Abstract
A novel two-dimensional hydrophilic interaction chromatography/reversed phase liquid chromatography–tandem mass spectrometry system was developed to analyze seven categories of antibiotics residues in dairy products. The seven categories were β-lactams, tetracyclines, macrolides, aminoglycosides, amphenicols, quinolones, and sulphonamides, and involved 20 antibiotics. Samples of milk powder or milk samples were extracted by mixed matrix solid phase dispersion using C18 and CN material. The analytes were eluted by acetonitrile and water, and were dried with rotary evaporation. Finally, the residues were dissolved with mobile phase and then analyzed. Under sample pretreatment conditions, chromatographic and mass spectrometric parameters were optimized. Twenty analytes showed good linearities with correlation coefficients of 0.9945 ~ 0.9998. The limits of detection and quantification for milk powder and milk samples were 0.10 ~ 2.40 and 0.33 ~ 7.92 μg/kg, respectively. The proposed method has been applied to the determination of antibiotics residues in milk powder and milk samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antibiotics are widely used against bacteria, fungi, spirochetes, mycoplasma, chlamydia, and other pathogenic microorganisms. At present, antibiotics are generally divided into eight categories: β-lactams, tetracyclines, macrolides, aminoglycosides (AGs), amphenicols, quinolones, sulfonamides, and polypeptides (Kennedy et al. 1998).
As the most common veterinary drugs, different kinds of antibiotics were often mixed and then used to prevent and treat diseases or to promote growth in animal husbandry (Gentili et al. 2005). Therefore, it is particularly important to establish a method that can analyze multi-antibiotic residues simultaneously, rapidly, and accurately. At present, a number of researches have been conducted in this field. For instance, Aguilera-Luiz had reported an analysis method for multi-residue determination of macrolides, quinolones, tetracyclines, and sulfonamides in milk by ultra-high-pressure liquid chromatography–tandem mass spectrometry (Aguilera-Luiz et al. 2008). Carretero et al. (2008) and Chico et al. (2008) applied liquid chromatography–tandem mass spectrometry to detect β-lactams, macrolides, quinolones, and tetracyclines in pork or chicken, respectively. Granelli detected 19 antibiotics simultaneously in muscle and kidney samples using a method that involved five species of antibiotics and could also measure more types of antibiotics (Granelli et al. 2009; Granelli and Branzell 2007).
Among the eight species of antibiotics, AGs have the strongest polarity and lack chromophores, which makes the analysis very difficult when using traditional reversed phase liquid chromatography (RPLC) with UV detection. Researchers have solved this problem by applying ion-pair reagents and derivatization agents for detection. Nevertheless, the derivatization steps make the analytical process more time-consuming and quantitative analysis problematic (van Holthoon et al. 2009; Gong et al. 2012). Hydrophilic interaction chromatography (HILIC), combined with mass spectrometry (MS), has been receiving great attention for AG analysis as there is no need for derivatization or addition of ion-pairing reagents. Another reason for the increasing popularity of HILIC is its excellent suitability for coupling to MS (Kahsay et al. 2014). Like normal phase liquid chromatography, HILIC employs conventional polar stationary phases such as silica, amino, or cyano, but the mobile phase used is similar to those employed in the RPLC mode.
Although AGs can be detected by HILIC-MS, it is a new challenge to detect AGs and other antibiotics simultaneously for the huge differences among their polarities. To separate antibiotic mixture including a huge range of polarities, we employed two-dimensional hydrophilic interaction chromatography/reversed phase liquid chromatography–tandem mass spectrometry (2D-HILIC/RP-MS/MS), which has been tried by some researchers (Jandera et al. 2012) but never been employed to analyze AGs and other antibiotics simultaneously. In the present study, we established a 2D-HILIC/RP-MS/MS system via the double-three mobile phase channels and added a pump whose flow rate can be variable. This system is coupled with mass spectrometry to analyze 20 antibiotics of 7 classes, which include 7 aminoglycoside antibiotics; 2 penicillin and cephalosporin of β-lactams, respectively; 1 amphenicols; and 2 tetracyclines, macrolides, quinolones, and sulfonamides, respectively. All these substances can be separated and detected simultaneously. Sample preparation employed mixed matrix solid phase dispersion extraction technology involving the use of C18 and CN particles. The reported method is suitable for rapid screening of antibiotics in dairy products and the high-throughput detection of multi-antibiotic residues. This method is simple and rapid, producing accurate and sensitive detection results by mass spectrometry.
