Abstract
Sandwich enzyme-linked immunosorbent assay (ELISA) of sulfonamides based on double-competitive interaction between haptenized protein as a captured antigen and analyte for binding to immobilized and enzyme-labeled antibodies was developed. This experimental assay format was examined in analytical properties and matrix effect resistance in comparison with usual ones: indirect, direct antigen-coated and antibody-coated ELISAs. All four assay formats were designed on the basis of interactions between the previously prepared monoclonal antibody and immunizing hapten, 4-(4-(4-aminophenylsulfonamido) phenyl)butanoic acid, providing the uniform output optical signal for correct comparison of each assay characteristic. The sensitivity (IC50) of the developed competitive sandwich assay was rather below 100 ng/ml for 11 sulfonamides which was suitable for their determination in milk, muscles and animal sera at established maximum residue limit concentration. Comparative examination did not reveal changes in assay specificity, and advantages in sensitivity, matrix effect resistance, and procedure duration before commonly used assay formats. Moreover, new design of assay was shown to be three-fold more consumable in antibody reagents in comparison with direct assay formats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the past several decades, the use of antibodies against various low-molecular-weight compounds allowed to create sensitive and specific methods which were suitable for purposes of food safety control, veterinary and sanitary inspection, therapeutic drug and environmental monitoring, doping control and forensics, as well as in research (Shankaran et al. 2007; Yang and Carlson 2004; Tsai and Lin 2005; Barroso et al. 2012; Losoya-Leal et al. 2015).
Some attractive features of immunochemical methods are that they offer an alternative to chromatography assays in cost-effectiveness, possess high-throughput functionality and advantages in simple sample pretreatment.
Immunoassay of small molecules is most commonly based on competitive interactions between analyte and analyte-like reagent for antibody binding. The analyte-like reagent is the key reagent that determines the type and assay format. It may be immobilized on inert or sensory solid phase (Shankaran et al. 2007), or may represent a tracer, analyte or analyte-like compound labeled with reporter molecule: radioisotope (Yang and Carlson 2004), enzyme (Tijssen 1985), fluorophore (Smith and Eremin 2008) or nanoparticle (Huang et al. 2016).
Another type of test, conventional sandwich immunoassay configuration, is based on two-antibody binding detection. Two recognition sites on the target molecule spatially separated to avoid steric hindrance for two-antibody binding are the key factors for test-system operation. So, the main targets having multiple epitopes are large molecules (> 5000 Da) and corpuscles such as bacteria and viruses (Shankaran et al. 2007; Huang et al. 2016). Although, sandwich immunoassay is generally accepted as unsuitable for measurement of low-molecular-weight analytes, several kinds of sandwich assays for detection of haptens have been reported (Shen et al. 2014).
Noncompetitive variants have been developed on the basis of anti-metatype homologous, heterologous antibodies and peptides selected against hapten–mAb immunocomplex using classical immunization (Towbin et al. 1995), the autonomously diversifying library system (Omi et al. 2015), and phage display random peptide libraries (Inaba et al. 2009), respectively. An open-sandwich immunoassay (OS-IA) was created by Ihara et al. (2009) for glucocorticoid analysis as a result of immobilized VL and enzyme-labeled VH antibody fragments binding together in the presence of analyte.
Competitive variant of sandwich immunoassay of small analyte was realized using synthetic hapten-duplex structure. Such synthetic bis-hapten analog could be captured by immobilized and labeled antibodies, and this interaction could be inhibited by free hapten in dose-dependent manner (Ali et al. 1992). The mentioned works utilizing the sandwich principle for small analytes declared the substantially improved specificity of determination and higher sensitivity. To realize the mentioned advantages, more than one high-specific antibody or analyte-recognizing agents are required to construct noncompetitive sandwich immunoassay. The additional reagent should be generated individually and specially selected. The design of competitive sandwich variant described by Ali et al. (1992) is complicated with task to synthesize and purify bis-hapten analog.
The present study is designed to compare new sandwich double-competitive ELISA with conventional direct and indirect competitive formats developed using the same immunoreagents to reveal advantages in analytical properties and resistance to matrix interference. The numerous members from sulfonamide (SA) family of antibacterials were taken as models of low-molecular-weight analytes.
Methods
Reagents and Chemicals
Bovine serum albumin (BSA), N-hydroxysuccinimide (NHS), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), and horseradish peroxidase (HRP) were from Sigma (St. Louis, MO). Dimethylformamide (DMF) was from Serva (Heidelberg, Germany). Gelatin (Gel) was purchased from Bio-Rad (Hercules, CA, USA), and rabbit anti-mouse IgG-HRP (anti-mIgG-HRP) was from Imtek (Moscow, Russia).
