Abstract
Milk can be adulterated illegitimately with formaldehyde, melamine, urea, and sugars for preservation, protein content enhancement, and taste improvement, respectively. In the present study, formaldehyde, melamine, urea, and sugars (fructose, glucose, lactose, sucrose) were analyzed using ultra-fast high performance liquid chromatography in 30 fresh and pasteurized milk samples. Satisfactory chromatographic retention and peak shape investigated and method has been validated by means of recovery experiments in samples spiked at two levels. Average recoveries ranged between 95–105 % with excellent precision; RSD lower than 5 % was achieved. Among the tested samples, formaldehyde was detected in all samples while seven and 20 samples were positive for melamine and sucrose, respectively. Monitoring of these adulterants in milk could help to reduce the human health hazards. Exposure to formaldehyde, melamine, and urea from consumption of milk was estimated, and these were less than 3.3, 1, and 700 μg/mL, respectively, which is an oral reference dose suggested by the World Health Organization and US Food and Drug Administration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Milk is one of the most important foods in daily life providing rich nutrients which are produced by lacteal secretion from mammal’s mammary glands. Verities of milk contains all essential ingredients although differ in odor, taste, and chemical composition. It is an exclusive healthy food which cannot be replaced by any other food required for maintenance of life of both adults and infants. Milk contains good quality carbohydrates, fat, protein, minerals, and vitamins in significant amount especially amino acids which are needed for proper growth of adults and infants (Afzal et al. 2011; Finete Vde et al. 2013; Awan et al. 2014). Sources of fresh milk are the cow, goat, buffalo, sheep, reindeer, and camel. Due to the lack of proper facilities, both the quality and quantity of fresh milk is not satisfactory regarding to the health of consumers. In populous or metro cities where there is a shortage of fresh milk, people prefer to use pasteurized or packed milk. The process of pasteurization was attributed to ultra-high temperature (UHT) which is applied as a safety measure to kill the pathogens (Miller et al. 2000). The pathetic conditions of delivering the fresh or raw milk are one of the causes of a variety of diseases. Fifteen renowned companies (national and multinational) are operative in Pakistan for manufacturing of pasteurized milk, but little is known whether these have any procedure for treatment of raw milk other UHT procedure. Usually, milk is adulterated to maintain the viscosity and thicknesses as well as carbohydrates, fat, protein, and the adulterants which are usually starch, rice flour, urea, skimmed milk, vegetable oil, glucose, whey powder, animal fat, melamine, and detergents. The simplest and oldest form of adulterant in milk is water to increase the volume for profitable commodity, but contaminated water has potential risk to human health regarding the waterborne diseases (Campos Motta et al. 2014; Singuluri and Sukumaran 2014). Most of the milk provision in Pakistan is of buffalo (62 % of total milk production) which is more liable to water adulteration than cow’s milk because it has more fat content (Imran et al. 2008; Afzal et al. 2011; Mansour et al. 2012). Pond water is also added by some unscrupulous persons to increase the specific gravity of milk because it is a rich source of nitrates. Consumption of such water adulterated milk by humans may cause stomach disorder in elders and serious health hazards in infants and children where milk is their basic diet (Singh and Gandhi 2015). Refrigeration (ice used may be contaminated) of fresh milk is done to avoid spoiling, especially in the summer season during transportation and chemicals such as formalin, sodium carbonate/bicarbonate/hydroxide, and/or calcium hydroxide are added to enhance its shelf life. Cosmetic nature and foamy appearance of milk is maintained by the addition of some detergents; for whiteness and genuine appearance, potassium and calcium salts of thioglycolic acids are also added (Afzal et al. 2011; Soomro et al. 2014). The present work was conducted on fresh and pasteurized or packed milk samples for detection of adulterants in the second largest city (Lahore) of the country. Study of detection of adulteration is of great importance in daily life. With the help of this study, the natural composition of milk will be insured which is necessary for better growth and development of a community. In the present study, physical characteristics (pH, acidity, TDS, EC, and moisture) along with the chemical adulterants (formaldehyde, melamine, urea, and sugars) are investigated by ultra-fast liquid chromatography (UHPLC) coupled to photodiode array and differential refractive index detectors.
