Abstract
A slurry sampling method was described for the determination of total fluorine in flour via molecular absorption of calcium monofluoride (CaF) at 660.440 nm using a high-resolution continuum source graphite furnace atomic absorption spectrometer (HR-CS GF AAS). The slurries were prepared in 1 % Triton X-100, kept in an ultrasonic bath for 15 min, mixed in a shaker for 5 min (750 rpm), and then vortexed for 30 s shortly before sampling. The samples were mixed with 40 μg of Ca in the graphite furnace, pyrolyzed at 900 °C, and the absorbances for CaF were measured at 2200 °C. The LOD and characteristic mass of the method were 0.22 and 0.16 ng, respectively. The F concentrations of certified reference bush branches and leaves were determined in the uncertainty limits of the certified values. Finally, the F concentrations of different types of flours were determined applying the optimized conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Flour is one of the most commonly used food ingredient in the world since it is the main component of bakery and pastry. The foods made mainly from flour such as bread, cake, biscuit, confectionery, macaroni, etc. are consumed very frequently in large amounts by adults and children all over the world. Therefore, the flour content influences human body in their whole life. Fluorine is considered as an essential addition to our daily diet for preventing dental cavities but long term exposure of high concentrations of this element may cause cancer, dental fluorosis, and bone fractures (USA Public Service Department of Health and Human Services 1991). The range of optimal fluoride intake for adults, 1.5 to 4 mg per day, was adjusted for children and adolescents aged 1 to 19 by relating these limits to body weight and energy intake (Marthaler 1992).
Slurry sampling is assumed to be an intermediate sampling technique between solid sampling and digestion techniques (Baysal et al. 2008; Borges et al. 2014a; de Andrade et al. 2014; Nakadi et al. 2013; Ye et al. 2015; Soares and Nascentes 2013; Vignola et al. 2010). Like all the sample preparation methods, slurry sampling has some advantages as well as disadvantages compared to the others. Its advantages over digestion are (i) no analyte loss or contamination during sample preparation; (ii) no (or minimum) acidic wastes and no hazardous toxic or corrosive vapors during sample preparation, i.e., environmentally friendly; and (iii) less expensive with respect to consumption of energy, reagents, and consumables used during digestion. The advantages of slurry sampling over solid sampling can be summarized as follows: (i) slurry can be diluted easily, (ii) modifier is more effective (effective mixing and better contact with the analyte), (iii) sample introduction is easy and does not need special tools, and (iv) analyte standard addition technique can be applied easily. However, slurry sampling is not free of problems. The preparation of a homogeneous slurry remaining stable for quite a long time needs some efforts and many parameters should be optimized carefully after a series of tests. Moreover, some samples cannot be slurried (e.g., sand, many minerals, and inorganic samples are precipitated at once and cannot be homogeneously dispersed) (Ozbek and Akman 2012b). Since the literature is full of papers and reviews on slurry sampling determination of metal and metalloids using conventional AAS, they will not be repeated here again.