Experimental
Instruments and Reagents
UltiMate 3000 high-performance liquid chromatography with DGP-3600SD pump, one ten-port valve and one six-port valve (ThermoFisher, USA); AXP auxiliary pump (ThermoFisher, USA); 3200 Qtrap mass spectrometer (AB Sciex, USA); R-200 rotary evaporator (BÜCHI, Switzerland); Vortex genius3 mixer (IKA, Germany); 2034 vacuum pump (Welch, USA); Visiprep™ DL solid phase extraction system (Sepulco, USA); 6-ml empty SPE cartridge and SPE frits (Agilent, USA); Chromatorex C18 bonded silica sorbent (40 ~ 75 μm, Fuji, Japan); and Cleanert CN/SCX/silica sorbent (the average particle size is 45 μm, Agela Technologies, USA) were used.
Ceftiofur, ampicillin, cloxacillin, spectinomycin, streptomycin, dihydrostreptomycin, gentamicin C1a, amikacin, kanamycin, enrofloxacin, ciprofloxacin, tilmicosin, josamycin, doxycycline, oxytetracycline, and chloramphenicol were purchased from the Dr. Ehrenstorfer GmbH (Germany); cefalexin was purchased from Fluka (USA); sulfamethoxypyridazine and sulfachloropyridazine were purchased from Sigma (USA); and isepamicin was purchased from Shifeng Biological Technology (China). Acetonitrile and methanol (HPLC grade) were purchased from Dikma (China). Ammonium acetate and formate (HPLC grade) were purchased from Dikma (China). Ultra-pure water was used in the present study. Oxalic acid and Na2EDTA were of analytical reagent grade. Stock solutions of the standards were prepared in methanol at a concentration of 1.0 mg/ml. The prepared stock solutions were stored at −20 °C, and under this condition, cephalexin and ampicillin can be preserved for 14 days, while other antibiotics can be preserved for 3 months.
Sample Preparation
0.50 g milk or milk powder was weighed in a mortar, and the mixed sorbent which contained 0.50 g C18, 0.50 g CN particles, and 1.00 g Na2EDTA were added. Afterwards, all these were mixed well by a pestle in the mortar, and the mixture was transferred to a pre-fritted 6-ml empty SPE cartridge whose bottom was performed in a solid-phase extraction system model Visiprep DL. The sorbent was pressed by a syringe plunger until no obvious gap was observed. In order to ensure that the hydrophilic and lipophilic antibiotics can be completely eluted, the cartridge was eluted at a flow rate within 3 ml/min by 2.5 ml acetonitrile, 2.5 ml water, 2.5 ml acetonitrile, and 2.5 ml water successively. After that, the eluent was collected and rotary-evaporated to near dryness at 40 ± 1 °C, adding 20-mmol/l ammonium acetate + acetonitrile + formic acid (5 + 5 + 1) solution to 1.0 ml. It was passed through 0.2-μm hydrophilic filter immediately after vortex blending for about 1.0 min.
LC-MS/MS Analysis
Experimental Conditions of Liquid Chromatography
0.2% formic acid, acetonitrile, and 20 mmol/l oxalic acid were used as mobile phases A, B, and C of pump 2, respectively; 20-mmol/l ammonium acetate solution with 0.4% formic acid, acetonitrile with 0.4% formic acid, and water were used as mobile phases A, B, and C of pump 1, respectively. Water was used as mobile phase of AXP pump, with different rates of 0.50 ml/min between 0.70 and 1.30 min and 0.20 ml/min between 6.50 and 11.00 min. Chromatographic column 1: Inspie HILIC (100 × 2.1 mm, 3 μm, Dikma, China). Chromatographic column 2: Spursil C18-EP (100 × 2.1 mm, 3 μm, Dikma, China). Chromatographic column T: Oasis HLB (20 × 2.1 mm, 25 μm, Waters, USA). The column temperature was set at 30 °C, and the injection volume was 10 μl. Ninety percent acetonitrile with 10% water solution were employed as washing liquid of needle. Valve switching and mobile phase gradient were detailed in Tables 1
, 2
, and 3
.
