Abstract
The aim of this research was to study the application of MIR spectroscopy as an alternative to conventional methods to determine fat and protein content. Samples of the main species used to produce meat products were analyzed, showing all of them absorption bands at similar wavenumbers though with different intensity. Correlation analysis of absorption intensities showed that bands around 2925, 2854, and 1746 cm−1 are associated with fat content, whereas bands around 3288, 1657, and 1542 cm−1 are associated with proteins. During the validation process, prediction models of fat and protein content were successfully obtained with R 2 0.9173 and 0.7534, respectively. Finally, a good result (R 2 = 0.8829) was obtained on the estimation of the lipid content when the information at only one wavenumber was used.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food production demands high standards in quality assurance and process control. Consequently, appropriate analytical tools for food analysis both during and after production are required to satisfy this demand. Desirable features of such tools include speed, precision, reliability, selectivity, ease-of-use, minimal or no sample preparation, avoidance of sample destruction, and low cost (Karoui et al. 2010).
Protein and fat content are two parameters that are usually controlled during meat production. At present, the protein content of a sample can be measured by different chemical methods such as the Kjeldahl (which is the official standard (ISO 937:1978 1978)), Biuret, Lowry, or Bradford method. However, the chemical methods present several limitations, namely, time consumption and the production of a large amount of pollution residues during the main assay as well as during the required additional analyses. The fat content and the fatty acid profile can be measured by different chemical methods: Soxhlet extraction (ISO 1443:1973 1973), Babcock, Gerber, or gas chromatography. Nevertheless, these methods have several shortcomings, such as time consumption, sample preparation, and complex operations (Wu et al. 2008).
To overcome the limitations of classical methods of protein and fat content analysis, alternative methods to determine the chemical composition are searched and desired. Among these methods, the infrared spectroscopy techniques emerge as promising candidates, offering at the same time a high analysis speed and environmental sustainability as they do not use any hazardous substances for the environment (Wu et al. 2008). Near-infrared (NIR) spectroscopy is already widely used in processing operations to determine protein, fat, and other compounds (moisture, carbohydrates) content (Wold et al. 2011). However, the number of food products is still limited. For instance, the Foss Foodscan equipment, a specific meat analyzer, gives good results with little sample preparation. Nevertheless, NIR spectra are more complex, their signal is poorer, and they have a higher number of overlaps compared to the mid-infrared (MIR) ones (Sun 2009). However, this part of the spectrum is not commonly employed to determine the fat and protein content of meat samples.
The Fourier transform mid-infrared (FT-MIR) spectroscopy has already been used for the detection of microbiologically spoiled or contaminated meat products, for the authentication of the production method, specially the animals’ type of breeding, and for the detection of meat adulterations (Karoui et al. 2010). Al-Jowder et al. (1997) proposed to differentiate between raw minced chicken, pork, and turkey meats using principal component analysis (PCA). Later, the same authors succeeded in differentiating between muscle and offal tissue samples, as well as in detecting adulteration of raw and cooked beef containing 20% adulterants (Al-Jowder et al. 1999; Al-Jowder et al. 2002). In 1997, Rannou and Downey also performed a study where they tried to identify samples of pork, chicken, and turkey using NIR, MIR, and the combination of both spectral information. These authors suggested that the models produced by MIR data appear to be less accurate than those obtained from NIR spectra although the reduced number of principal components used by the former may suggest a potentially more robust model. Moreover, Ellis et al. (2002) explored the use of FT-MIR measurements to improve the accuracy and speed of the detection of microbial spoilage in meat. Their study involved comminuted chicken breasts, which were left to spoil at room temperature for 24 h. Each hour, the FT-MIR measurements were collected and the authors reported that absorbance peaks between 1500 and 700 cm−1 were positively correlated with spoilage, although no single peak was obvious. Currently, most investigations are focused on the development of a technique to detect very small amounts of adulterant. An example of this is the investigation developed by Meza-Marquez et al. (2012) in which detection of the adulteration of meat with clenbuterol was investigated.