Fluorine, strictly speaking fluoride, has been determined by various methods such as potentiometry (Kissa 1983), spectrometry (American Public Health Association 1995), ion chromatography (Umile and Huber 1993), and capillary electrophoresis (Wang et al. 1997). Since the resonance atomic absorption and emission lines of fluorine are located at vacuum UV region, its determination by atomic absorption spectrometers (AAS), inductively couple plasma atomic emission spectrometers (ICP-AES) is not possible. Moreover, fluorine’s high ionization potential makes its quantification by inductively coupled plasma mass spectrometers (ICP-MS) hardly possible (Ozbek and Akman 2012a). The drawbacks and limitations of those methods were extensively discussed in the literature (Gleisner et al. 2010). However, there are some studies in the literature to determine fluorine by a LS-AAS instrument via molecular absorption of diatomic molecules formed between fluorine and a metal, namely molecule forming element in an atomizer, flame, or graphite furnace. The molecular absorption of the diatomic molecule at one of its structured rotational lines is measured using a suitable HCL so that one of the emission lines of HCL should overlap with the rotational absorption line for the diatomic molecule. For this purpose, Ga (Dittrich 1978), Al (Butcher 1993; Tsunoda et al. 1979), and Mg (Dittrich and Vorberg 1982) were selected as molecule-forming elements. Recently, owing to the production of high-resolution continuum source atomic absorption spectrometers, total fluorine (ionic and covalently bonded) in all solvents (organic and inorganic) could be determined via molecular absorption of its diatomic molecules. For this purpose, same principle is used, the sample or standard is mixed with a molecule-forming element and the diatomic molecules of the analyte with a molecule-forming element are generated in the gas phase of the graphite furnace or flame. The molecular absorption for the diatomic molecules at one of its fine rotational lines is measured. There were many studies for determination of fluoride with different molecule-forming elements, i.e., GaF (Gleisner et al. 2010, 2011; Kruger et al. 2012), AlF (Bücker and Acker 2012), SrF (Ozbek and Akman 2012a), BaF (Ozbek and Akman 2014), and calcium monofluoride (CaF) (Borges et al. 2014b, 2016; Huang et al. 2014; Machado et al. 2015; Mores et al. 2011; Ozbek and Akman 2015). Welz et al. (2009) reviewed the determination of sulfur, phosphorus, and the halogens using line source conventional flame and graphite furnace AAS and HR CS-AAS. Since fluorine generally forms volatile compounds, its loss at high temperatures during digestion, especially when the vessels of microwave oven are opened, is very likely (Borges et al. 2016). Therefore, slurry sampling determination of fluorine without any digestion is advantageous.
In this study, a method for the slurry sampling determination of total fluorine (ionic and covalently bound) in flour via molecular absorption of CaF in a graphite furnace of a high-resolution continuum source atomic absorption spectrometer was described. Flour is found to be an appropriate sample to prepare homogenous and stable slurries while its fluorine concentration has never been investigated before. The conditions to provide maximum sensitivity as well as a stable and homogeneous slurry were optimized.
Exprerimental
Instruments
An Analytik Jena ContrAA 700 high-resolution continuum source atomic absorption spectrometer equipped with a 300-W xenon short arc lamp (XBO 301, GLE, Berlin, Germany), high-resolution double monochromator, consisting of a prism for predispersion and an echelle monochromator for highresolution, and a charge-coupled device (CCD) array detector was used for all measurements throughout this study. Absorbances for CaF were measured at 606.440 nm with 3 pixels (central pixel ±1). All solutions and slurries were introduced in pyrolytically coated graphite tubes with integrated PIN platform (Analytik Jena Part No. 407-A81.025) by means of MPE 60 auto sampler (Analytik Jena, Jena, Germany) as 10 μL. PRECISA XR 205SM-DR with precision as ±0.00002 g (for 5 g, ten measurements) was used as a balance.
Reagents and Solutions
In all dilutions, high purity water with 18.2 μΩ cm resistivity obtained from a TKA reverse osmosis and a TKA deionizer system (TKA Wasseraufbereitungsysteme GmbH, Niederelbert Germany) was used. Ten thousand micrograms per milliliter of fluorine and calcium stock solutions were prepared from NaF and Ca(NO3)2·4H2O and diluted daily.
Samples
For this study, different types of flours, namely wheat, rice, corn, and whole wheat flours of different origins, were purchased from markets in Istanbul, Turkey. For validation purposes, the bush branch standard reference material (NCS DC 73349) was provided from the China National Analysis Center for Iron and Steel, Beijing, China.
Procedure
Prepared slurries and 4000 μg mL−1 of calcium prepared from Ca(NO3)2·4H2O were co-injected into the graphite tube as 10 μL from each. Fluorine determination was performed by HR-CS GF AAS via molecular absorption lines of CaF at 606.440 nm. The slurries of flour and standard reference material were prepared as 1.0 % (w/v) (0.1 g in 10 mL) in 1 % Triton X-100 without adding any acid. The samples kept in an ultrasonic bath for 15 min, mixed in a shaker for 5 min, and then vortexed for 30 s, shortly before transferring to the vials. The slurries were transferred to vials, put on the autosampler tray and immediately pipetted to the furnace. The optimized graphite furnace program is given in Table 1. Analyses were performed both by linear calibration and standard addition. All results were given as the mean of at least three replicates.