Parameters of Mass Spectrometry
Detection was implemented by electrospray ionization in positive mode with a ion source voltage of 3500 V, interface temperature of 550 °C, nitrogen curtain and collision gas flow rates of 25.00 and 5.00 psi, and nebulized gas and auxiliary heating gas flow rates of 50.00 and 50.00 psi, respectively. The voltage of detector was set at 2000 V. The ionization mode was electrospray ionization (ESI) positive. The parameters for determination of the 20 antibiotics were detailed in Table 4
.
Under the optimized liquid chromatographic and mass spectrometric conditions described above, the multiple-reaction monitoring (MRM) chromatogram of blank milk sample spiked the mixed standard (the concentration of each compound was 20 ng ml−1) (Fig. 1
MRM chromatogram of blank milk sample spiked the mixed standard for the 20 antibiotics. 1 sulfamethoxypyridazine, 2 sulfachloropyridazine, 3 chloramphenicol, 4 cloxacillin, 5 ceftiofur, 6 enrofloxacin, 7 ciprofloxacin, 8 ampicillin, 9 doxycycline, 10 cefalexin, 11 oxytetracycline, 12 josamycin, 13 tilmicosin, 14 spectinomycin, 15 streptomycin, 16 dihydrostreptomycin, 17 gentamicin C1a, 18 amikacin, 19 kanamycin, 20 isepamicin
).
Results and Discussion
Optimization of Sample Preparation
Matrix solid phase dispersion (MSPD) technology, simple, rapid, and high-efficient, is suitable to be applied in the sample preparation of veterinary drug multi-residue detection in animal-originated food (Wang et al. 2011). In view of the huge differences among their polarities of the seven classes of antibiotics, a new technique, mixed matrix solid phase dispersion (MMSPD), was proposed; namely, 0.5 g CN particles, 0.5 g particle C18, and 1.0 g Na2EDTA were mixed to compose the extracted material.
Three kinds of polar particles—silica, SCX, and CN which were all 45 μm in the average particle size—were mixed with 0.5 g C18 particle and 1.0 g Na2EDTA, respectively, and were compared in the extraction efficiency of AGs. The extraction efficiency was measured by the recoveries of the milk powder and milk samples. Results showed that the seven AGs had hardly been extracted or eluted when SCX or silica particle was employed, while the approving recoveries could be obtained when CN particle was applied, with 81.3% (streptomycin)—98.3% (ciprofloxacin) of the milk powder sample and 78.4% (streptomycin)—97.0% (chloramphenicol) of the milk powder. The result may be related to the CN particle in which the silica is bonded with cyanopropyl because it possesses normal and reversed phase retention function simultaneously. When the analyte was dissolved in non-polar solvent and eluted by polar solvent, the CN particle showed its normal phase effect with strong retention to polar compounds. Both silica and SCX have strong retention to highly polar compounds, and the analytes were hardly eluted.