The substances of sulfonamides (SAs), sulfamethazine (SMZ), sulfalene (SLE), sulfasalazine (SSZ), sulfadimethoxine (SDM), phtalylsulfathiazole (PST), sulfathiazole (STZ), sulfisoxazole (SIZ), sulfaethidole (SET), sulfamethizole (SMT), sulfamethoxazole (SMX), sulfachloropyridazine (SCP), sulfadiazine (SDZ), sulfamonomethoxine (SMM), sulfamerazine (SMR), sulfamethoxypyridazine (SMP), sulfadoxine (SDX), sulfaquinoxaline (SQX), sulfapyridine (SPY) and sulfanitran (SNT) were purchased from Chimmed (Moscow, Russia). Stock solutions of SA substances (1 mg/ml) were prepared using methanol and stored at − 20 °C. All the standard solutions were diluted from stock solutions with PBST (phosphate-buffered saline buffer containing 0.05% Tween). The coating buffer was 0.05 M carbonate-bicarbonate buffer (CBB, pH 9.6). TMB/H2O2-containing solution was a product of Biotest Systems (Moscow, Russia) and used as ready-to-use substrate mixture.
Bovine and porcine sera were from the following different manufacturers: Biolot (St. Petersburg, Russia), Pan Eco (Moscow, Russia), PAA The Cell Culture Company (France) and GE Healthcare Life Science (New Zealand). Skim milk powder was from Fluka (Switzerland). Beef, pork and milk samples were purchased from a private organic farming. All other chemical reagents were of analytical grade.
Hapten Synthesis
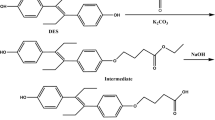
Sulfonamide derivatives, BS (6-(4-aminobenzenesulfonylamino)butanoic acid), HS (6-(6-aminobenzenesulfonylamino)hexanoic acid), TS (2-(2-(4-aminophenylsulfonamido)thiazol-5-yl)acetic acid), CS (4-(4-aminobenzenesulfonylamino)benzoic acid) and PB (4-(4-(4-aminophenylsulfonamido) phenyl)butanoic acid), were synthesized and described in detail previously (Zhang et al. 2006; Wang et al. 2013). The derivative PB was designated as SA10 in previous work. The structures of SA derivatives are presented in Fig. 1.
Preparation of Conjugated Antigens Based on Sulfonamide Derivatives and Protein Carriers
Ten micromole quantity of SA derivatives, BS (2.6 mg), HS (2.9 mg), TS (3.1 mg), CS (2.9 mg), PB (3.3 mg) and TS (3.1 mg) were dissolved in DMF; each one was mixed with EDC and NHS (12 μmol) in 1 ml of whole volume and vigorously agitated using a magnet stirrer for 3 h at room temperature. Then, the activated haptens were added drop-wise to protein carriers in CBB at рН 9.6 and incubated under stirring overnight at 4 °С. The molar ratios between BSA and PB or TS at conjugation were taken as 1/60 and were 1/100 for BS, HS and CS. Gelatine-based conjugates were prepared using 10- and 30-fold molar excesses of haptens over protein.
Preparation of Tracer Based on Haptens Conjugated to Horseradish Peroxidase
The haptens PB and TS were activated with NHS/EDC as described in previous texts and then mixed for conjugation with HRP at the ratio from 5:1 to 15:1 mol/mol. The mixtures were stirred for 3 h at room temperature.
The resultant conjugates were purified from unreacted substances by exhausting dialysis against three changes of 5 l of water.
Preparation of Antibody-Peroxidase Conjugate
The conjugation was conducted accordingly to the procedure of Nakane and Kawaoi (1974) but slightly modified. To horseradish peroxidase (10 mg, 0.25 μmol) in 1 ml of distilled water, 0.3 ml of sodium periodate (5.35 mg, 25 μmol) was added and stirred for 20 min. During oxidizing, the color of solution changed from brown to green and returned to brownish after addition of 0.5 ml of 0.1% ethylene glycol. Then, the oxidized enzyme was purified by ultrafiltration using a 30-kDa cut-off membrane (Ultracent-30 Toyosoda). The retentate was four times filled up to that of the initial volume with water and repeatedly centrifuged at 4000 rpm for 20 min. Monoclonal antibody, McAb 4D11 (Wang et al. 2013), was isolated from ascitic fluid using ammonium persulfate precipitation and reconstituted with CBB (pH 9.6). Bright brown solution of HRP was drop-wise added to the solution of McAb (8 to 1 mol/mol). After 2 h stirring, reaction mixture was supplemented with 0.1 ml of sodium borohydride (2 mg/ml) and 1 h later extensively dialyzed against PBS at pH 7.4. Prepared conjugate was stabilized with BSA (up to 5 mg/ml) and glycerol (up to 50%) and stored at − 20 °C.
