positive electrospray voltage was 4000 V, and negative electrospray voltage was 3500 V. All of these three compounds were performed in multi-reaction monitoring (MRM) mode and the residues were calculated based on quantitative fragment ions. The detailed parameters are shown in the Table 1.
Sample Pretreatment
The samples were prepared by modified QuEChERS method that could reduce the time to complete the extraction and cleanup procedure with high sensitivity and good repeatability. In this study, the apple samples and soil samples had the same preparation method as follows: 10 g of homogenized samples was placed in a 50-ml centrifuge tube, and 5 ml water and 10 ml acetonitrile were added into the tube. Then the tube was tightly capped and mixed for 1 min by a vortex mixer. A combination of 1 g sodium chloride and 4 g anhydrous magnesium was added to the tube and kept swirling for 1 min, then the tube was centrifuged for 3 min at the speed of 4000 rpm to acquire a well-defined separation of the acetonitrile phase.
A volume of 1 ml acetonitrile layer was transferred into a 5-ml centrifuge tube equipped with 200 mg anhydrous magnesium and 50 mg C18. The tube was shaken vigorously for 1 min and was centrifuged at 10,000 rpm for 3 min to stratify completely. The supernatant was filtered into an autosampler via a 0.22-mm syringe filter and was then stored at 4 °C until analyzed by a RRLC-MS/MS analyzer. The above pretreatment procedure was carried out in triplicate.
Method Verification
The standard mixture solution prepared previously was added into blank soil and apple matrix. The recovery was evaluated in contrast with the matrix extracting solution against the substrate standard at three different concentrations, and each has five parallels. The matrix extraction and the substrate standard use the same pesticide mix; the volume ratio of the pesticide mix was not more than 10%. These samples were treated and analyzed according to the previously described method, and the blank controls were also investigated to compensate the matrix effect.
Field Trials
The field trials contain the degradation dynamics and ultimate residue experiments in apple and soil that were conducted at three representative locations: Chang Ping region of Beijing (116.46 E, 39.92 N, north of China), Lai Yang city of Shandong province (120.99 E, 36.97 N, east of China), and Su Zhou city of Anhui province (116.58 E, 33.38 N, south of China), from June to September in 2016.
The characteristics of the soil used for the field trials at the three locations had been investigated, and the results are as follows: Beijing soil type was sandy brown soil, with about 2.70% organic matter, pH = 7.32, and a CEC (cation exchange capacity) of about 29.7 cmol kg−1. The soil from Shandong province was clay loam with the parameters as follows: 3.89% (organic matter), 6.73 (pH), and 16.7 cmol kg−1 (CEC). The soil from Anhui province was sandy loam with the following parameters: 1.71% (organic matter), 6.8 (pH), and 36.4 cmol kg−1 (CEC).
Dissipation Kinetic Trials
The field trials were designed according to NY/T 788-2004 (Guidelines on Pesticide Residue Trials) that was issued by the Ministry of Agriculture, P. R. China. There were five experimental plots, and each of them was 30 m2 with no application history of pyraclostrobin and prochloraz usage; each plot had three replications. A buffer zone of about 1 m distance was adopted to separate the plots from different plots. During the trial period, other fungicides were forbidden to use on these apple trees.
To survey the dissipation trends of pyraclostrobin and prochloraz in soil and apple, 40% emulsion in water (EW) of pyraclostrobin and prochloraz was diluted by water and then was separately sprayed on the blank soil and apple at a dosage of 300 mg active ingredient per kilogram (mg a.i. kg−1) (twice the recommended high dosage). Water was sprayed on the same size plots simultaneously as the control. The representative soil and apple samples (each 2 kg) were collected randomly from no less than five points of each plot at interval times of 0 (2 h after treatment), 1, 3, 5, 7, 10, 14, 21, and 30 days and were mixed uniformly. After sampling, the samples were put into polyethylene bags, labeled, and stored at − 20 °C until analysis.