The objective of the present study was to propose a new method based on MIR for simultaneous quantification of fat and protein content.
Materials and Methods
Sample Preparation
Samples with different protein and fat content belonging to several animal species (beef, chicken, cow, fighting bull, foal, hen, lamb, pork, rabbit, and turkey) were analyzed. The total number of samples employed was 28, and they were acquired in a local butcher’s shop from an EU-licensed commercial abattoir. Samples were first cleaned, removing the remaining skin and fat that could interfere in the analysis, and they were minced by a Solac Quick Mix (N202 of 500 W) mincer to homogenize them. After that, samples were divided in two fractions, one for the chemical composition analysis and the other one for the FT-MIR spectroscopy analysis, and kept frozen in the laboratory freezers at −20 °C until they were analyzed. Before the analysis, samples were gently thawed at 4 °C for 24 h.
The chemical composition of the meat samples under study was obtained using the official reference methods for total fat (ISO 1443:1973 1973), protein (ISO 937-1978), and moisture content (ISO 1442:1973 1973).
For the spectral analysis, the KBr-pressed-disc technique was used with 2.0 mg of sample dispersed in 198 mg of KBr. Due to the high percentage of moisture in meat and the high intensity absorbance of the bands that appeared with the vibration of the water bonds, samples were dried in order to be able to observe the specific bands from protein and lipids in more detail. The disks were dried in a furnace at 105 °C for at least 8 h. To verify that this drying time was enough, a blank sample of KBr was prepared and measured using the same procedure as the samples, checking that the absorbance of bands related to water was negligible. Each meat sample was analyzed in duplicate. Bovine serum albumin (96% purity, Sigma-Aldrich) and foal fat were also analyzed to obtain the specific bands of the protein and lipid compounds.
Spectra Acquisition
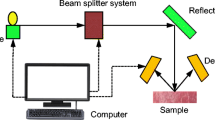
Fourier transform infrared (FTIR) spectra were acquired using a Bruker FTIR Vertex 80v spectrometer (Ettlingen, Germany). The spectrometer was equipped with a Globar source (operation bandwidth, 6000–50 cm−1), a beamsplitter of KBr (10,000–400 cm−1), and DLaTGS detector (10,000–250 cm−1). All measurements (backgrounds and samples) were done in vacuum conditions so as to avoid adsorption due to atmospheric gases. For each sample, 32 scans in the 4000–400 cm−1 spectral range were recorded with a resolution of 4 cm−1.
The reliance of the preparation of the samples with the KBr-pressed-disc technique and the drying process was checked by comparing the response of the couple of disks of each sample. The wavenumbers with higher absorption intensities were selected, and the standard deviation between the pair of absorption intensities was calculated.
Data Analysis
The chemical bonds associated with each peak of the FTIR spectra were determined by analyzing fat and protein samples, by correlations among the bands with larger intensity, and by comparing the wavenumbers with the literature (Table 2).
Then, the analysis was particularized to the fat and protein content of the samples and prediction models were built, using the results obtained by the standard methods (ISO 1443-1973 1973 and ISO 937-1978 1978) as reference values. The models were built using a specific program of chemometrics, OPUS Quant 2 (Bruker, Ettlingen, Germany). The calibration models were developed using the partial least square (PLS) regression method and validated by cross-validation. The main advantage of cross-validation is that a small number of samples are required because the same set of samples is used to calibrate and validate the method. Before starting the calibration, one sample is excluded from the entity of samples and the remaining samples are used to calibrate the system. Once the model is built, it is tested using the excluded sample. Then, the cycle is repeated separating a different sample until all samples have been used for validation once. The optimum number of factors in the PLS calibration models was indicated by the lowest number that gave the minimum value of the root mean square error (RMSE) in cross-validation, in order to avoid overfitting of the models. In each variable analyzed, the range of wavenumbers with more information in their absorbance and the best pretreatment method were selected using the OPUS Quant 2 program. These models enable a quantitative estimation of the fat and protein content of the different samples depending on the absorbance intensity.