Selection of HILIC
The family of HILIC stationary phases with various support materials and surface chemistries has been continuously enlarged, to address specific separation problems. The basic types of HILIC stationary phases include plain silica (Grumbach et al. 2008; Fountain et al. 2010), hybrid particle (Nováková et al. 2010; Chauve et al. 2010), amide (Liu et al. 2008a, b; Nguyen et al. 2010), amino (Jandera et al. 2010), diol (Hemström and Irgum 2006), and zwitterionic (Goucher et al. 2010) stationary phases. Unmodified bare silica gel column and hybrid particle-column, with the similar retention mechanism, have been used to separate alkaline polar substances (Dejaegher and Vander Heyden 2010). The mobile phase of the two kinds of column consists of organic solvents and water mixture which produces silica-OH groups and negatively charged acidic surface. For the solubility of unmodified bare silica gel groups in high pH solution, the application of hybrid particle-column is more extensive than that of unmodified bare silica column. Aminopropyl-silica stationary phases have been available to the analysis of anionic polar analytes and carbohydrate substances (Jandera 2008) because of the positive electricity on its surface. Because the amide-silica column has acceptor sites and donor sites for hydrogen atoms, it has been employed to retain acidic analytes and separate sugars and other carbohydrates (Nguyen et al. 2010). The retention mechanism of diol-silica group is related to the distribution of analytes between mobile phase and the adsorbed diffuse water layer at the surface of a polar stationary phase in highly organic environment, the –OH groups of stationary phase and the hydrogen bonding between solutions (Hemström and Irgum 2006). The retention effect of diol-silica stationary phase to ionic compounds is weaker for that there is hardly any ionic reaction between analytes and the HO-silica group (Wu et al. 2008). Zwitterionic stationary phase, with strong base (quaternary ammonium) and acid (sulfonic acid) groups of molar ratio 1:1, has retention ability to acidic and basic polar compounds theoretically. For this reason, it has been applied extensively, and its application in AG separation has been reported (Buszewski and Noga 2012; Kumar et al. 2012).
In our study, we used Atlantis HILIC-silica (100 × 2.1 mm, μm, Waters), ZIC-HILIC (50 × 2.1 mm, 3.5 μm, Merck), and Inspie HILIC (100 × 2.1 mm, 3 μm, Dikma) to analyze basic polar compounds—AGs, respectively. By comparison, the Inspie HILIC can separate the seven AGs better. The poor analysis result of ZIC-HILIC may be related to the positively and negatively charged functional groups which make its total net charge closed to zero. This also explains why the changes in pH of mobile phase have little influence on separation. Given the above theories, the separation effect of ZIC-HILIC may be inferior to that of hybrid particle-column when positively charged basic polar compounds were analyzed.
Selection of Separation Mode
There are reports about the separation orthogonality of HILIC and RP (Liu et al. 2008a, b). Among the 20 antibiotics in the present study, 5 antibiotics were not retained at HILIC column at all, 7 AGs and tilmicosin could not be separated by C18 column, while other 7 antibiotics among them could be retained at the two columns, and the influences of the composition of mobile phase and the changes in AXP pump flow rate at the 7 antibiotics were different from each other.
The 20 antibiotics were divided into three groups (Table 4). Group 1 consisted of five antibiotics that were not retained at HILIC column at all, group 2 consisted of seven antibiotics that were retained both at HILIC column and C18 column, and group 3 consisted of seven AGs and tilmicosin that were not retained at C18 column at all. All these antibiotics were detected at different phases. As condition A of Fig. 2 shows, after injection, compounds in the groups 2 and 3 were retained at HILIC column while substances in the group 1 passed through HILIC column within the dead time of 0.70 min. During 0.70–1.30 min, 0.5 ml/min of water was provided by AXP pump to dilute the high proportion of organic mobile phase. Afterwards, group 1 antibiotics were retained at column T. During 1.30–6.50 min (condition B), group 1 antibiotics were carried to C18 column by pump 2 mobile phase; after separation at C18 column, they were detected by mass spectrometry. During 6.50–11.00 min (condition A) and 11.01–14.00 min (condition B), the same method was applied to separate and detected group 2 compounds with the flow rate of AXP pump being 0.20 ml/min. During 14.00–20.00 min (condition C), group 3 antibiotics that were retained at HILIC column were eluted to mass spectrometry detector by pump 1 mobile phase
.