Competitive ELISA Procedure
Competitive enzyme-linked immunosorbent assay was conducted in four different formats according to the following scheme:
-
Format 1 (indirect cELISA): immobilized protein-SA + [SA + anti-SA] + anti-mIgG-HRP;
-
Format 2 (direct cELISA): immobilized protein-SA + [SA + anti-SA-HRP];
-
Format 3 (direct cELISA): immobilized anti-SA + [SA + SA-HRP].
Interaction between McAb 4D11 (anti-SA) and antigens (protein-SA) and their optimal concentrations were firstly determined in noncompetition (without free SA) checkerboard titration procedure. Corresponding reagents that should be immobilized on the polystyrene 96-well plates (Costar, USA) were prepared in serial concentrations in CBB at pH 9.6 and were incubated 100 μl/well for a night at 4 °C. After thrice repeated washing with PBS containing 0.05% Tween 20 (PBS-T), the wells were filled with 100 μl solution of binding agent (1/antibody, 2/antibody-peroxidase conjugate (anti-SA-HRP) or 3/hapten-peroxidase conjugate (SA-HRP)) diluted in 1%BSA-PBS-T in the range of concentrations and 100 μl PBST. The immunoreaction was lasted for 1 h at room temperature, and then, unbound reagents were washed out. The procedure of ELISA format 1 means registration of bound McAb and included the additional incubation step (1 h, 37 °C) with 100 μl of rabbit anti-mouse IgG conjugated with horseradish peroxidase. Then, TMB-H2O2-containing ready-to-use substrate mixture was used for visualization of immunological interaction. The color development reaction was terminated after 30 min by addition of 100 μl of 1 M sulfuric acid, and absorbance was registered at 450 nm using StatFax 2100 reader (Awareness Technologies, USA).
Competitive Sandwich ELISA Procedure
One more type of assay that combined the steps of ELISA formats 2 and 3 applied in the present study was developed. The following scheme shows the principle of interactions in this assay format:
-
Format 4. Sandwich cELISA: immobilized anti-SA + [SA + protein-SA + anti-SA-HRP].
To construct sandwich assay, the parameters established for assay format 2 and format 3 served as initial reference points and then were optimized. Monoclonal antibody was coated on the plates in the same concentration which was accepted as optimal in ELISA format 3. Then, the optimal relationship between concentrations of hapten conjugate and anti-SA-HRP was determined in checkerboard titration. The solutions of reagents (each in 50 μl) were mixed in the wells with 100 μl of PBST and incubated at room temperature for 2 h. The following completion of the enzymatic reaction and data registration were analogic to the previously discussed description.
Several assay variants with combinations of reagents displaying the absorbance intensity of 0.8–1.2 were compared in the corresponding competitive ELISA formats using PB (immunizing hapten) or representative of SAs as competitors. The combination that demonstrated the better sensitivity of SA determination was selected for the following experiments.
Assay Performance
A number of SAs were analyzed in each assay format in the range of concentrations (10,000–0.01 ng/ml). Concentration-dependent inhibition of antibody binding (B/B0 × 100) was presented in the form of sigmoid standard curves using OriginPro 8.0 software. For assessment and comparison of sensitivity of determination in developed assay formats, the IC50 (half-inhibition concentrations) values were determined for each analyte (Burkin and Galvidis 2009).
Cross-reactivity (CR) was expressed by the following equation:
Since the hapten used in conjugate synthesis was not an analyte but an SA derivative, the “main analyte” was taken as the most active SA, the nearest structurally related to hapten.
Matrix Effect Estimation, Sample Pretreatment and Recovery Experiments
The foodstuffs of animal origin are the potential test subjects that may be contaminated with the residues of sulfonamides as one of the most commonly used veterinary antibacterials (Dmitrienko et al. 2014). So, such complex matrixes as milk, muscle extracts and animal sera were chosen for evaluation of their effect on immunodetection of SAs. The extent of matrix interferences on optical signal in each assay format was estimated using the dilution method and was expressed in relative antibody binding (B/Bo). So, the examined blank samples of matrix were gradually diluted with assay buffer (PBST) and tested in each system. The corresponding factor of matrix dilution which was enough to avoid the differences in absorbance between control (PBST) and test (diluted matrix) wells was chosen for sample preparation.
Biofluids were simply diluted with assay buffer, and for preparation of beef and pork extracts, the tissue samples were firstly homogenized using a blender. Then, 1 g portion was thoroughly stirred with 4 ml of PBST and left for extraction overnight at 4 °C. Tissue debris was pelleted by centrifugation at 3000 rpm for 10 min, and supernatants were analyzed.
Blank samples of tissue homogenates and fluids were fortified with known quantity of SA. The samples were treated and diluted appropriately and tested in ELISAs. A ratio between the measured and the fortified concentration was accepted as assay recovery.