Finally, to simplify the prediction models, the possibility of a more direct way of obtaining the protein and lipid content of the samples was studied. For that reason, the absorption values at four different wavenumbers were selected: 2925 and 1744 cm−1 related to the fat content and 1657 and 1542 cm−1 related to the protein content. The selection was performed based on the literature (Table 2) and on the information obtained from analyzing fat and protein samples. In all cases, the spectral information was not preprocessed and the prediction models built were cross-validated.
Results and Discussion
Chemical Composition of the Samples
Table 1 includes average values, standard deviation, as well as highest and lowest value for fat, protein, and moisture content of the samples. The data was sorted out following an increasing fat content. The average fat content varied from a minimum value of 1.10% for hen to a maximum of 9.60% for cow. The fat content showed more variability than moisture or protein content, both for a given animal or among animals. These differences can be explained by several factors, including gender, age at slaughtering, or the type of breeding and feeding that the animal had followed (De Smet et al. 2004). Protein content ranged from 18.30% for lamb to 23.60% for foal, being 3.2% the highest difference in the protein concentration within the same type of animal. Moisture content varied from 69.40% for cow meat to 75.10% for turkey, and the largest difference between samples of the same specie was found in lamb meat (7.8%).
Assignment of the Representative Bands of the Meat FTIR Spectra
Figure 1 shows the average spectra of the samples analyzed. All have absorption bands at similar wavenumbers but different intensity, which denotes the same components but different concentration. The FTIR spectra can be divided in two parts. The first one, between 3750 and 2750 cm−1, corresponds to the vibrational resonances of bonds that contain hydrogen atoms, such as C-H, O-H, and N-H. The range between 1900 and 400 cm−1 is associated with stretching vibrations of C=C, C=O, C-C, C-N, C-O, and C-O; bending and twisting resonance modes; and it also includes the fingerprint region from 1500 to 400 cm−1 (Al-Jowder et al. 1999; Karoui et al. 2010). The assignment of representative bands (Table 2) was performed by analyzing fat and protein samples, correlation matrixes, and taking into account literature (Pretsch et al. 2000; Skoog et al. 2008).
Figure 2 shows the FTIR spectra of the fat (foal fat) and protein (bovine serum album) samples that have been normalized to the maximum in each case to facilitate the comparison. The lipid spectrum from the foal fat sample confirmed that bands at 2925, 2854, and 1746 cm−1 are associated with fats. In the protein spectrum, high absorption intensities appear at 3288, 1657, and 1542 cm−1. In contrast, this figure shows that the normalized absorption spectrum of beef is more complex than those for foal fat and bovine serum album. The beef spectrum had peaks corresponding to both protein and lipid characteristic bond vibration modes, as expected.
In addition, the peaks between 3500 and 3000 cm−1 can be associated with the proteins since they do not appear in the lipid spectrum. In a similar way, the peaks between 3000 and 1746 cm−1 can be associated with the lipids because they do not appear in the protein spectrum. Finally, the peaks around 1657 and 1542 cm−1 can be associated with the proteins as they do not appear in the lipid spectrum. The rest of spectral peaks appear both in the lipid and protein absorption spectra, so they cannot be definitely identified either with proteins or lipids.
To confirm these relationships, a correlation matrix analysis using the absorbance at the relevant wavenumbers was performed to differentiate them (Table 3). The level of significance was set at 0.05 for all the correlations performed. The absorbance ∼3288 cm−1 was positively related to the peaks ∼1657 cm−1 (0.97) and ∼1542 cm−1 (0.99). This correlation agrees with the results obtained in the spectra analysis. Previous studies showed that the broadband of moderate intensity that appears around 3288 cm−1 is associated with the stretching vibration of N-H bonds typical from the protein amino acids. If samples are not dried, the N-H bond band can be masked by the water hydroxyl (O-H) bond vibration. Moreover, the band ∼1657 cm−1 is related to the N-H bond vibration of protein amino groups and the alkenes double-bond (C=C) stretching vibration. The band at 1542 cm−1 is associated with the amides II, a mixture of N-H bond bending and C-H bond stretching vibration (Carbonaro and Nucara 2010; Hernández-Martínez 2013; Meza-Márquez 2010; Papadopoulou 2011; Rohman 2011; Shiroma and Rodriguez-Solona 2009; Wu et al. 2008).