Terminal Residue Trials
To investigate the final residues of pyraclostrobin and prochloraz in soil and apple, 40% emulsion in water (EW) of pyraclostrobin and prochloraz were sprayed at two dosage levels, 150 mg a.i. kg−1 (high recommended dosage) and 300 mg a.i. kg−1 (twice the recommended high dosage). Each dosage level was sprayed three times and four times on soil and apple, respectively. The interval of sampling was 7 days, thus the representative soil and apple samples were randomly collected at 7, 14, 21, and 28 days after application. More than five points of soil were collected to a depth of 0–15 cm in each plot and then the samples were screened through 40-mesh sieves. No less than four apples were gathered randomly from each plot and then cut into four petals; two opposite angles of them were taken to chop into small pieces (< 1 cm). All the samples were packed into polyethylene bags, labeled, and stored in the dark at − 20 °C until further analysis.
Theoretical Calculation
In this study, the metabolite was transformed into its parent to calculate the residue of prochloraz. The molecular weights of prochloraz and 2,4,6-trichlorophenol were 376 and 197. The quotient of two formula weights which keep three valid digits is the conversion coefficient to convert 2,4,6-trichlorophenol into prochloraz residues (De et al. 1997; Polese et al. 2006). The conversion formula is listed below in which the concentration of total prochloraz (C total) means the sum of prochloraz (C parent) and 2,4,6-trichlorophenol (C metabolite) multiplied conversion factor, namely 1.91.
where the C total (mg kg−1) represents the total residue of prochloraz and C parent (mg kg−1) and C metabolite (mg kg−1) represent the residue of prochloraz and its secondary metabolite.
Results and Discussion
Instrument Condition Optimization
2,4,6-Trichorophenol had an easier trend to lose a hydrogen ion and became negative ion. Thus, the ideal mobile phase should satisfy both positive ions and negative ions of chromatograph. In this experiment, pure water, 0.1% formic acid, and 20 mM ammonium acetate were compared to investigate the optimum mobile phase, as to prochloraz and pyraclostrobin; these three mobile phases showed inconspicuous effects, but ammonium acetate could let 2,4,6-trichlorophenol have the highest signals. Ammonium acetate is a strong electrolyte and its aqueous solution was shown to be electrically neutral, hence, ammonium acetate was the optimal mobile phase.
As the collision energy increased, the fragment ions of prochloraz and pyraclostrobin increased. Among them, the highest signals were chosen as quantitative ions of prochloraz and pyraclostrobin, of which the molecular weights were 308.1 and 194, respectively. While in 2,4,6-trichorophenol, the precursor ion lessened when the collision energy was heightened, the appropriate product ions still cannot be detected. Thus, the MRM mode with collision energy of zero was adopted to detect 2,4,6-trichorophenol; meanwhile, prochloraz and pyraclostrobin used multi-reaction monitoring (MRM) mode with their ideal collision energy.
Cleanup Sorbent Selection
In the QuEChERS method, dispersive solid-phase extraction (d-SPE) is often used to remove impurities and interferences to raise the signal-to-noise ratio (S/N) of target analyses. Meanwhile, the ideal d-SPE should have little effect on the compounds that were analyzed (Lehotay et al. 2010). According to the amount and type, C18, GCB, PSA, and CNTs were evaluated in apple. The results in Fig. 2 show the recoveries with the amount of GCB from 20 to 100 mg, C18 from 20 to 100 mg, PSA from 20 to 100 mg, and CNTs from 5 to 25 mg; each sorbent had five gradients with same equal intervals. As the GCB, PSA, and CNTs increase, recoveries decreased dramatically. Though C18 had a little effect on the recoveries of prochloraz, it was the optimum sorbent. In this study, 50 mg C18 was chosen to purify 1 ml extract acetonitrile phase to obtain an optimal cleanup effect.
Method Validation
According to the anterior researches, the matrix effects provide much challenge in developing reliable multi-residue quantified methods, and the matrix effects cannot be eliminated (Niessen et al. 2006). Thus, the matrix-matched calibration curves were used a common external standardization method to compensate in accuracies and reduce signal changes, namely, using cleaned and blank extract to prepare the calibration curve. The external standard method was used to calculate the content of these three compounds in actual samples, and it is more suitable to test a huge number of samples than internal standard method. The results showed a good linearity with all the correlation coefficients (r) higher than 0.99 (Table 2). By comparing the slopes of matrix-matched standard calibrations and those from pure acetonitrile curves, it is easy to obtain that both the pyraclostrobin and prochloraz had the signal enhancement while 2,4,6-trichlorophenol had signal suppression.