Optimization of Mobile Phase
Mobile Phase of Pump 2
To separate efficiently and obtain sensitive mass spectrometry signal, 0.2% formic acid and acetonitrile were applied as mobile phase of group 1 antibiotics. However, oxytetracycline and doxycycline of tetracycline in group 2 compounds showed the tailing peak at the process of analysis since the two-keto structure that was prone to interact with silica-OH. Some researches reported that this condition can be improved by adding chelators like oxalic acid (Nakazawa et al. 1999), succinic acid (Cristofani et al. 2009), and citric acid (Zurhelle et al. 2000) in mobile phase. In the present study, the peaks of doxycycline and oxytetracycline were improved greatly by adding oxalic acid in mobile phase, but the high concentration of oxalic acid would influence the separation and peak shape of other substances. During 11.21–12.70 min, 5% acetonitrile with 95% 20-mmol/l oxalic acid solution was employed as mobile phase; afterwards, acetonitrile and 0.2% formic acid was applied in gradient. Under these conditions, the peak shape of group 2 substances was improved.
Mobile Phase of Pump 1
We observed that a high proportion of the organic phase (95 or 90%) can elute groups 1 and 2 antibiotics to column T efficiently, while group 3 compounds were not eluted from HILIC column. This result may be related to the weak polarity of groups 1 and 2 antibiotics and the strong retention of HILIC column to the polar substances.
Optimization of AXP Pump
The starting time of AXP pump and its flow rate could influence the separation of target antibiotics to some extent. If it started too early or the flow rate was too rapid, the backpressure generated thereof would then influence negatively the separation of HILIC column through the three-way valve (T-piece), with group 1 antibiotics not being eluted from T-piece to column T successfully. Four flow rates of AXP pump 0.00, 0.25, 0.50, and 0.75 ml/min were compared, with the dead time of system being considered; this result showed that AXP was opened at 0.71 min at the flow rate of 0.50-ml/min flow.
The AXP conditions on analysis of group 2 antibiotics were optimized by the same procedure. It showed that group 2 antibiotics could be separated efficiently with AXP working time 6.50–11.00 min and 0.20-ml/min flow rate.
The multiple-reaction monitoring information of several typical antibiotics was given in Fig. 3
MRM chromatogram of standard applying solution for several typical antibiotics. a Sulfachloropyridazine with 20 ng/ml; b chloramphenicol with 20 ng/ml; c cloxacillin with 150 ng/ml; d ciprofloxacin with 150 ng/ml; e ampicillin with 15 ng/ml; f doxycycline with 1.0 ng/ml; g tilmicosin with 50 ng/ml; h spectinomycin with 30 ng/ml
.
Validation
Method linearity was assayed by performing calibration curves using blank milk powder samples spiked (matrix-matched calibration) with 1.0 ml target antibiotics in the range from 0.2, 0.5, 2.0, 5.0, 20 to 100 ng/ml, respectively. After sample preparation, LC separation, and MS detection under the optimum conditions, linear regressions were determined by the relation between concentration of target antibiotics and peak area of quantification ion. LODs and limits of quantification (LOQs) were calculated using the lowest concentrations of the analytes for which quantitative ion signal-to-noise ratios were 3 and 10, respectively (Table 5
).
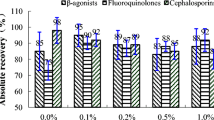
The average recoveries and relative standard deviation were performed at three levels (0.5, 1.0, and 1.5 MRL), by the blank milk powder or milk sample being spiked with 25, 50, and 75 μg/kg of each antibiotic, respectively. The MRL value was determined according to that of cephalosporin antibiotic 50 μg/kg because its MRL value was the minimum among the 20 antibiotics. The average recoveries and relative standard deviation of the 20 antibiotics were detailed in Table 6
.
At the level of 25 μg/kg, average recoveries of the milk powder and milk sample were 72.5% (doxycycline)—97.2% (ciprofloxacin) and 70.1% (doxycycline)—96.8% (ciprofloxacin), and relative standard deviation was 4.2% (tilmicosin)—8.8% (tetracycline) and 3.7% (tilmicosin)—9.9% (tetracycline), respectively. At the level of 50 μg/kg, average recoveries of the milk powder and milk sample were 77.0% (doxycycline)—102.1% (ciprofloxacin) and 76.4% (tetracycline)—98.9% (ciprofloxacin), and relative standard deviation was 2.8% (kanamycin)—6.9% (tetracycline) and 4.0% (chloramphenicol)—7.8% (tetracycline), respectively. At the level of 75 μg/kg, average recoveries of the milk powder and milk sample was 82.4% (tetracycline)—104.7% (enrofloxacin) and 79.0% (tetracycline)—105.8% (ciprofloxacin), and relative standard deviation was 3.6% (chloramphenicol)—7.2% (tetracycline) and 4.2% (streptomycin)—8.8% (doxycycline), respectively.