The absorbance ∼2925 cm−1 was correlated with the signals present ∼2854 cm−1 (0.96), 1746 cm−1 (0.96), and 1162 cm−1 (0.96). According to several authors, these wavenumbers are related to typical vibration modes of the lipids fatty acids. The signals at ∼2925 and ∼2854 cm−1 correspond to the symmetric and asymmetric stretching vibration of C-H bonds, respectively. These bonds are typical of the methyl (CH3) and methylene (CH2) groups in proteins and fatty acids. And the narrow peak ∼1746 cm−1 is associated with the stretching vibration of carbonyl bond of esters and free fatty acids (Guillen 2000; Hernández-Martínez 2013; Ordoudi et al. 2014; Papadopoulou 2011; Rohman 2011; Shiroma and Rodriguez-Solona 2009).
This correlation matrix (Table 3) confirms the existence of different isolated vibration modes that enable the identification of the lipid and protein molecules of the meat samples. Also, a new investigation line can be developed with a possible quantification of these substances in a faster way.
However, even though the rest of bands in the range have minor absorption intensities than those described above and cannot be associated either to the protein or the lipid content of the samples, they provide key information. For example, the different bands which appear around 3000 cm−1 are related to different C-H bond vibration modes. The low-intensity signal at 3006 cm−1 is associated with the C-H bond vibration of the unsaturated fatty acids cis double-bond (C=CH). The peak ∼1465 cm−1 is associated with scissoring bending vibration mode of C-H bonds. The band at ∼1379 cm−1 is related to the fatty acids carboxyl group (COO-) and the amino acid side chains. The band at 1239 cm−1 is related to two different functional groups: the C-N bond stretching vibration and the N-H bond bending vibration. In a similar way, the peak around 1162 cm−1 is associated with the C-O bond stretching vibration and the C-H bond bending vibration. The peak at 1117 cm−1 is assigned to bending and twisting vibration of the fatty acids.
Several closely packed peaks of low absorption intensity appeared between 1000 and 400 cm−1. From all of them, the peak at ∼721 cm−1 is highlighted because of its higher intensity. This peak is caused by the overlapping of the methylene (-CH2) rocking vibration and the out of plane vibration of cis-disubstituted olefins. These bonds that are typical from the alkenes have a cis conformation.
Precision of the Spectral Measurements
As it has been stated in the “Materials and Methods” section, two different disks of each sample were prepared and measured to validate the KBr-press-disc method. Figure 3 shows the two spectra of the same lamb sample, and in Table 4, the relative standard deviation of the higher absorption intensities are compared. As it can be observed on this table, a relative standard deviation of absorbance between 0.13% at ∼1116 cm−1 and 4.80% at ∼1656 cm−1 was obtained, evidencing the high repeatability of the technique.
Estimation of the Fat and Protein Content of the Samples: Calibration and Validation Results
Regression models were built to estimate the fat and protein content of the samples analyzed from the spectral information. A summary of the results obtained in each model is shown in Table 5, and Fig. 4 shows the correlation between the values determined by reference analysis and the values predicted by MIR spectroscopy for the lipid and protein content.
In the fat model, a multiplicative scatter correction of the spectral information was chosen during the optimization process, and the following wavenumbers related with the fat content of the samples were selected: 3000–2800, 2300–1850, and 981–489 cm−1. In this model, it was necessary to employ 11 factors, obtaining in the validation process a determination coefficient (R 2) of 91.73% and a root mean square error of cross-validation (RMSECV) of 0.711. As the R 2 value was greater than 90%, the model can be used to predict the fat content of meat and meat products.