Materials and Methods
Instrumentation
HPLC Shimadzu Nexera X2 (Japan) equipped with LC20AD pump, SPD-M20A diode array, RIO10A RI detectors, DGU-20A5 degasser (online), and variable volume loop injection valve were used. The separations were achieved on Acquity BEH-C18 column (100 × 2.1 mm, 1.7 μm particle size) and Phenomenex Luna NH2 column (250 × 4.6 mm, 5.0 μm particle size). Shimadzu LC program, LabSolutions, analysis data system was used for the integration of peak areas.
Collection of Samples
Fifteen milk samples (pasteurized) were collected from local market shelves in the capital city Lahore (31° 33′ 16.58″ N, 74° 21′ 25.77″ E) Punjab-Pakistan. Each sample was of 250 mL in tetra packing, and none of sample exceeded the storage period of 15 days. Fresh milk samples (15 No’s) were collected from dairy shops randomly. Three sites (Fig. 1) were selected for fresh milk sampling, i.e., Kasur-Lahore (Feroz pur road), Sheikhupura-Lahore (Sheikhupura road), and Raiwind-Lahore (Raiwind road) because from these three sides the fresh milk is provided to dairy shops. On these three roads, many industries like pharmaceutical, textile, leather, and chemicals are situated and these industries effluent their wastes along these roads. All the samples were stored at 4 °C and before analysis kept overnight at 25 ± 2 °C.
Physical Characteristics
pH was determined by multi-meter (Orion 5 star, Thermo scientific, UK) by direct immersing the electrode in milk sample. Twenty milliliters of milk was weighed and dissolved it in 75 ml of high purity water (0.01 μS/cm prepared by using purification system Millipore system by Milli-Q, USA). 0.05 M sodium hydroxide (Merck, Germany) was used for free acidity by plotting the neutralization curve; and pH was determined at equivalence point and acidity in milliequivalents per kilogram of milk was obtained as ten times the volume of sodium hydroxide. For lactone acidity, excess sodium hydroxide was added to milk solution and back titrated with 0.025 M sulphuric acid (Merck, Germany) for plotting of neutralization curve. Summation of both free and lactone acidity is equivalent to total acidity. TDS in parts per million and EC in milliSiemens per centimeter were measured by Orion 5 star multi-meter (Thermo scientific, UK). Twenty grams of milk was suspended in 75.0 mL of high purity water in a volumetric flask and makes the volume up to 100 mL with the same solvent. Two grams of milk in a china dish was placed in a furnace (Thermolyne™, Thermoscientific-UK) and commenced drying at temperature 105–110 °C. After 1 h, the dish was cooled at room temperature and moisture content was calculated as grams per 100 g of milk (AOAC 2000).
Chemical Adulterants Analysis
Formaldehyde and Melamine
Formaldehyde and melamine were determined by liquid phase extraction followed by HPLC-DAD by slight modification in earlier method reported (Kaminski et al. 1993; Deabes and El- Habib 2012). For formaldehyde, 2.0 mL solution of hexane (Sigma-Aldrich, USA) and 2, 4-dinitrophenyhydrazine (DPPH) from Merck, Germany, was added to 5.0 g of milk sample. Reaction mixture was allowed to stand for 30 min at room temperature. The solution was filtered and washed with hexane, and the solvent was evaporated. The solid matrix was dissolved in acetonitrile (Sigma-Aldrich, USA) for HPLC analysis. The mobile phase comprising of an acetonitrile to water ratio (50:50 v/v) set at a flow rate of 0.45 mL/min and injection volume of 3.0 μL was injected through a column maintained at 40 °C. The wavelength selected was 365 nm. For melamine, 5.0 g of milk sample was mixed with 20 mL of methanol (Sigma-Aldrich, USA) and sonicated for 10 min and then the matrix was centrifuged at 4500 rpm. The supernatant was mixed into a solution of methanol and water (4:1). HPLC conditions remain the same as mentioned in formaldehyde analysis except the λ max is 220 nm and mobile phase is comprised of methanol and water (80:20 v/v). The same procedure was adopted for standard formaldehyde and melamine solutions.