In general, LOD is defined as the lowest concentration that could be detected, and it was determined in each matrix at a signal-to-noise ratio (S/N) of 3:1. LOQ was determined based on a S/N ratio of 10:1 (Bao et al. 2012; Zou et al. 2012). In this study, the lowest concentration was defined as LOQ which could represent the sensitivity of the method, namely 0.005 mg kg−1. If the response value was below the LOQ, the data was viewed as inexact and would be replaced by the value of LOQ. The LOD was determined by different matrices with a S/N ratio of 3:1; the specific data is shown in Table 2. Figure. 3a and b shows the typical HPLC-MS/MS chromatograms of 2,4,6-trichlorophenol, prochloraz, and pyraclostrobin at 0.5 mg/kg in matrix-matched standard of apple and soil. As shown in Fig. 3, there were no interference peaks in this region of the chromatograph and the analysis time of the three compounds was < 3.9 min.
Five parallel extractions at four different levels in apple and soil matrices were executed to evaluate the accuracy and repeatability of the versatile method. Accuracy is expressed as recovery (%) with its RSD% expressed as precision (Lu et al. 2016). The recoveries (n = 5) of pyraclostrobin in apple ranged from 88.1 to 105.2% with the RSDs ranged 1.1–5.1 and in soil from 84.2 to 105.3% with the RSDs ranged 4.7–9.6. As to prochloraz in apple and soil, the recoveries ranged from 78.1 to 103.7% with the RSDs ranged 4.3–5.9 and from 82.7 to 96.8% with the RSDs ranged 1.6–7.7, respectively. Concerning 2,4,6-trichlorophenol, the recoveries of apple ranged from 83.3 to 107.6% with the RSDs ranged 2.6–6.5 and from 84.8 to 106.7% with the RSDs ranged 3.4–8.9 of soil. The RSDs in apple are all below 6.5 (Table 3) which showed great precision.
The method showed superb sensitivity, accuracy, linearity, and repeatability which could satisfy the demand of a real sample test to assess the security and validity of the formulation usage.
Real Sample Detection
Dissipation Behaviors of Pyraclostrobin and Prochloraz in Apple and Soil
In the three representative locations, pyraclostrobin initial concentrations at 0 day (2 h after application) in soil and apple ranged 0.23–0.92 mg kg−1 and 0.151–0.357 mg kg−1. The degradation half-lives of pyraclostrobin in soil and apple ranged 13.44–19.8 days and 7.99–15.04 days, respectively. As to prochloraz, the initial concentrations in soil and apple ranged 0.507–2.556 mg kg−1 and 0.176–0.450 mg kg−1, respectively, with the degradation half-lives of prochloraz ranged 8.89–18.02 days and 5.79–12.38 days. The soil and apple samples from Shandong fields dissipated faster with lower half-lives, and compared to the other two locations, it may apply to the specific climate. The specific data and each degradation equation are exhibited in Table 4.
The initial concentrations of pyraclostrobin and prochloraz in apple were smaller than soil. Meanwhile, the half-lives of these two fungicides were acceptable to apple growth cycle. The two fungicides have different degradation pathways in apple and soil, and the acquired data indicated that both pyraclostrobin and prochloraz dissipated faster in apple than in soil. Some external factors, like rainfall, temperature, wind speed, sunlight, etc., have an effect on the experiment results. Besides, the soil type of three locations can significantly influence the initial concentration and degradations of these two pesticides. Except these abiotic factors, the number and the type of microbes in soil and air can influence the initial concentrations and half-lives of pyraclostrobin and prochloraz.
Residue Distributions of Pyraclostrobin, Prochloraz, and 2,4,6-Trichlorophenol in Apple and Soil
The residues of total prochloraz in apple at Beijing, Anhui, and Shandong locations were all below 0.2 mg kg−1, and the content of pyraclostrobin in apple at three locations were all below official MRLs (2 mg kg−1 for prochloraz and 0.5 mg kg−1 for pyraclostrobin) recommended by China after 21 days of application. Apparently, it would be safe for apple consumption at the pre-harvest intervals (PHI, 21 days) based on the recommended dosage.