Results and Discussion
Preparation of Hapten Conjugates
Five SA derivatives (Fig. 1), functionalized with carboxyl groups, were designed for conjugation to carrier/enzyme to expose common moiety of SA molecules (Fig. 2) for group-specific immunoaffinity recognition. Each one was coupled through carboxyl with amines of BSA and Gel using NHS/EDC method. The ratios between proteins and haptens taken in synthesis were different, i.e., from 1/60 or 1/100 mol/mol for BSA-haptens to 1/10 and 1/30 for Gel-haptens. The changes in protein spectra typical for haptens were registered in the resultant BSA-antigens that were the evidence of successful conjugate formation (Fig. 3).
The values of absorption increment in conjugate spectra in the regions of 260–270 nm characteristic to hapten maxima were used to calculate the hapten content in conjugates as a ratio mole of hapten per mole of BSA (Table 1).
The similar changes in the Gel-based antigens were less pronounced because of less hapten load; however, their binding with antibody confirmed the hapten attachment. The prepared conjugates were examined as coating and captured antigens. Tracers, the products of conjugation of peroxidase with haptens, were also checked up directly in binding with immobilized antibodies (format 3).
Optimization of ELISA Formats
Different hapten loads in the resultant coating antigens, in SA-HRP conjugates as well as in captured antigens influenced the sensitivity of corresponding assay variants. Thus, the best sensitivity of SA determination served as a criterion for choosing a conjugate with optimal hapten load.
Format 1.
A panel of protein-hapten conjugates varied in hapten load was examined as coating antigens in cross-titration with McAb 4D11. TS-based conjugates lacked binding activity. All the other four groups of coating antigens demonstrated good activity, and the best sensitivity values of assay were found for conjugates prepared with minimal hapten load, namely 10-fold excess, and indicated as Gel-SA × 10 (Table S1).
Format 2.
The hapten load and concentration of coating conjugate found for realization of the most sensitive version of indirect ELISA format 1 were accepted as optimal parameters for direct antigen-immobilized assay. The preparation of McAb 4D11 labeled with horseradish peroxidase was diluted (1:2000) to provide absorbance of reaction about 1.0. The higher concentration of McAb-HRP resulted in higher optical signal but worse sensitivity. More dilution makes the assay system more sensitive but decreasing of the signal could give inaccuracy of measurements.
Format 3.
The interactions between immobilized McAb and peroxidase conjugates with different hapten loads were examined to choose the preferable parameters of reagents. Different molar ratio between HRP and PB at synthesis (1/5 and 1/15) was found to make no difference for assay sensitivity, whereas 10-fold decreasing of coating McAb concentration (from 1/1000 to 1/10,000) resulted in almost 10-fold improvement in sensitivity of hapten determination (Fig. S1). Like the results in the previous texts (format 1), TS-labeled peroxidase did not bind by immobilized McAb. The fact of binding failure between TS and anti-PB McAb was additionally confirmed by inhibitory inactivity of TS; so, these interactions were ignored in the following experiments.
Format 4.
Competitive sandwich assay of low-molecular-weight analytes is an experimental format studied here which may be considered as combination of formats 2 and 3. Thus, the parameters optimized for formats 2 and 3, namely concentration of immobilized McAb (1:10,000) and working titer of McAb-HRP conjugate (1:2000), served as starting points and then were corrected.
Protein carriers conjugated with haptens are commonly used as coating antigens, and in such capacity, they were assessed in format 1. Because these conjugates bear more than one hapten determinant, except for Gel-PB × 10 (Table 1), they could interact with several antibody molecules. In our attempt to design sandwich assay using these model constructions, we intended to reveal possible advantages because of double-competitive interactions. For optimization parameters of this complex system, cross-titration between captured antigen and McAb-HRP was conducted in McAb-coated plates. The known principle of competitive immunoassay, unlike noncompetitive ones, is that the lower concentrations of binding reagents permit the higher sensitivity. Nevertheless, the concentrations of reagents should be possibly low and permit output signal high enough to avoid measuring inaccuracy. Absorbance value equal to 1.0 was taken as satisfying to this requirement and serves a common measurement rate in the other formats as well.