Urea
One milliliter of milk was added to 0.5 mL of trichloroacetic acid (Sigma-Aldrich, USA). and mixture was centrifuge at 5000 rpm for 10 min at 4 °C. 0.75 mL of supernatant was diluted with 0.25 mL distilled water (Milli-Q system, USA). Then, 0.25 mL of acidic p-dimethylaminobenzaldehyde (used 20 % HCl) was added and stirred vigorously for 10 min at room temperature (Czauderna and Kowalczyk 2012). The same procedure was adopted for standard urea solutions. The mobile phase comprising of phosphate buffer of pH 7 (sodium phosphate dibasic and phosphoric acid) and methanol (70:30 v/v) set at flow a rate of 0.45 mL/min and injection volume of 3.0 μL was injected through column maintained at 25 °C. The wavelength was selected at 370 nm.
Sugars
Two milliliters of milk sample was added to 1.5 mL of distilled water, and the diluted milk was incubated at 60 °C for 10 min. Then, 2.5 mL each of potassium ferricyanide (0.5 M) and zinc acetate (0.5 M) and 1.0 mL of acetonitrile was added. The mixture was allowed to stand for 1 h at room temperature then centrifuged at 10,000 rpm for 10 min at 20 °C. The supernatant was filtered through a membrane filter (0.45 μm). The extract contains the sugars and was injected (20 μL) into HPLC system (Sharma et al. 2009). Mobile phase comprised of acetonitrile and water (70:30 v/v) at a flow rate of 1.0 mL/min, and the temperature of column and RI cell was maintained at 40 °C.
Validation Studies
Linearity
The linearity of proposed method was determined over a concentration range of 0.25–8.0 μg/ml (0.25, 0.50, 1.0, 2.0, 4.0, 8.0 μg/mL) for formaldehyde and for melamine, 2.5–15.0 μg/mL (2.5, 5.0, 7.5, 10.0, 12.5, 15.0 μg/mL) for urea, 100–200 μg/mL (100, 120, 140, 160, 180, 200 μg/mL) for fructose, glucose, sucrose, and 5–10 mg/mL (5, 6, 7, 8, 9, 10 mg/mL) for lactose.
Accuracy and Precision
Accuracy and precision (repeatability and reproducibility) studies were performed by spiking the milk samples at two different levels (L1 and L2) for different days. Repeatability (intra-day precision) and reproducibility (inter-day precision) were determined on same day and three consecutive days, respectively. Spiked concentration for formaldehyde and melamine is 1.5 and 2.4 μg/mL, 7.5 and 12.5 μg/mL concentration spiked for urea, while 10 and 15 μg/mL concentration spiked for each of fructose, glucose, sucrose, and lactose. Five milliliters of milk sample was spiked for accuracy and precision studies, and all measurements were made in triplicate.
Limit of Detection and Limit of Quantitation
The limit of detection (LOD) is the minimum amount of analyte in a sample detectable and larger than uncertainty associated with it, and the limit of quantitation (LOQ) is the amount quantitatively measured with suitable precision and accuracy. LOD and LOQ were determined by standard deviation of the response based on the slope of the calibration by six injections of six working standards each of formaldehyde, melamine, urea, fructose, glucose, sucrose, and lactose under the optimized chromatographic conditions, and calibration curve method (Qadir et al. 2015a, b; Ali et al. 2015; Ahmed et al. 2014) is used for calculation by the following equations:
Where, yB is intercepts of regression line and sB is standard deviation of intercepts of regression line.
Statistical Analysis
Statistical analysis was performed on SPSS version 15 (SPSS Inc., Chicago, IL, USA) for Windows; analysis of variance (ANOVA) was performed for significant difference (p <0.05) with post hoc multiple comparisons.