Regarding the protein model, the wavenumbers between 3200–2999 and 1951–1850 cm−1 were selected to obtain the regression line due to its relation with the protein content of the samples. Also, by using the optimizer, it was determined that it was necessary to obtain the first derivate of the different samples spectra. Using nine factors, a 75.34% determination coefficient (R 2) was obtained and the root mean square error was 0.934.
Figure 5 shows the results obtained for the prediction models when only the absorption intensity at one wavenumber was used. For the fat content, the determination coefficient (R 2) obtained during the cross-validation process with the first absorption intensity (2925 cm−1) was 88.29%. In the second case (1744 cm−1), this coefficient was 83.44%. In both cases, the results were not as good as the ones obtained with Opus Quant 2 (Bruker, Ettlingen, Germany). However, the R 2 coefficients were high enough to consider the model accurate to predict the fat content of the samples.
A similar analysis was performed with absorption intensities at wavenumbers typical from the proteins bonds (1657 and 1542 cm−1). However, the determination coefficients were not high enough to consider the method accurate. The absence of a correlation can be attributed to the effect of the protein environment on the amide bands because the hydrogen bonds are sensitive to protein concentration and ionic strength of the media (Etzion et al. 2004).
Conclusion
The results of this research show that FT-MIR is a suitable technique to estimate the fat content of meat samples. Moreover, using the information of the absorption intensity at only one wavenumber, the lipid content of the samples can also be estimated with good accuracy using a simplified method. However, the results obtained for the protein content are less accurate because it ranges less than the lipids contents in the different animal species. Nevertheless, both prediction models can be improved with the analysis of an increase number of samples.
References
Alamprese C, Casale M, Sinelli N, Lanteri S, Casiraghi E (2013) Detection of minced beef adulteration with turkey meat by UV-vis, NIR and MIR spectroscopy. Lwt-Food Sci Technol 53:225–232
Al-Jowder O, Kemsley EK, Wilson RH (1997) Mid-infrared spectroscopy and authenticity problems in selected meats: a feasibility study. Food Chem 59:195–201
Al-Jowder O, Defernez M, Kemsley EK, Wilson RH (1999) Mid-infrared spectroscopy and chemometrics for the authentication of meat products. J Agric Food Chem 47:3210–3218
Al-Jowder O, Kemsley EK, Wilson RH (2002) Detection of adulteration in cooked meat products by mid-infrared spectroscopy. J Agric Food Chem 50:1325–1329
Arce L (2009) Feasibility study on the use of infrared spectroscopy for the direct authentication of Iberian pig fattening diet. Anal Chim Acta 636:183–189
Carbonaro M, Nucara A (2010) Secondary structure of food proteins by Fourier transform spectroscopy in the mid-infrared region. Amino Acids 38:679–690
De Smet S, Raes K, Demeyer D (2004) Meat fatty acid composition as affected by fatness and genetic factors: a review. Anim Res 53:81–98
Ellis DI, Broadhurst D, Kell DB, Rowland JJ, Goodacre R (2002) Rapid and quantitative detection of the microbial spoilage of meat by Fourier transform infrared spectroscopy and machine learning. Appl Environ Microbiol 68:2822–2828
Etzion Y, Linker R, Cogan U, Shmulevich I (2004) Determination of protein concentration in raw milk by mid-infrared Fourier transform infrared/attenuated total reflectance spectroscopy. J Dairy Sci 87:2779–2788
Guillen MD (2000) Some of the most significant changes in the Fourier transform infrared spectra of edible oils under oxidative conditions. J Sci Food Agric 80:2028
Hernández-Martínez M (2013) Prediction of total fat, fatty acid composition and nutritional parameters in fish fillets using MID-FTIR spectroscopy and chemometrics. LWT Food Sci Technol 52:12–20