Results and Discussion
In order to prepare a homogeneous slurry and provide its homogeneity during the analysis, the effects of surfactant, mixing method, and slurry concentration, i.e., the ratio of the amount of sample to slurry volume, were investigated.
At first, the experimental conditions to provide a satisfactory homogeneity were optimized. In order to lower the surface tension and to improve the dispersion and stability of the slurry, the effects of different surfactants such as Tween 20, Triton X-100, and Triton X-114 were investigated. After a series of studies, it was found that the RSD values (N 10) for the slurries prepared in 1 % Triton X-100 (subjected to ultrasonication for 15 min, mixed in a shaker for 5°min, and then vortexed for 30 s, shortly before pipetting) were below 10 % and remained stable during the whole analysis. Moreover, the absorbances for slurry portions taken from different depths of autosampler vials were almost the same and did not change significantly. This is an evidence that a homogeneous slurry was prepared and a precipitation did not occur at least during the sampling.
The effect of HNO3 on the sensitivity was checked. When the slurries were prepared in 1.0 mol L−1 of HNO3, the results were lower compared to acid-free slurries, which can be attributed to losses of fluorine from slurry or during the drying or pyrolysis stages in the graphite furnace and absolutely in good agreement with the finding of Borges et al. (2016). The other acids, e.g., HCl, H2SO4, H3PO4, etc. were not tried because they may cause some nonspectral interferences for the formation of CaF due to competitive reactions of their anions with Ca (Huang et al. 2014).
Choice of Molecule-Forming Metal and Wavelength
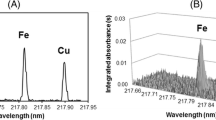
Fluorine cannot be determined directly by conventional atomic absorption spectrometry because its atomic absorption line is in vacuum UV range around 95 nm. In order to determine fluorine, a diatomic molecule of fluorine formed in gas phase and its molecular absorption is measured. This diatomic molecule should be stable (i.e., its bond dissociation energy should be higher than 500 kJ mol−1), and its sensitivity should be high as much as possible to maintain lower LOD values. Since the bond dissociation energy of CaF is around 550 kJ mol−1 and its applicability is well explained (Borges et al. 2014b; Ozbek and Akman 2015), CaF was selected as a suitable diatomic molecule for this study. The wavelength-resolved absorption spectrum obtained from a flour sample in the vicinity of 606.440 nm, which is a part of the rotational fine structure of X2Σ+-A2Π electronic transition of CaF, is depicted in Fig. 1.
One of the advantages of using CaF is that calcium acts both as the molecule-forming reagent and as a chemical modifier, so that no other reagent had to be added. Besides, the molecular absorption band is in the visible range of the spectrum, where only very few atoms have absorption lines, minimizing the possible spectral interferences. One of the possible interference caused by Cl (Huang et al. 2014), a broadband of CaCl formed in the presence of Cl overlapped with the CaF line at 606.440 nm. However, the sensitivity of CaCl absorption band was very low and the concentration of Cl in flour slurries was not high enough to cause a detectable spectral interference. In the spectrum of the slurried flour sample in Fig. 1, spectral interference due to overlapping of CaF line (660.440 nm) with the molecular absorption band of CaCl as well as another line or band originated from sample was not detected even in the near vicinity of CaF line at 606.440 nm.
Optimization of Ca and Calibration
The determination of F was performed via molecular absorption of CaF formed in a graphite furnace of HR-CS AAS. For this purpose, calcium was pipetted on the slurry or standards in the graphite furnace and the molecular absorption for CaF were measured at its most sensitive rotational line of 606.440 nm. The effect of the amount of Ca on CaF sensitivity is depicted in Fig. 2. When 40 μg of Ca was used, the sensitivities for CaF in slurries and standards were reached to maximum and remained almost constant at higher concentrations of Ca. Actually, the maximum sensitivity for matrix-free standards were obtained above 20 μg of Ca. However, the absorbances for CaF in the slurry samples reached to maximum and stable values above 25 μg. Therefore, with safety of margin, 40 μg of Ca was used throughout this study. Due to the competitive reactions of Ca and F with matrix components in the condensed and gas phases, e.g., Ca with nonmetals and anions and F with metals and cations, some nonspectral interferences may inevitably occur depending on the matrix components and their concentrations (Ozbek and Akman 2015).