Results and Discussion
Physical Characteristics
pH of milk is correlated with non-fat solids and lactose contents, but it is not influenced by lactation number, month, and season of calving. Non-fat and fatty solids are accounted for total acidity of milk; lactic acid almost shares the 25 % acidity of milk (Ahmad et al. 2013). Total acidity and pH of tested fresh and pasteurized milk samples are presented in Table 1. The mean value of total acidity for fresh milk and pasteurized milk obtained 0.26 and 0.24 while the pH was 6.71 and 6.73, respectively. TDS and EC are interlinked with each other and important parameters to determine the physical characteristics of milk. The TDS and EC (Table 1) obtained was in the range of 10.21–12.63 and 1.3–5.5 for fresh milk and 10.4–12.6 and 1.9–5.8 for pasteurized milk, respectively, which is similar to earlier reported. The moisture content was found in the range of 80.4–86.6 and 75.9–85.4 for fresh and pasteurized milk, respectively.
Formaldehyde and Melamine
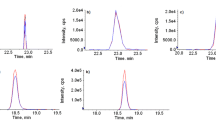
Parameter of linear regression equation (linear range, correlation coefficient) and detection limits (LOD and LOQ) for both the adulterants are presented in Table 2. The linear range of 0.25–8.0 μg/mL for both formaldehyde and melamine was selected, and LOD was found to be 0.012 and 0.051 μg/mL, respectively (Table 2). Both the analytes were successfully eluted at the retention time of 3.42 min and 4.21 min (Fig. 2). Accuracy of method was assessed by recovery studies. Known amount of formaldehyde (37 % Sigma-Aldrich, USA) and melamine (Sigma-Aldrich, USA) was spiked into 5.0 mL of milk sample matrix, and measurement was made in triplicate for 3 days. The mean recovery rates (Table 3) for 1.5 and 2.4 μg/mL spiked formaldehyde in 3 days were 99.5 and 100.7 %, respectively, while recovery rates for same spiked melamine were found 98.9 and 99.2 %, respectively. Precision of method was also determined by measuring the spiked concentration as already mentioned. Intra-day precision (Table 3) in terms of % RSD for two spiked concentration was 2.3 and 2.6 %, respectively, for formaldehyde and 1.7 and 1.8 % obtained for melamine while the results of inter-day precision (Table 3) were obtained as 2.9 and 3.2 %, respectively, for formaldehyde while 2.5 % obtained for melamine for both spiked levels. Formaldehyde (Table 4) was found in both fresh and pasteurized milk, in ranges between 0.27–3.12 μg/mL and 0.33–1.76 μg/mL, respectively. Due to the antiseptic nature of formaldehyde, it is added deliberately and illegally in raw milk to increase its shelf life. Formaldehyde content in raw milk ranges between 1–3.3 μg/mL according to WHO (Sandhu et al. 1984; WHO 1989). One sample of fresh milk was quantified as borderline concentration of formaldehyde (3.12 μg/mL). Formaldehyde should not be added to milk although it preserves the milk for a long time at room temperature and also increase its heat stability. Even in small amount, formaldehyde is highly toxic and classified as carcinogenic. A maximum 0.2 mg/kg oral dose is suggested by Environmental Protection Agency (EPA) of the USA. Various illnesses such as vomiting, abdominal pain, diarrhea, shallow respiration, decrease in body temperature, memory loss, weak irregular pulse, insomnia, mood and balance alteration, unconsciousness, and blindness due to damage of the optical nerve can be caused by ingestion of formaldehyde in humans (USEPA 1999; Afzal et al. 2011). Data in Table 4 presented that only four samples of fresh milk (0.16, 0.21, 0.09, and 0.18 μg/mL) and three samples of pasteurized milk (0.19, 0.22, and 0.23 μg/mL) were positive for melamine contents. In recent years, melamine became topic of discussion because hundreds of thousands of pets died due to melamine-contaminated feeds. There was a urinary stone outbreak in Chinese children who consumed melamine tainted milk. Melamine is illegally added to milk to increase protein contents. Due to its high nitrogen contents (66.6 %), the addition of 1 g melamine in 1 kg of milk increased up to 0.4 % protein contents. Melamine also contains reaction by products, viz, ammeline, ammelide, and cyanuric acid which are also toxic to humans (Tyan et al. 2009; Hau et al. 2009). Maximum residue limit (MRL) for melamine suggested by the European Union (EU) and Food and Drug Administration (FDA-USA) is 2.5 and 0.25 mg/kg, respectively, whereas the former stressed that infant formula should be completely free from it. Experts from WHO/FAO stated a safety margin for infant formula is 1 mg/kg. Melamine adulterated milk ingestion in humans may cause renal failure, urolithiasis, bladder cancer, and even death particularly for vulnerable individuals such as young children and infants (Jawaid et al. 2013). The concentrations found in both the tested samples are within the permissible level suggested by regulatory authorities (WHO and EU).