Figure 4 demonstrates that optical signal value depends on concentration of captured antigen, hapten load and concentration of McAb-HRP. Because of single-step interaction between three reagents “immobilized anti-SA–protein-SA–anti-SA-HRP,” the titration curves of captured antigens are bell-shaped. For each system of interaction, the maximum absorbance (curve peaks) corresponds to the optimal concentration of captured antigen when the binding sites on the immobilized antibody are completely occupied with protein-PB. Unsaturated binding sites on the immobilized antibody as well as superfluous quantity of detecting McAb-HRP cause weak inhibitory effectiveness of competitor and poor sensitivity. The higher concentrations of protein-PB result in the formation of two types of immunocomplexes instead of a sandwich-complex. Thus, the optimal concentrations of reagents that provide adequate absorbance level (≈ 1.0) were determined in standard checkerboard titration and found to be 3 and 1 μg/ml for Gel-PB × 10 and Gel-PB × 30, respectively (Fig. 4a, b). Surprisingly, that in spite of hapten load < 1.0, the Gel-PB × 10 appeared to be also suitable as captured antigen. The possible reason of this phenomenon was dissimilar molecular mass of gelatin or non-uniform hapten conjugation. The optimal ratios between BSA-PB × 60 and McAb-HRP were also obtained in cross-titration procedure (Fig. 4c), but they were out of accepted absorbance level of 1.0. The exact concentrations of reagents that permitted optical density equal to 1.0 were obtained by the calculation using equations presented in plot (Fig. S2). Such the concentration for BSA-PB × 60 was found to be 0.042 μg/ml, and the titer of McAb-HRP was 1:720. Thus, three assay systems being optimized and output signal-equalized were estimated in their sensitivity to reveal the role of hapten load in captured antigen. As can be seen from comparative data presented in Fig. 5, the higher index of hapten/carrier ratio in captured conjugates was more preferable for sensitive determination of SAs exemplified by SMX, SCP and SMZ.
Comparative sensitivity estimation of sulfonamides in sandwich competitive ELISA (format 4) based on protein-hapten conjugates with different hapten load. Each IC50 value was determined from the standard curves obtained from the average values (n = 3) for corresponding SA analytes in ELISA format 4. The number in conjugate designation indicates excess of hapten over carrier at synthesis
Comparative Assay Format Performance
Four competitive ELISA formats were designed based on single pair of interacting reagents, namely McAb 4D11 and immunizing hapten PB. Coating and captured antigens (protein-PB) and reporter molecules (4D11-HRP and PB-HRP) necessary for assay format construction were prepared using the mentioned elements. Thus, only the assay design made a difference in comparative SA analysis and could be identified. A number of SAs (Fig. 2) taken in a range of concentrations (10,000–0.01 ng/ml) were tested in each assay format. Using the standard curves plotted for each analyte, the corresponding IC50 values were determined (Table 2).
Sulfonamides with five-member ring at N1 position (except for SMX) were weak competitors (IC50 > 100 ng/ml) in every assay format. The radicals in six-member ring nearest to N1 position were a crucial hindrance for antibody binding (SDX and SLE). The compared formats also failed to recognize analytes with substituents at N4 position (PST and SSZ). The exception was SNT that was found to be the most active inhibitor among the tested SAs. Surprisingly, that acetyl substituent at N4 position of SNT was not an obstacle for 4D11 binding. Acetyl substitution at this site of molecule is specific for SA biotransformation (Vree et al. 1985). Thus, such McAb specificity may be useful for simultaneous metabolite detection, as confirmed by example of acetyl-SMZ (Wang et al. 2013). No nitrogen substituents in the second six-atom ring of SNT make its structure the most related to that of immunizing hapten PB (Figs. 1 and 2). For this reason, activity of SNT was accepted as reference (100%) for cross-reactivity examinations. The sensitivity (IC50) of SA determination in each assay format was not different sufficiently; nevertheless, it could be noted that antibody-coated direct ELISA demonstrated the priority in sensitivity which gradually decreased in the row of formats 3 > 2 > 4 > 1 (Table 2). This correlation remained the same for each analyte. As a result, the number of SAs which was detected with sensitivity 100 ng/ml and below could be augmented using assay format design from 11 (formats 1, 2 and 4) to 12 in format 3. High sensitivity determination (IC50 < 10 ng/ml) could be reached for 6, 8, 9 and 7 SAs depending on assay formats 1, 2, 3 and 4, respectively.
Comparison of assay formats was carried out and reported before, but the results obtained from the different research groups were contradictory. So, Wang et al. (2015) compared direct (streptavidin immobilized) and indirect (tetracycline-BSA immobilized) competitive formats of biotinylated aptamer-based enzyme-linked assay (ELAA) for tetracycline determination and found 10-fold higher sensitivity in indirect format owing to signal amplification mediated by biotin/streptavidin-HRP interaction (Wang et al. 2015) Analogical comparison of direct and indirect formats of ELAA and ELISA of ochratoxin A revealed the opposite tendency. Both direct assays demonstrated 10-fold advantage in sensitivity in comparison with the indirect ones (Barthelmebs et al. 2011). Indirect immunoassay formats were found to be preferable for citrinin (Abramson 1996), sulfathiazole (Pastor-Navarro et al. 2004) and bisphenol A (Lu et al. 2012). ELISA for triazophos (Jin et al. 2008) and data of the present study are evidence in the favor of direct assay. Thus, the properties of reporter system for amplification of resultant signal in each particular case determine the sensitivity advantages. Furthermore, the coating properties, universal usage or availability of reagents and being time-consuming may determine the preference when choosing of assay format.