In this study, in order to test the effect of nonspectral interferences due to flour matrix on CaF molecular absorbance, the recovery of an analyte added to the slurry was investigated. The analyte added to the slurry was quantitatively recovered >90 % which showed that conditions, the sensitivities of CaF in the matrix-free aqueous solution and slurried samples, were not significantly different. In other words, at optimized conditions, the effects of nonspectral interferences on CaF formation efficiency were at negligible level.
Effect of Slurry Concentration
The homogeneity of F distribution in a slurry was tested by measuring the precision (RSD) of F concentrations for repetitive pipettings taken from the same slurry. The precisions of F in the same slurry were satisfactory (RSD 5–10 %, N 10) which is in the range of RSDs of matrix-free standards. This means that the fluorine distribution in the slurry was satisfactorily homogeneous due to an effective homogenization/stabilization procedure applied. In addition, the F concentrations obtained in a time interval of 30 min. did not significantly change (RSD 10–15 %) which proved that the distribution of solid particles in the slurry remained stable in a reasonable time. On the other hand, the F concentrations in different depths of slurry were almost the same which meant that the distribution of flour in the slurry was homogeneous. In another set of experiments, the precision of the mean F concentrations of slurries prepared from different sample portions in a flour package were compared. It was found that the RSD was around 10 % for the means of more than three slurries prepared from different portions from the same flour package.
As shown from Fig. 3, a good linearity was obtained up to 0.1 g of flour in 10 mL, i.e., 1.0 % (w/v) of slurry concentration. To determine the F concentrations in real samples of flours, the slurries were prepared as 1.0 % (w/v). In order to eliminate the errors caused by heterogeneity, every result is given as mean F concentration of three different slurry samples, which were analyzed three times. The precision of each data point was calculated as the pooled standard deviation of three slurries.
Optimization of Graphite Furnace Program
The pyrolysis and volatilization (molecule formation) curves for CaF obtained from a slurry and standard F solution (prepared from NaF) are depicted in Fig. 4. The general behaviors of curves for slurries and standards are almost the same. The maximum sensitivity was obtained when a pyrolysis temperature of 900 °C and a volatilization temperature (molecule formation step) of 2200 °C were applied.
In a previous paper, it was shown that during the determination of F via SrF, gas-phase combination reactions between Sr and F played an important role for the formation of SrF, which can be adopted for the formation of other diatomic molecules as well (Ozbek and Akman 2013). The sensitivity for CaF highly depends on the formation rate of CaF as well as the stability of CaF molecule (dissociation energy) and its removal rate in the gas phase. It is likely that at low volatilization temperatures, the concentrations of Ca and F in the gas phase did not reach to the maximum which results in lower CaF concentrations in the gas-phase. On the other hand, at higher temperatures, the combination efficiency of Ca and F is less likely as well as the CaF formed is more effectively removed from the furnace due to excessive thermal expansion. Under the effects of various processes, the maximum CaF concentration was reached at 2200 °C. The graphite furnace program given in Table 1 was applied for the determination of F via MAS of CaF.
Figures of Merit
The figures of merits are given in Table 2. The limit of detection (LOD) and limit of quantitation (LOQ) for the determination of F in flour were determined as the three and ten times the standard deviation (σ) for ten repetitive pipettings of the blank solution (1 % Triton-X 100) with 10 μL of 4000 mg L−1 Ca, respectively, whereas the characteristic mass (m0) was calculated as the concentration of F corresponding to 0.0044 A, i.e., 0.0044/slope of calibration graph. The LOD and m0 values were 0.22 ± 0.02 and 0.16 ± 0.02 ng, respectively.
The flour seems to be homogeneously distributed in the package as well as in the slurry and finally remained stable in a reasonable time (30 min). The F contents of a standard reference material (bush brunches) and various kinds of flours sold in Turkey were determined, and the results are given in Table 3. As can be seen in Table 3, the fluorine concentrations in various kinds of flour samples changes in a small range between 8.01 and 10.22 μg g−1.