Urea
The linear range selected for determination of urea in milk was 2.5–15 μg/mL, and detection limit was found to be 0.027 μg/mL (Table 2). The urea was eluted at the retention time of 5.12 min (Fig. 3) and parameters of linear regression are presented in Table 2. For accuracy and precision studies, 7.5 and 12.5 μg of standard urea were spiked to milk samples separately; recovery rates (Table 3) and % RSD was determined. The mean recovery rates for 7.5 and 12.5 μg spiked urea in 3 days were obtained 99.1 and 98.5 %, respectively. Intra-day precision (Table 3) in terms of % RSD for two spiked concentration was 1.8 and 2.8 %, respectively, while the results of inter-day precision (Table 3) were obtained as 3.0 and 2.7 %, respectively. The urea contents in fresh milk obtained in ranges between 505–625 μg/mL while pasteurized milk contained in ranges between 520–678 μg/mL. Non-protein nitrogen source (55 %) of milk is urea. Milk is adulterated with urea because it is cheaper, easily available, and a rich source of nitrogen. It is added to milk for non-fat solid content standardization, increase whiteness, consistency, and shelf life. Typically, the contents of urea in milk are 180–400 mg/L; excess supply of crude protein in animal feeds contribute to milk urea concentration. The maximum acceptable limit for urea in milk is 700 mg/L (Kohn 2000; Trivedi et al. 2009). Higher level of urea in feeds influences the milk production as well as fertility. Urea exceeding the upper limit is harmful especially for pregnant women, children, and young girls where it hastens the puberty process; it also causes severe illnesses such as acidity, malfunctioning of the kidney, ulcer, osteoporosis, and cancer. At high temperature, boiling of milk containing urea decompose to acetic acid, ammonia, and carbonic acid and may be converted into biuret which causes strong irritation in the urinary tract and decrease in blood pressure of humans (Singh and Gandhi 2015). In the present study, none of the sample exceeded the upper limit of 700 mg/L.
Sugars
The amino column was used to elute the sugars because sugars can interact with amines; four sugars, viz. fructose, glucose, sucrose, and lactose were eluted in isocratic mode using RI detector at retention times of 4.91, 5.5, 8.1, and 9.2 min, respectively (Fig. 3). The linear ranges used for the four sugars were 100–200 μg/mL (fructose, glucose, sucrose) and 5–10 mg/mL (lactose). The detection limit for the four sugars 0.21 μg/mL (fructose), 0.31 μg/mL (glucose), 0.26 μg/mL (sucrose), and 3.6 μg/mL (lactose) was investigated (Table 3). For accuracy and precision studies, 10 and 15 μg of each sugar was spiked to milk samples separately and recovery rates and % RSD were determined. The mean recovery rates for 10 and 15 μg spiked standards in 3 days were obtained 99.9 and 99.4 % (fructose), 98.7 and 99.1 % (glucose), 101.9 and 101.6 % (lactose), and 99.2, 100.2 % (sucrose). Repeatability (Table 3) in terms of % RSD for two spiked concentration was 3.1 and 3.5 % for fructose, 2.2 and 2.6 % for glucose, 1.2 and 1.4 % for lactose, and 2.4 and 2.1 % for sucrose, respectively, while the results of reproducibility (Table 3) were obtained as 3.3 and 3.7 % for fructose, 2.4 and 2.6 % for glucose, 2.8 and 2.6 % for lactose, and 2.9 % (both spiked levels) for sucrose, respectively. Data in Table 5 presents the sugar contents present in milk samples under investigation. The concentration of fructose ranged between 176–216 μg/mL for fresh milk and 175–215 μg/mL for pasteurized milk; glucose ranged between 102–132 μg/mL for fresh milk while 109–131 μg/mL for pasteurized milk samples. Sixty and 67 % of fresh and pasteurized milk samples were identified positive for sucrose contents; the maximum amount of sucrose was found to be 3.9 and 5.5 μg/mL, respectively. The lactose content in the range between 2.9–4.6 g/100 mL and 2.7–3.8 g/100 mL was found in fresh and pasteurized milk samples, respectively. These values of sugars obtained in tested samples are comparable with values reported earlier in literature (Afzal et al. 2011; Czauderna and Kowalczyk 2012). Still no regulatory body like WHO, EU, USFDA suggested the permissible level of these sugars reported in this study. But the milk is adulterated with glucose and sucrose to mask the addition of extraneous water in milk and improve the taste. Lactometer reading is also managed by the addition of these sugars by masking the addition of water. High contents of sucrose or glucose may contribute to diabetics in the elevation of blood sugar. It can be hazardous for peoples who already are diabetic or have a cardiac problem (Malik et al. 2006; Singh and Gandhi 2015).