Conclusion
An accurate and repeatable method was first established to simultaneously detect 2,4,6-trichlorophenol, prochloraz, and pyraclostrobin in soil and apple. The method showed satisfactory performance in accuracy, precision, and reproducibility. Based on this method, the dissipation behaviors of prochloraz and pyraclostrobin in apple ecosystem were investigated following pseudo-first-order kinetic models. All of the terminal residues of three compounds in apple with an interval of 21 days were far below the official MRLs recommended by China. The current study was not only important to provide guidance on reasonable use of these fungicides in apple ecosystem but also facilitates the trade export of apple for China.
Abbreviations
- LODs:
-
Limits of detection
- LOQs:
-
Limits of quantification
- MRLs:
-
Maximum residue limits
- PHI:
-
Pre-harvest interval
- RRLC-MS/MS:
-
Rapid resolution liquid chromatography tandem mass spectrometry
- RSDs:
-
Relative standard deviations
- EW:
-
Emulsion in water
References
Aktar MW, Sengupta D, Purkait S, Ganguly M, Paramasivam M (2008) Degradation dynamics and dissipation kinetics of an imidazole fungicide (prochloraz) in aqueous medium of varying pH. Interdiscipl Toxicolog 1(3–4):203
Arts ICW, Hollman PCH, Bueno de Mesquita HB et al (2001) Dietary catechins and epithelial cancer incidence: the Zutphen elderly study. Int J Cancer 92:298–302
Bajpai VK, Choi SW, Cho MS et al (2009) Isolation and morphological identification of apple anthracnose fungus of Colletotrichum sp. KV-21. Korean J Environ Agric 28:442–446
Bao Y, Liu Q, Chen J, Lin Y, Wu B, Xie J (2012) Quantification of nerve agent adducts with albumin in rat plasma using liquid chromatography-isotope dilution tandem mass spectrometry. J Chromatogr A 1229:164–171 (in Chinese)
Bartlett DW, Clough JM, Godwin JR et al (2002) The strobilurin fungicides. Pest Manag Sci 58:649–662
Boyer J, Liu RH (2004) Apple phytochemicals and their health benefits. Nutr J 3:1–15
Cengiz MF, Catal M, Erler F, Bilgin AK (2014) Rapid and sensitive determination of the prochloraz residues in the cultivated mushroom, Agaricus bisporus (Lange) Imbach. Anal Methods UK 6(6):1970–1976
Davis PA, Polagruto JA, Valacchi G, Phung A, Soucek K, Keen CL et al (2006) Effect of apple extracts on NF-B activation in human umbilical vein endothelial cells. Exp Biol Med 231:594–598
De PM, Taccheo BM, Damiano V, Fabbro D, Bruno R (1997) Simplified determination of combined residues of prochloraz and its metabolites in vegetable, fruit and wheat samples by gas chromatography. J Chromatogr A 765(1):127
de Oliveira LA, Pacheco HP, Scherer R (2016) Flutriafol and pyraclostrobin residues in Brazilian green coffees. Food Chem 190:60–63
Feskanich D, Ziegler RG, Michaud DS et al (2000) Prospective study of fruit and vegetable consumption and risk of lung cancer among men and women. J Natl Cancer Inst 92:1812–1823
Fulcher JM, Wayment DG, White PM Jr et al (2014) Pyraclostrobin wash-off from sugarcane leaves and aerobic dissipation in agricultural soil. J Agric Food Chem 62(10):2141–2146
Fuse JI, Kanamori H, Ideyoshi N (2010) Determination of prochloraz and its metabolites in fruits and vegetables by GC. J Food Hyg Soc Jpn 41:61–651
Guo X, Wu W, Song N et al (2017) Residue dynamics and risk assessment of pyraclostrobin in rice, plants, hulls, field soil, and paddy water. Hum Ecol Risk Assess 23:67–81
Hameed BH (2007) Equilibrium and kinetics studies of 2,4,6-trichlorophenol adsorption onto activated clay. Colloids Surf A 307(1–3):45–52
Hameed BH, Tan IAW, Ahmad AL (2008) Adsorption isotherm, kinetic modeling and mechanism of 2,4, 6-trichlorophenol on coconut husk-based activated carbon. Chem Eng J 144:235–244