Analysis of CRM and Various Flour Samples
The F concentration in certified reference material (CRM) (bush branch standard reference material, NCS DC 73349) found by linear calibration using aqueous standards was in the uncertainty limits of the certified value which shows that the optimized conditions for slurry sampling were appropriate. Obviously, the interaction of Ca with other anions in the CRM did not cause an interference. Nevertheless, the fluorine concentrations determined in the flour samples by the linear calibration technique were compared with those found by the standard addition technique in which the fluorine in the sample and that added to the sample are present in the same environment and interacted with the sample concomitants, i.e., the effects of competitive reactions with sample concomitants are the same for the analyte in the sample and the calibrants added for calibration. Therefore, if there were significant differences between the results found by the two techniques, the results found by the standard addition technique would be accepted to be more reliable because the analyte added to the sample is exposed to the chemical and physical effects of matrix as well. To compare the results obtained by the two calibration techniques more reliably, the same slurry was used for both linear calibration and standard addition techniques. According to Student’s t test, there were no significant differences between the results obtained by the two calibration techniques at 95 % confidence level (p > 0.05). This shows that the competitive reactions of F and Ca with sample components do not cause any significant nonspectral interference in fluorine determination in flour. On the other hand, standard addition is inadequate for some systematic errors due to calibration imperfection of laboratory wares such as balance, pipette, and volumetric flask, as well as impurities of standards. However, all those errors are independent of the sample matrix. By considering the results obtained by standard addition technique and analysis of a CRM, it can be concluded that there were no systematic errors and non-spectral interferences. Therefore, the F concentrations in different kinds of flour samples were reported according to aqueous calibrants. Nevertheless, this is valid for the samples analyzed and not a general conclusion. For the determination of F in many other real samples, the contents of which are unknown, a cross check by standard addition is strongly advised to test the nonspectral interferences. In case of any significant difference, standard addition technique should be preferable.
Each result was given as the average of three different slurries prepared from different portions of the same package, and each slurry was measured three times whereas the overall uncertainty was given as the pooled standard deviation of three different slurries prepared from each package with three repetitive pipetting of each slurry.
Conclusion
In this study, a practical method was described for determination of the total fluorine (ionic and covalently bound) concentrations in different types of flours via molecular absorption of CaF formed in the graphite furnace of a HR-CS GFAAS with slurry sampling. The flour samples were simply slurried in water, effectively homogenized and introduced to the graphite furnace. The advantages of slurry sampling, e.g., no risk of analyte loss during sample preparation, less time required for sample preparation, no toxic or corrosive reagents (environmentally friendly), no time and energy consumption, etc., were benefited in this study. In slurry sampling, there is no need to digest the samples which provided a great advantage because in acidic medium, fluorine losses may occur. The calcium acted both as a molecule-forming element and as a modifier as well. Therefore, there was no need to add an extra modifier which is another advantage of the method. Moreover, since CaF molecular absorption band is in the visible range of the spectrum, there are minimum spectral interferences from other atoms and molecules. Flour products are very commonly consumed all over the world. Therefore, the levels of components in flour are important for human life. The fluorine concentrations of various kinds of flour samples were varied in small range between 8.01 and 10.22 μg g−1 which are far from daily fluorine intake limits.