The developed one-step sandwich ELISA based on double-competitive interaction between analyte and haptenized protein antigen for binding with pair of antibodies, immobilized and enzyme-labeled, did not perform advantages in sensitivity before commonly used assay formats. Double-competitive analyte interaction with capture and second antibody in related sandwich was reported to be rather effective because of the capability to inhibit simultaneous binding of both antibodies that resulted in sensitivity improvement (Ali et al. 1992). However, another opinion is that increasing of interacting reagent concentration should weaken the inhibition activity of analyte. The sandwich assay in the present case deals with double quantity of reagents (at least two PB-haptens in the conjugate and two 4D11 antibodies) that diminishes the possible positive effect.
Cross-reactivity profile of analytes was not expected to be modified with assay design because the main reactants remained the same. The obtained data confirmed that format-dependent fluctuations of CR were not significant (Table 2).
Matrix Effect Estimation and Sample Pretreatment
Easy sample pretreatment is one of the advantageous features of immunoassays. Nevertheless, matrix influence on antigen-antibody binding may result in false analyte detection. A simple dilution of test samples may be often effective to diminish and overcome matrix effect problem. The degree of influence displayed by milk, sera and tissue extract matrixes on SA immunodetection was examined to find more resistance to matrix effect assay format.
As can be noticed from comparative data (Fig. 6), the ELISA format 2 displayed the most homogenous results for every type of matrix, while the assay format 3 demonstrated the opposite tendency, great variability of results for the same tested samples. This may indicate that adsorbed form of antigen and soluble form of antibody (format 2 design) are more resistant to matrix influence. The comparison between indirect and direct ELISAs 1 and 2 which were equal in immobilized antigen and soluble form of antibody let us think that variability was introduced by additional step reaction with anti-mIgG-HRP. On the other hand, the variability in both compared antibody-coated formats dominated in 3 over 4. This suggests that observed effect was due to PB-HRP that influenced the matrix. Rather intensive effect was registered in format 3 for sera samples (Fig. 6d). High sensitivity of format 3, the most sensitive for target molecule and, consequently, the most influenced by different interference factors, may only partially explain this effect. Low-molecular-weight bioactive substances like vitamins, hormones and peptides were removed from sera samples by dialysis; however, this did not change the degree of serum matrix effect. An inactivation of serum thermolabile components such as complement was achieved by incubation at 56 °C for 40 min but also gave no result (data not shown). Thus, the observed interference was the feature of sera samples, effect of which was due to macromolecular matrix inhibiting interaction between immobilized antibody and haptenized enzyme.
Matrix dilution-dependent influence on optical signal in competitive ELISA formats 1–4. Each symbol indicates an individual blank sample of pork (a) and beef (b) extracts, milk (c) and bovine/porcine sera (d) matrix. Empty symbol represents the skim milk powder. The horizontal bar refers to the average (n = 5), while the box shows ±the standard deviation
It should also be noted that the degree of matrix effect manifestation of milk samples was more pronounced in all assay formats. This influence was not completely eliminated under dilution, only slightly diminished (Fig. 6c). The similar case was described for immunoassay of SAs in milk previously (Jiang et al. 2013), when the only procedure of milk protein removal reduced the matrix effects sufficiently.
In our study, “skim milk powder” reagent reproduced adequately the matrix effect of liquid milk samples, especially in formats 1 and 2. This was the reason for using this reagent as a diluent for standards to mimic milk matrix as before (Galvidis and Burkin 2010).
The studied experimental competitive sandwich assay (format 4) represented a hybrid variant combining formats 2 and 3. That is why the variability of data in format 4 was assessed as intermediate, not so pronounced as in format 3, at the same time less homogeneous than in format 2. However, the degree of matrix effect was more similar to that of format 2 and differed from that of format 3 owing to the absence of PB-HRP. The latter was identified to be a crucial factor susceptible to undesired matrix influence.
Recovery Experiments
The developed ELISA formats were compared in recovery experiments for SA determination in food matrixes prepared accordingly the dilution procedure avoiding the influence on antibody-hapten binding characteristics. Several blank matrix samples were fortified with SAs of different structures to compare the suitability of different assay systems for determination of broad spectrum of analytes (Fig. 7). Milk samples were analyzed at two fortification levels of SMZ, 100 and 25 μg kg−1, which correspond to the maximum residue limits (MRLs) for SAs in milk established in the EU and in the Russian Federation and China (EU Council Regulation 2010; Sanitary Regulation in Russian Federation 2010; Regulation in People’s Republic of China 2002). Using the skim milk reagent for milk matrix mimicry, it was enough to dilute milk samples 10-fold and recover SMZ at both MRL levels (Table 3). Recovery of SAs from meat was exemplified by swine muscles spiked with SQX and measurement in extracts after 10-fold dilution. Because of marked matrix effect discovered for sera samples, to avoid it, the samples should be diluted 50-fold. The experiments showed the satisfactory recovery rate for widely used SAs. A higher variation of data observed in format 4 may be a result of more complex interactions in this type of assay.