ISO 1442:1973 (1973) Meat and meat products—determination of moisture content
ISO 1443:1973 (1973) Meat and meat products—determination of total fat content
ISO 937:1978 (1978) Meat and meat products—determination of nitrogen content (Reference method)
Karoui R, Downey G, Blecker C (2010) Mid-infrared spectroscopy coupled with chemometrics: a tool for the analysis of intact food systems and the exploration of their molecular structure-quality relationships—a review. Chem Rev 110:6144–6168
Meza-Márquez OG (2010) Application of mid-infrared spectroscopy with multivariate analysis and soft independent modeling of class analogies (SIMCA) for the detection of adulterants in minced beef. Meat Sci 86:511–519
Meza-Marquez OG, Gallardo-Velazquez T, Osorio-Revilla G, Dorantes-Alvarez L (2012) Detection of clenbuterol in beef meat, liver and kidney by mid-infrared spectroscopy (FT-mid IR) and multivariate analysis. Int J Food Sci Technol 47:2342–2351
Ordoudi SA, de los Mozos Pascual M, Tsimidou MZ (2014) On the quality control of traded saffron by means of transmission Fourier-transform mid-infrared (FT-MIR) spectroscopy and chemometrics. Food Chem 150:414–421
Papadopoulou O (2011) Contribution of Fourier transform infrared (FTIR) spectroscopy data on the quantitative determination of minced pork meat spoilage. Food Res Int 44:3264–3271
Pretsch E, Bühlmann P, Affolter C (2000). Structure determination of organic compounds: tables of spectral data. Springer
Rannou H, Downey G (1997) Discrimination of raw pork, chicken and turkey meat by spectroscopy in the visible, near- and mid-infrared ranges. Anal Commun 34:401–404
Rohman A (2011) Analysis of pork adulteration in beef meatball using Fourier transform infrared (FTIR) spectroscopy. Meat Sci 88:91–95
Shiroma C, Rodriguez-Solona L (2009) Application of NIR and MIR spectroscopy in quality control of potato chips. J Food Compos Anal 22:596–605
Skoog DA, Crouch SR, Holler FJ, Anzures MB (2008) Principles of instrumental analysis. Cengage Learning Latin America
Sun DW (2009) Infrared spectroscopy for food quality analysis and control. Elsevier Science
Wold JP, O’Farrell M, Høy M, Tschudi J (2011) On-line determination and control of fat content in batches of beef trimmings by NIR imaging spectroscopy. Meat Sci 89:317–324
Wu D, Feng SJ, He C, He Y (2008) NIRS and MIRS technique for the determination of protein and fat content in milk powder - art. no. 66251S. International Symposium on Photoelectronic Detection and Imaging 2007. Related Technologies and Applications 6625:S6251–S6251
Wu D, He Y, Shi J, Feng S (2009) Exploring near and midinfrared spectroscopy to predict trace iron and zinc contents in powdered milk. J Agric Food Chem 57:1697–1704
Acknowledgements
In memoriam of our beloved friend Mario Sorolla Ayza.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Spanish Government through the Consolider Engineering Metamaterials under Contract CSD2008-00066 and Contract TEC2011-28664-C02. The FTIR spectrometer was financed by the Spanish Ministry of Economy and Competitiveness in the frame of the project CEI10-2-2005. M. Beruete acknowledges funding by the Spanish Government under the research contract program Ramón y Cajal RYC-2011-08221.
Conflict of Interest
M. Lozano declares that she has no conflict of interest. P. Rodríguez-Ulibarri declares that he has no conflict of interest. J. C. Echeverría declares that he has no conflict of interest. M. Beruete declares that he has no conflict of interest. M. Sorolla declares that he has no conflict of interest. M. J. Beriain declares that she has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Lozano, M., Rodríguez-Ulibarri, P., Echeverría, J.C. et al. Mid-Infrared Spectroscopy (MIR) for Simultaneous Determination of Fat and Protein Content in Meat of Several Animal Species. Food Anal. Methods 10, 3462–3470 (2017). https://doi.org/10.1007/s12161-017-0879-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-0879-1