Conclusion
In present study, chemical adulterants, viz, formaldehyde, melamine, urea, and sugars especially sucrose added to milk illegally were successfully analyzed by UHPLC coupled to diode array and differential refractive index detectors. Out of 30 samples, one sample has borderline content of formaldehyde of 3.12 μg/mL while remaining samples are in compliance with contents suggested by WHO and USFDA. Melamine and urea were also in the safe range given by EU and WHO/FAO. Natural occurring of urea in milk should be investigated. With knowledge that there is endogenous urea present in milk, a proper regulation should be put in place against use of urea for protein content enhancement. The monitoring of deliberately added formaldehyde for preservation, melamine and urea for protein contents enhancement, and sucrose for taste improvement by using proper analytical technique is very important.
References
Afzal A, Mahmood MS, Hussain I, Akhtar M (2011) Adulteration and microbiological quality of milk. Pak J Nutr 10:1195–1202
Ahmad S, Anjum FM, Huma N, Sameen A, Zahoor T (2013) Composition and physico-chemical characteristics of buffalo milk with particular emphasis on lipids, proteins, minerals, enzymes and vitamins. J Anim Plant Sci 23:62–74
Ahmed M, Qadir MA, Shahzad S, Waseem R, Tahir MS (2014) Validation of UV-HPLC method for simultaneous quantification of organic acids in disinfectants for haemodialysis machines. Int J Chem Phar Sci 2:536–540
Ali A, Ahmed M, Mahmud T, Qadir MA, Nadeem K, Saleem A (2015) Stability indicating HPLC method for simultaneous determination of aminophylline and chlorpheniramine maleate in pharmaceutical formulations. Ind J Pharm Sci 77:415–421
AOAC (Association of Official Analytical Chemists) (2000) Official methods of analysis international, 17th edn. AOAC, Washington, DC
Awan A, Naseer M, Iqbal A, Ali M, Iqbal R, Iqbal F (2014) A study on chemical composition and detection of chemical adulteration in tetra pack milk samples commercially available in Multan. Pak J Pharm Sci 27:183–186
Campos Motta TM, Hoff RB, Barreto F, Andrade RBS, Lorenzini DM, Meneghini LZ, Pizzolato TM (2014) Detection and confirmation of milk adulteration with cheese whey using proteomic-like sample preparation and liquid chromatography–electrospray–tandem mass spectrometry analysis. Talanta 120:498–505
Czauderna M, Kowalczyk J (2012) Simple, selective, and sensitive measurement of urea in body fluids of mammals by reversed-phase ultra-fast liquid chromatography. Czech J Anim Sci 57:19–27
Deabes MM, El- Habib R (2012) Determination of melamine in infant milk formula, milk powder and basaa fish samples by HPLC/DAD. J Environ Anal Toxicol 2:137
Finete Vde L, Gouvêa MM, Marques FF, Netto AD (2013) Is it possible to screen for milk or whey protein adulteration with melamine, urea and ammonium sulphate, combining Kjeldahl and classical spectrophotometric methods? Food Chem 141:3649–3655
Hau AKC, Kwan TH, Li PKT (2009) Melamine toxicity and the kidney. J Am Soc Nephrol 20:245–250
Imran M, Khan H, Hassan SS, Khan R (2008) Physicochemical characteristics of various milk samples available in Pakistan. J Zhejiang Univ Sci B 9:546–551
Jawaid S, Talpur FN, Sherazi STH, Nizamani SM, Khaskheli AA (2013) Rapid detection of melamine adulteration in dairy milk by SB-ATR–Fourier transform infrared spectroscopy. Food Chem 141:3066–3071