Method Application
Five milk powder samples, five milk samples, and five raw milk samples (extruded from the breast of cows without any treatment) were bought from different supermarkets in the city of Chengdu (China); 37.6 μg/kg ampicillin, below the EU MRL standard of 50 μg/kg, was detected in one of the raw milk samples. The total ion current chromatogram and the product ion chromatogram of positive sample were shown in Fig. 4
.
References
Aguilera-Luiz MM, Vidal JL, Romero-González R, Frenich AG (2008) Multi-residue determination of veterinary drugs in milk by ultra-high-pressure liquid chromatography-tandem mass spectrometry. J Chromatogr A 1205:10–16
Buszewski B, Noga S (2012) Hydrophilic interaction liquid chromatography (HILIC)—a powerful separation technique. Anal Bioanal Chem 402:231–247
Carretero V, Blasco C, Picó Y (2008) Multi-class determination of antimicrobials in meat by pressurized liquid extraction and liquid chromatography-tandem mass spectrometry. J Chromatogr A 1209:162–173
Chauve B, Guillarme D, Cléon P, Veuthey JL (2010) Evaluation of various HILIC materials for the fast separation of polar compounds. J Sep Sci 33:752–764
Chico J, Rúbies A, Centrich F, Companyó R, Prat MD, Granados M (2008) High-throughput multiclass method for antibiotic residue analysis by liquid chromatography-tandem mass spectrometry. J Chromatogr A 1213:189–199
Cristofani E, Antonini C, Tovo G, Fioroni L, Piersanti A, Galarini R (2009) A confirmatory method for the determination of tetracyclines in muscle using high-performance liquid chromatography with diode-array detection. Anal Chim Acta 637:40–46
Dejaegher B, Vander Heyden Y (2010) HILIC methods in pharmaceutical analysis. J Sep Sci 33:698–715
Fountain KJ, Xu J, Diehl DM, Morrison D (2010) Influence of stationary phase chemistry and mobile-phase composition on retention, selectivity, and MS response in hydrophilic interaction chromatography. J Sep Sci 33:740–751
Gentili A, Perret D, Marchese S (2005) Liquid chromatography-tandem mass spectrometry for performing confirmatory analysis of veterinary drugs in animal-food products. Trends Anal Chem 24:704–733
Gong Q, Ding L, Zhu S, Jiao Y, Cheng J, Fu S, Wang L (2012) Determination of ten aminoglycoside residues in milk and dairy products using high performance liquid chromatography-tandem mass spectrometry. Chinese J Chromatography 30:1143–1147
Goucher E, Kicman A, Wolff K, Smith N, Jickells S (2010) Hydrophilic stationary phases: a practical approach for the co-analysis of compounds with varying polarity in biological matrices. J Sep Sci 33:955–965
Granelli K, Branzell C (2007) Rapid multi-residue screening of antibiotics in muscle and kidney by liquid chromatography-electrospray ionization-tandem mass spectrometry. Anal Chim Acta 586:289–295
Granelli K, Elgerud C, Lundström A, Ohlsson A, Sjöberg P (2009) Rapid multi-residue analysis of antibiotics in muscle by liquid chromatography-tandem mass spectrometry. Anal Chim Acta 637:87–91
Grumbach ES, Diehl DM, Neue UD (2008) The application of novel 1.7 microm ethylene bridged hybrid particles for hydrophilic interaction chromatography. J Sep Sci 31:1511–1518
Hemström P, Irgum K (2006) Hydrophilic interaction chromatography. J Sep Sci 29:1784–1821
Jandera P (2008) Stationary phases for hydrophilic interaction chromatography, their characterization and implementation into multidimensional chromatography concepts. J Sep Sci 31:1421–1437
Jandera P, Hájek T, Skeríková V, Soukup J (2010) Dual hydrophilic interaction-RP retention mechanism on polar columns: structural correlations and implementation for 2-D separations on a single column. J Sep Sci 33:841–852