Standard curves for determination of sulfamethazine (a), sulfaquinoxaline (b) and sulfamethoxazole (c) in four competitive assay formats. Standard concentrations of SQX, SMX in PBST and SMZ in PBST containing 1% skim milk powder were analyzed in indirect ELISA (format 1), direct antigen-coated ELISA (format 2), direct antibody-coated ELISA (format 3) and sandwich ELISA (format 4). Each value represents the average from three replicates, and error bars are the standard deviations
Conclusion
The present study aimed to develop a new sandwich double-competitive ELISA for determination of sulfonamide analytes and investigate its possible advantageous analytical properties in comparison with usual formats of competitive immunoassay. The principle of experimental format was one-step sandwich ELISA involving competition between target analyte and haptenized protein (dendrimer, particle, virus or bacteria) for simultaneous binding with immobilized and enzyme-labeled antibodies. The reference methods were competitive indirect, direct antigen-coated and antibody-coated ELISA formats. For comparison, all four assay formats were designed on the base of interactions between the same monoclonal antibody and immunizing hapten PB. The uniform reaction level (OD ≈ 1.0) was taken for correct comparison of characteristics of each assay. The duration of experimental assay format was 2.5 h, equal to that of indirect ELISA. This format showed to be three times more consumable for immobilized antibody (1/3000 vs. 1/10,000) and for McAb-HRP (1/720 vs. 1/2000) in comparison with direct assay formats. Prospective positive effect from double-competitive interaction was not confirmed in present study. Comparative examination did not reveal advantages in sensitivity, specificity and matrix effect resistance before commonly used assay formats. However, improvement of analytical characteristics may be expected in the following experiments when using heterologous haptens or pair of different antibodies.
References
Abramson D (1996) Determination of citrinin in barley by indirect and direct enzyme immunoassay. J AOAC Int 79(6):1325–1329
Ali E, Sengupta J, Dhar TK (1992) Sandwich immunoassay of small molecules. I. Investigation with testosterone as model hapten. J Immun Meth 147:173–179
Barroso O, Handelsman DJ, Strasburger C, Thevis M (2012) Analytical challenges in the detection of peptide hormones for anti-doping purposes. Bioanalysis 4(13):1577–1590
Barthelmebs L, Jonca J, Hayat A, Prieto-Simon B, Marty J-L (2011) Enzyme-linked aptamer assays (ELAAs) based on a competition format for a rapid and sensitive detection of ochratoxin A in wine. Food Control 22:737–743
Burkin MA, Galvidis IA (2009) Improved group determination of tetracycline antibiotics in competitive enzyme-linked immunosorbent assay. Food Agric Immunol 20(3):245–252
Dmitrienko SG, Kochuk EV, Apyari VV, Tolmacheva VV, Zolotov YA (2014) Recent advances in sample preparation techniques and methods of sulfonamides detection—a review. Anal Chim Acta 850:6–25
EU Council Regulation (2010) N 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off J Eur Union L15:1–72
Galvidis IA, Burkin MA (2010) Monoclonal antibody-based enzyme-linked immunosorbent assay for the aminoglycoside antibiotic kanamycin in foodstuffs. Russ J Bioorg Chem 36(6):722–729
Huang X, Aguilar ZP, Xu H, Lai W, Xiong Y (2016) Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: a review. Biosens Bioelectron 75:166–180
Ihara M, Suzuki T, Kobayashi N, Goto J, Ueda H (2009) Open-sandwich enzyme immunoassay for one-step noncompetitive detection of corticosteroid 11-deoxycortisol. Anal Chem 81:8298–8304
Inaba J, Nakamura S, Shimizu K, Asami T, Suzuki Y (2009) Anti-metatype peptides, a molecular tool with high sensitivity and specificity to monitor small ligands. Anal Biochem 388(1):63–70
Jiang W, Wang Z, Beier RC, Jiang H, Wu Y, Shen J (2013) Simultaneous determination of 13 fluoroquinolone and 22 sulfonamide residues in milk by a dual-colorimetric enzyme-linked immunosorbent assay. Anal Chem 85:1995–1999
Jin RY, Gui WJ, Guo YR, Wang CM, Wu JX, Zhu GN (2008) Comparison of monoclonal antibody-based ELISA for triazophos between the indirect and direct formats. Food Agric Immunol 19(1):49–60