Kaminski J, Atwal AS, Mahadevan S (1993) Determination of formaldehyde in fresh and retail milk by liquid column chromatography. J AOAC Int 76:1010–1013
Kohn R (2000) Caution needed when interpreting MUNs. Hoard’s Dairyman 145:58
Malik VS, Schulze MB, Hu FB (2006) Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr 84:274–288
Mansour AIA, El-Loly MM, Ahmed RO (2012) A preliminary detection of physical and chemical properties, inhibitory substances and preservatives in raw milk. Internet J Food Saf 14:93–103
Miller GD, Jarvis JK, McBean LD (2000) The importance of milk and milk products in the diet, handbook of dairy foods and nutrition second edition (Wolinsky editor). CRC Press, Boca Raton, Florida, USA
Qadir MA, Ahmed M, Haq I, Ahmed S (2015a) Improved limit of detection and quantitation development and validation procedure for quantification of zinc in insulin by atomic absorption spectrometry. Pak J Pharm Sci 28:875–879
Qadir MA, Ahmed M, Hussain WA, Tahir MS (2015b) Development and validation of new HPLC method for simultaneous estimation of L-lysine hydrochloride and L-carnitine-L-tartrate in pharmaceutical dosage form. Ind J Pharm Sci 77: 434–38.
Sandhu JS, Nasir N, Narayanaswamy M, Kapur OP (1984) Study on the effect of formalin as a preservative on different constituents of raw milk samples during storage. J Food Sci Technol 21:424–425
Sharma R, Rajput YS, Poonam DG, Tomar ST (2009) Estimation of sugars in milk by HPLC and its application in detection of adulteration of milk with soymilk. Int J Dairy Technol 62:514–519
Singh P, Gandhi N (2015) Milk preservatives and adulterants: processing, regulatory and safety issues. Food Rev Int 31:236–261
Singuluri H, Sukumaran MK (2014) Milk adulteration in Hyderabad, India—a comparative study on the levels of different adulterants present in milk. J Chromatogr Separat Tech 5:1–3
Soomro AA, Khaskheli M, Memon MA, Barham GS, Haq IU, Fazlani SN, Khan IA, Lochi GM, Soomro RN (2014) Study on adulteration and composition of milk sold at Badin. Intl J Res Appl Nat Social Sci 2:57–70
Trivedi UB, Lakshminarayana D, Kothari IL, Patel NG, Kapse HN, Makhija KK, Patel PB, Panchal CJ (2009) Potentiometric biosensor for urea determination in milk. Sens Actuators B 140:260–266
Tyan YC, Yang MH, Jong SB, Wang CK, Shiea J (2009) Melamine contamination. Anal Bioanal Chem 395:729–735
US Environmental Protection Agency (1999) Integrated Risk Information System (IRIS) on formaldehyde; National Center for Environmental Assessment. Office of Research and Development, US Environmental Protection Agency, Washington, DC
World Health Organization (1989) Environmental health criteria 89., Formaldehyde
Funding
There has been no financial support for this work that could have influenced its outcome.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed Consent
Not applicable.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s12161-016-0541-3.
Rights and permissions
About this article
Cite this article
Ahmad, S.A., Ahmed, M., Qadir, M.A. et al. Quantitation and Risk Assessment of Chemical Adulterants in Milk Using UHPLC Coupled to Photodiode Array and Differential Refractive Index Detectors. Food Anal. Methods 9, 3367–3376 (2016). https://doi.org/10.1007/s12161-016-0534-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0534-2