Jandera P, Hájek T, Staňková M, Vyňuchalová K, Česla P (2012) Optimization of comprehensive two-dimensional gradient chromatography coupling in-line hydrophilic interaction and reversed phase liquid chromatography. J Chromatogr A 1268:91–101
Kahsay G, Song H, Van Schepdael A, Cabooter D, Adams E (2014) Hydrophilic interaction chromatography (HILIC) in the analysis of antibiotics. J Pharm Biomed Anal 87:142–154
Kennedy DG, McCracken RJ, Cannavan A, Hewitt SA (1998) Use of liquid chromatography-mass spectrometry in the analysis of residues of antibiotics in meat and milk. J Chromatogr A 812:77–98
Kumar P, Rubies A, Companyó R, Centrich F (2012) Hydrophilic interaction chromatography for the analysis of aminoglycosides. J Sep Sci 35:498–504
Liu M, Chen EX, Ji R, Semin D (2008a) Stability-indicating hydrophilic interaction liquid chromatography method for highly polar and basic compounds. J Chromatogr A 1188:255–263
Liu Y, Xue X, Guo Z, Xu Q, Zhang F, Liang X (2008b) Novel two-dimensional reversed-phase liquid chromatography/hydrophilic interaction chromatography, an excellent orthogonal system for practical analysis. J Chromatogr A 1208:133–140
Nakazawa H, Ino S, Kato K, Watanabe T, Ito Y, Oka H (1999) Simultaneous determination of residual tetracyclines in foods by high-performance liquid chromatography with atmospheric pressure chemical ionization tandem mass spectrometry. J Chromatogr B Biomed Sci Appl 732:55–64
Nguyen HP, Yang SH, Wigginton JG, Simpkins JW, Schug KA (2010) Retention behavior of estrogen metabolites on hydrophilic interaction chromatography stationary phases. J Sep Sci 33:793–802
Nováková L, Kaufmannová I, Jánská R (2010) Evaluation of hybrid hydrophilic interaction chromatography stationary phases for ultra-HPLC in analysis of polar pteridines. J Sep Sci 33:765–772
van Holthoon FL, Essers ML, Mulder PJ, Stead SL, Caldow M, Ashwin HM, Sharman M (2009) A generic method for the quantitative analysis of aminoglycosides (and spectinomycin) in animal tissue using methylated internal standards and liquid chromatography tandem mass spectrometry. Anal Chim Acta 637:135–143
Wang L, Li YQ, Wang HB, Guan YL (2011) Simultaneous determination of twenty veterinary drug residues in milk and meat using matrix solid phase dispersion-ultra performance liquid chromatography-tandem mass spectrometry. Chinese Journal of Anal Chem 39:203–207
Wu J, Bicker W, Lindner W (2008) Separation properties of novel and commercial polar stationary phases in hydrophilic interaction and reversed-phase liquid chromatography mode. J Sep Sci 31:1492–1503
Zurhelle G, Müller-Seitz E, Petz M (2000) Automated residue analysis of tetracyclines and their metabolites in whole egg, egg white, egg yolk and hen's plasma utilizing a modified ASTED system. J Chromatogr B Biomed Sci Appl 739:191–203
Acknowledgements
This study was funded by the China Postdoctoral Science Foundation (2012M521703). We thank Prof. Dongtao Lin of the Sichuan University for copyediting the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
Lian Wang declares that he has no conflict of interest, Bixia Yang declares that she has no conflict of interest, Xinshen Zhang declares that he has no conflict of interest, and Hongguo Zheng declares that he has no conflict of interest.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Wang, L., Yang, B., Zhang, X. et al. Novel Two-Dimensional Liquid Chromatography–Tandem Mass Spectrometry for the Analysis of Twenty Antibiotics Residues in Dairy Products. Food Anal. Methods 10, 2001–2010 (2017). https://doi.org/10.1007/s12161-016-0763-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0763-4