References
American Public Health Association (1995) Standard methods for the examination of water and wastewater. AWWA, WEF, Baltimore, MD, USA
Baysal A, Tokman N, Akman S, Ozeroglu C (2008) Slurry analysis after lead collection on a sorbent and its determination by electrothermal atomic absorption spectrometry. J Hazard Mater 150:804–808. doi:10.1016/j.jhazmat.2007.05.033
Borges AR, Becker EM, Dessuy MB, Vale MGR, Welz B (2014a) Investigation of chemical modifiers for the determination of lead in fertilizers and limestone using graphite furnace atomic absorption spectrometry with Zeeman-effect background correction and slurry sampling. Spectrochim Acta B At Spectrosc 92:1–8. doi:10.1016/j.sab.2013.11.001
Borges AR, Francois LL, Welz B, Carasek E, Vale MGR (2014b) Determination of fluorine in plant materials via calcium mono-fluoride using high-resolution graphite furnace molecular absorption spectrometry with direct solid sample introduction. J Anal At Spectrom 29:1564–1569. doi:10.1039/C4ja00067f
Borges AR, Duarte ÁT, Potes ML, Silva MM, Vale MGR, Welz B (2016) Fluorine in eye shadow: development of method using high-resolution continuum source graphite furnace molecular absorption spectrometry via calcium mono-fluoride with direct solid sample introduction. Microchem J 124:410–415. doi:10.1016/j.microc.2015.09.025
Bücker S, Acker J (2012) Spectrometric analysis of process etching solutions of the photovoltaic industry—determination of HNO3, HF, and H2SiF6 using high-resolution continuum source absorption spectrometry of diatomic molecules and atoms. Talanta 94:335–341. doi:10.1016/j.talanta.2012.03.052
Butcher DJ (1993) Determination of fluorine, chlorine, and bromine by molecular absorption spectrometry. Microchem J 48:303–317. doi:10.1006/mchj.1993.1104
de Andrade CK, dos Anjos VE, Felsner ML, Torres YR, Quináia SP (2014) Direct determination of Cd, Pb and Cr in honey by slurry sampling electrothermal atomic absorption spectrometry. Food Chem 146:166–173. doi:10.1016/j.foodchem.2013.09.065
Dittrich K (1978) Molekülabsorptionsspektrometrie bei elektrothermischer verdampfung in einer graphitrohrküvette : I. Grundlagen der methode und untersuchungen über die molekülabsorption von Ga- und In-halogeniden Molecular absorption spectrometry by electrothermal volatilization in a graphite furnace. Part 1. Basis of the method and studies of the molecular absorption of gallium and indium halides. Anal Chim Acta 97:59–68. doi:10.1016/S0003-2670(01)83275-X
Dittrich K, Vorberg B (1982) Molecular absorption spectrometry with electrothermal volatilization in a graphite tube.7. A study of molecular absorption of alkaline-earth halides and determination of traces of fluoride and chloride based on molecular absorption of Mgf and Mgcl molecules. Anal Chim Acta 140:237–248. doi:10.1016/S0003-2670(01)95470-4
Gleisner H, Welz B, Einax JW (2010) Optimization of fluorine determination via the molecular absorption of gallium mono-fluoride in a graphite furnace using a high-resolution continuum source spectrometer. Spectrochim Acta B At Spectrosc 65:864–869. doi:10.1016/j.sab.2010.08.003
Gleisner H, Einax JW, Mores S, Welz B, Carasek E (2011) A fast and accurate method for the determination of total and soluble fluorine in toothpaste using high-resolution graphite furnace molecular absorption spectrometry and its comparison with established techniques. J Pharm Biomed Anal 54:1040–1046. doi:10.1016/j.jpba.2010.12.013
Huang MD, Becker-Ross H, Okruss M, Geisler S, Florek S, Richter S, Meckelburg A (2014) Direct determination of fluorine in niobium oxide using slurry sampling electrothermal high-resolution continuum source molecular absorption spectrometry Spectrochimica Acta Part B:34–38
Kissa E (1983) Determination of fluoride at low concentrations with the ion-selective electrode. Anal Chem 55:1445–1448. doi:10.1021/ac00259a065
Kruger M, Huang MD, Becker-Ross H, Florek S, Ott I, Gust R (2012) Quantification of the fluorine containing drug 5-fluorouracil in cancer cells by GaF molecular absorption via high-resolution continuum source molecular absorption spectrometry. Spectrochim Acta B 69:50–55. doi:10.1016/j.sab.2012.02.004
Machado PM, Morés S, Pereira ÉR, Welz B, Carasek E, de Andrade JB (2015) Fluorine determination in coal using high-resolution graphite furnace molecular absorption spectrometry and direct solid sample analysis. Spectrochim Acta B At Spectrosc 105:18–24. doi:10.1016/j.sab.2014.08.001