Losoya-Leal A, Estevez MC, Martínez-Chapa SO, Lechug LM (2015) Design of a surface plasmon resonance immunoassay for therapeutic drug monitoring of amikacin. Talanta 141:253–258
Lu Y, Peterson JR, Gooding JJ, Lee NA (2012) Development of sensitive direct and indirect enzyme-linked immunosorbent assays (ELISAs) for monitoring bisphenol-A in canned foods and beverages. Anal Bioanal Chem 403:1607–1618
Nakane PK, Kawaoi A (1974) Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem 22(12):1084–1091
Omi K, Ando T, Sakyu T, Shirakawa T, Uchida Y, Oka A, Ise N, Aoyagi K, Goishi K (2015) Noncompetitive immunoassay detection system for haptens on the basis of antimetatype antibodies. Clin Chem 61(4):627–635
Pastor-Navarro N, García-Bover C, Maquieira A, Puchades R (2004) Specific polyclonal-based immunoassays for sulfathiazole. Anal Bioanal Chem 379:1088–1099
PRC Regulation (2002) Ministry of Agriculture of the People’s Republic of China (In Chinese) no. 235
Sanitary Regulation (2010) SanPiN 2.3.2.1078-01 hygienic requirements for safety and nutritive value of foodstuffs, Moscow, 2008. (In Russian), Supplement 22
Shankaran DR, Gobi KV, Miura N (2007) Recent advancements in surface plasmon resonance immunosensors for detection of small molecules of biomedical, food and environmental interest. Sensors Actuators B 121:158–177
Shen J, Li Y, Gu H, Xia F, Zuo X (2014) Recent development of sandwich assay based on the nanobiotechnologies for proteins, nucleic acids, small molecules, and ions. Chem Rev 114(15):7631–7677
Smith DS, Eremin SA (2008) Fluorescence polarization immunoassays and related methods for simple, high-throughput screening of small molecules. Anal Bioanal Chem 391:1499–1507
Tijssen P (1985) In: Burdon RH, Knippenberg PH (eds) Practice and theory of enzyme immunoassays, vol 15. Elsevier, Amsterdam
Towbin H, Motz J, Oroszlan P, Zingel O (1995) Sandwich immunoassay for the hapten angiotensin. II: a novel assay principle based on antibodies against immune complexes. J Immunol Methods 181:167–176
Tsai JS-C, Lin GL (2005) Drug-testing technologies and applications. 3.1. Immunoassays. In: Wong RC, Tse HY (eds) Drugs of abuse: body fluid testing. Humana Press Inc., New Jersey, pp 35–41
Vree TB, Hekster YA, Tijhuis M W (1985) Metabolism of sulfonamides. In Vree TB Pharmacokinetics of sulfonamides revisited. Antibiot Chemother. Basel, Karger 34:5–65
Wang Z, Beier RC, Sheng Y, Zhang S, Jiang W, Wang Z, Wang J, Shen J (2013) Monoclonal antibodies with group specificity toward sulfonamides: selection of hapten and antibody selectivity. Anal Bioanal Chem 405:4027–4037
Wang S, Liu J, Yong W, Chen Q, Zhang L, Dong Y, Su H, Tan T (2015) A direct competitive assay-based aptasensor for sensitive determination of tetracycline residue in honey. Talanta 131:562–569
Yang S, Carlson K (2004) Routine monitoring of antibiotics in water and wastewater with a radioimmunoassay technique. Water Res 38:3155–3166
Zhang H, Duan Z, Wang L, Zhang Y, Wang S (2006) Hapten synthesis and development of polyclonal antibody-based multi-sulfonamide immunoassays. J Agric Food Chem 54:4499–4505
Acknowledgements
The authors would like to thank the National Natural Science Foundation of China (Grant No. 31372475) for financial support.
Funding
Zhanhui Wang declares that he got grant from the NSFC (No. 31372475).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Maksim Burkin declares that he has no conflict of interest.
Rinat Nuriev declares that he has no conflict of interest.
Zhanhui Wang declares that he has no conflict of interest.
Inna Galvidis declares that she has no conflict of interest.
Ethical Approval
This article does not contain any studies involving human participants and animals.
Informed Consent
Not applicable.
Electronic supplementary material
ESM 1
(DOC 10199 kb)
Rights and permissions
About this article
Cite this article
Burkin, M.A., Nuriev, R.I., Wang, Z. et al. Development of Sandwich Double-Competitive ELISA for Sulfonamides. Comparative Analytical Characteristics and Matrix Effect Resistance. Food Anal. Methods 11, 663–674 (2018). https://doi.org/10.1007/s12161-017-1036-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-1036-6