Abstract
Mangiferin has high potential as a cancer chemopreventive agent; but readily available sources are scarce. Therefore, the concentration of mangiferin and its isomer, were quantitated in the leaves of five species of Coffee leaves from Brazil and Costa Rica respectively. The amount of total mangiferins in methanol extracts of the Brazilian species; was in the range 0.67–4.97 g/kg, whereas in the Costa Rican species, it lay in the range 0.85–4.01 g/kg. In 90 % of cases, mangiferin accounted for 80 % or more of total mangiferins; as opposed to isomangiferin. Infusion studies with powdered leaves of a commercial Brazilian species (Coffea arabica) shows that the release of mangiferins is temperature dependent and that release at 100 °C (1.6 ± 0.06 g/kg) is instantaneous, but approximately 50 % less compared to prolonged methanol extraction (3.05 ± 0.16 g/kg). Consumption of Coffee leaf tea brews, as a natural source of mangiferins, may contribute significantly to elevated intake of these potentially health-promoting phenolic compounds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tea and coffee, the most popular beverages in the world, have been consumed for thousands of years. Tea is produced by brewing the dried leaves and buds of Camellia sinensis. Annual production of about 1.8 million tons of dried leaf provides about 40 L of beverage per capita worldwide (Wang and Ho 2009). Coffee is the third most frequently consumed beverage in the world, after water and tea (Wang and Ho 2009). Once ripe, coffee berries are dried, roasted at various temperatures, and then ground and brewed. Many studies have shown the relation between the consumption of tea and coffee and their potential disease prevention properties, which may be due to their polyphenol content (Wang and Ho 2009).
There is a growing interest in disease prevention using natural products, especially polyphenolic compounds, as consumer awareness of their possible beneficial health effects increases. Mangiferin, as an example of the many polyphenolic compounds, appears to have considerable promise as a natural disease chemopreventant.
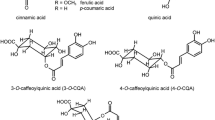
Mangiferin (1,3,6,7-tetrahydroxyxanthone-C2-ß-glucoside) (Fig. 1) is a C-glycosylated xanthone which is highly resistant to hydrolysis and is widely distributed in the plant kingdom as reviewed by Vyas et al. (2012). The following species are reported to contain mangiferin namely Anemmarhena asphodeloides, Aphloia theiformis, Arrabidaea patellifera, Arrabidaea samydoides, Bersama abyssinica, Bombax ceiba, Bombax malabaricum, Cratoxylum cochinchinens, Cyclopia genistoides, Cyclopia subternata, Folium mangiferae, Folium pyrrosiae, Gentiana lutea, Gentianella nitida, Gnidia involucrata, Hypericum perforatum, Mahkota dewa, Mangifera indica, Mangifera odorata, Mangifera persiciformis, Phaleria cumingii, Phaleria macrocarpa, Mangifera persiciformis, Polygala hongkongensis, Pyrrosia gralla, Rhizoma anemarrhenae, Rhizoma belamcandae, Salacia hainanensis, Salacia oblonga, Salacia reticulate, Swertia macrosperma, Swertia chirata, Swertia mussotii, Swertia punicea, Trichomanes reniforme, and Zizyphus cambodiana (Vyas et al. 2012).
However these plants apart from the Cyclopia family and Mangifera indica (Barreto et al. 2008) are not a viable source as a convenient provider of mangiferin for oral consumption. The Cyclopia family is indigenous to South Africa, and is popular for the preparation of a herbal tea termed “green honeybush.” This represents a viable natural source of mangiferin, in which amounts as high as 11.8 g/100 g extract (n.b. not g/100 g plant material) have been reported (De Beer and Joubert 2010), but the availability of the plant is extremely limited. In addition, Campa et al. (2012) have recently demonstrated mangiferin accumulation in the leaves of seven African Coffea taxa namely C. anthonyi, C. arabica, C. eugenioides, C. heterocalyx cf., C. pseudozanguebariae, C. salvatrix, and C. Sessiliflora which ostensibly represent an excellent natural source of this potentially disease-preventive agent, for which the following properties have been described.