Marthaler TM (1992) Age-adjusted limits of fluoride intake to minimize the prevalence of fluorosis. J Biol Buccale 20:121–127
Mores S, Monteiro GC, Santos FD, Carasek E, Welz B (2011) Determination of fluorine in tea using high-resolution molecular absorption spectrometry with electrothermal vaporization of the calcium mono-fluoride CaF. Talanta 85:2681–2685. doi:10.1016/j.talanta.2011.08.044
Nakadi FV, Rosa LR, da Veiga MAMS (2013) Determination of sulfur in coal and ash slurry by high-resolution continuum source electrothermal molecular absorption spectrometry. Spectrochim Acta B 88:80–84. doi:10.1016/j.sab.2013.04.011
Ozbek N, Akman S (2012a) Method development for the determination of fluorine in water samples via the molecular absorption of strontium monofluoride formed in an electrothermal atomizer. Spectrochim Acta B At Spectrosc 69:32–37. doi:10.1016/j.sab.2012.03.003
Ozbek N, Akman S (2012b) A slurry sampling method for the determination of iron and zinc in baby food by flame atomic absorption spectrometry. Food Addit Contam A 29:208–216. doi:10.1080/19440049.2011.631193
Ozbek N, Akman S (2013) Molecule formation mechanisms of strontium mono fluoride in high-resolution continuum source electrothermal atomic absorption spectromerty. Anal Sci 29:741–746
Ozbek N, Akman S (2014) Determination of fluorine in milk and water via molecular absorption of barium monofluoride by high-resolution continuum source atomic absorption spectrometer. Microchem J 117:111–115. doi:10.1016/j.microc.2014.06.013
Ozbek N, Akman S (2015) Determination of fluorine in Turkish wines by molecular absorbance of CaF using a high resolution continuum source atomic absorption spectrometer LWT. Food Sci Technol 61:112–116
Soares AR, Nascentes CC (2013) Method for determination of lead in hair dyes using slurry sampling graphite furnace atomic absorption spectrometry. Anal Lett 46:356–366. doi:10.1080/00032719.2012.710868
Tsunoda K, Chiba K, Haraguchi H, Fuwa K (1979) Platinum atomic lines for determination of ultratrace fluoride by aluminum monofluoride molecular absorption spectrometry. Anal Chem 51:2059–2061. doi:10.1021/ac50048a044
Umile C, Huber JFK (1993) Determination of inorganic and organic anions in one run by ion chromatography with column switching. J Chromatogr A 640:27–31. doi:10.1016/0021-9673(93)80164-4
USA Public Service Department of Health and Human Services (1991) Revıew of fluorıde: benefıts and rısks. publıc health servıce department of health and human servıces, USA
Vignola F, Borges DLG, Curtius AJ, Welz B, Becker-Ross H (2010) Simultaneous determination of Cd and Fe in sewage sludge by high-resolution continuum source electrothermal atomic absorption spectrometry with slurry sampling. Microchem J 95:333–336. doi:10.1016/j.microc.2010.01.014
Wang P, Li SFY, Lee HK (1997) Simultaneous determination of monofluorophosphate and fluoride in toothpaste by capillary electrophoresis. J Chromatogr A 765:353–359. doi:10.1016/S0021-9673(96)00926-0
Welz B, Lepri FG, Araujo RGO, Ferreira SLC, Huang MD, Okruss M, Becker-Ross H (2009) Determination of phosphorus, sulfur and the halogens using high-temperature molecular absorption spectrometry in flames and furnaces—a review. Analytica Chimica Acta 647:137–148. doi:10.1016/j.aca.2009.06.029
Ye P, Guo W, Zhang P, Jin L (2015) Heat-assisted slurry sampling GFAAS method for determination of lead in food standard reference materials. J Food Compos Anal 42:78–83. doi:10.1016/j.jfca.2015.03.007
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was not funded by any agencies/institutes.
Conflict of Interest
Nil Ozbek declares that she has no conflict of interest.
Suleyman Akman declares that he has no conflict of interest.
Ethical Approval
For this type of study, formal consent is not required. This study does not contain any studies with human participants or animals performed by any of the authors.
Informed Constent
Not applicable.
Rights and permissions
About this article
Cite this article
Ozbek, N., Akman, S. Optimization and Application of a Slurry Sampling Method for the Determination of Total Fluorine in Flour Using a High-Resolution Continuum Source Graphite Furnace Molecular Absorption Spectrometer. Food Anal. Methods 9, 2925–2932 (2016). https://doi.org/10.1007/s12161-016-0488-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0488-4