Hanafi A, Garau VL, Caboni P, Sarais G, Cabras P (2010) Minor crops for export: a case study of boscalid, pyraclostrobin, lufenuron and lambda-cyhalothrin residue levels on green beans and spring onions in Egypt. J Environ Sci Health B 45(6):493–500
Lafuente MT, Tadeo JL (2015) High performance liquid chromatography determination of prochloraz residues in citrus fruit. J Sep Sci 7(5):268–270
Lee DH, Kim D, Jeon Y et al (2007) Molecular and cultural characterization of Colletotrichum spp.causing bitter rot of apples in Korea. Plant Pathol J 23:37–44
Lehotay SJ, Kyungae S, Hyeyoung K et al (2010) Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J Chromatogr A 1217:2548–2560
Li H, Li J, Hou C et al (2010) A sub-nanomole level electrochemical method for determination of prochloraz and its metabolites based on medical stone doped disposable electrode. Talanta 83:591–595
Li K, Wu S, Xu S et al (2016) Oiling out and polymorphism control of pyraclostrobin in cooling crystallization. Ind Eng Chem Res 55:11631–11637
Lu P, Wu C, Shi Q et al (2016) A sensitive and validated method for determination of T-2 and HT-2 toxin residues in shrimp tissues by LC-MS/MS. Food Anal Methods 9:1–15
Navickiene S, Ribeiro ML (2005) An alternative LC-UV procedure for the determination of prochloraz residues in fruits. J Braz Chem Soc 16(2):157–162
Niessen WMA, Manini P, Andreoli R (2006) Matrix effects in quantitative pesticide analysis using liquid chromatography-mass spectrometry. Mass Spectrom Rev 25:881–899
Patyal SK, Sharma ID, Chandel RS et al (2013) Dissipation kinetics of trifloxystrobin and tebuconazole on apple (Malus domestica) and soil: a multi-location study from north western Himalayan region. Chemosphere 92:949–954
Polese L, Jardim EFG, Navickiene S et al (2006) Prochloraz residue levels in ginger submitted to Sportak 450 CE″ postharvest treatment. Eclética Quím 31(2):59–62
Shabeer TPA, Girame R, Hingmire S, Banerjee K, Sharma AK, Oulkar D et al (2015) Dissipation pattern, safety evaluation, and generation of processing factor (PF) for pyraclostrobin and metiram residues in grapes during raisin preparation. Environ Monit Assess 187(2):4268
Vinggaard AM, Nellemann C, Dalgaard M et al (2002) Antiandrogenic effects in vitro and in vivo of the fungicide prochloraz. Toxicol Sci 69:344–353
Yoon H, Liu RH (2007) Effect of selected phytochemicals and apple extracts on NF-B activation in human breast cancer MCF-7 cells. J Agric Food Chem 55:3167–3173
Zhang F, Wang L, Li Z, Wu D, Pan H, Pan C (2012) Residue dynamics of pyraclostrobin in peanut and field soil by QuEChERS and LC–MS/MS. Ecotoxicol Environ Saf 78(2):116–122
Zou Z, He Z, Li H, Han P, Tang J, Xi C, Li Y, Zhang L, Li X (2012) Development and application of a method for the analysis of two trichothecenes: deoxynivalenol and T-2 toxin in meat in China by HPLC-MS/MS. Meat Sci 90:613–617 (in Chinese)
Funding
This study was funded by the National Natural Science Foundation of China (grant no. 21677009) and the Natural Science Foundation of Beijing (grant no. 8162029).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
X.F. declares that she has no conflict of interest. S.Z. declares that he has no conflict of interest. X.C. declares that she has no conflict of interest. J.H. declares that she has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Fan, X., Zhao, S., Chen, X. et al. Simultaneous Determination of Pyraclostrobin, Prochloraz, and its Metabolite in Apple and Soil Via RRLC-MS/MS. Food Anal. Methods 11, 1312–1320 (2018). https://doi.org/10.1007/s12161-017-1065-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-1065-1