In addition to inhibition of bowel carcinogenesis (Yoshimi et al. 2001), mangiferin is reported to have potent cytoprotective and antigenotoxic effects against CdCl2-induced toxicity in HepG2 cells, on the basis of absorption spectra, which were attributed to a decrease in the levels of reactive oxygen species (Satish Rao et al. 2009), anti-allergic properties (Rivera et al. 2006), anthelminthic properties (Garcia et al. 2003), gastro-protective effects (Carvalho et al. 2007; Morais et al. 2012), potent hepatoprotective effects on CCl4-induced liver damage in mice (Rasool et al. 2012), chemopreventive and chemotherapeutic effects (Rajendran et al. 2008), and antiviral properties (Yoosook et al. 2000). Mangiferin has also been used in foodstuffs for treating diabetes (Wada 2007) and the treatment and prevention of neurodegenerative diseases and ageing symptoms (Matute Almau et al. 2007; Rao et al. 2012). Mangiferin has also been reported to have strong scavenging effects against reactive oxygen species (Barreto et al. 2008) which attack molecules including DNA, proteins, and lipids, contributing to the development of several chronic diseases including cancer (Tang et al. 2004). Mangiferin is considered non-toxic, because its reported oral LD50 value in mice is 400 mg/kg (Jagetia and Baliga 2005).
The aim of this work was the quantitation of mangiferin and isomangiferin (Fig. 1) in methanol extracts of Coffea arabica leaves from Brazil and Costa Rica grown under various environmental conditions and infusion of a commercial source to establish a readily available natural source for future human intervention trials.
Materials and Methods
Chemicals
Acetic acid, dimethyl sulfoxide, n-hexane and methanol were purchased from Merck (Darmstadt, Germany); and acetonitrile from Fluka/Riedel de Haen (Seelze, Germany) while mangiferin was obtained from Extrasynthese (Lyon Nord, Genay, France). All solutions were made up in either double distilled water or methanol.
Plant Material
The coffee leaves of nine cultivars (C. arabica) were obtained from Brazil (Table 1) and Costa Rica (Table 2) as listed. The material was obtained from several different sources in Brazil: Ceará (Guaramiranga), Minas Gerais (Bom Sucesso and Manhumirim), and from Costa Rica (Herredura and Turrialba): the Turrialba cultivars were from CATIE (Solutions for Environment and Development). In addition, one commercial source of coffee tea leaves was used for comparison.
Preparation of Plant Material
Coffee leaves were freeze-dried (Christ, Gefriertrocknungsanlangen, Osterode, Germany) to constant weight. Dried samples were pulverized by blending to a fine homogeneous powder in a Moulinex coffee grinder prior to extraction.
Coffee Leaf Extraction
The various samples of C. arabica leaves (10 g of freeze-dried powder) were extracted in duplicate for 3 h with n-hexane in a soxhlet apparatus to remove lipids. After drying, the material was further extracted, three times for 3 h with methanol, the solutions pooled and evaporated to dryness at 40 °C by rotary evaporation under reduced pressure. The extracts were dissolved in methanol and analyzed by analytical reverse-phase high-performance liquid chromatography electrospray ionization mass spectrometry (HPLC-ESI-MS).
Coffee Leaf Infusion
In order to assess the efficiency of mangiferin extraction by infusion, varying amounts (100–1000 mg) of coffee leaf powder from the commercial source (3.05 ± 0.16 g/kg mangiferins) were transferred to 15 mL Falcon tubes in duplicate, and 10.0 mL of double distilled boiling water were added and maintained in a water bath at 100 °C for 20 min. The solutions were filtered, and 1.0 mL was set aside for HPLC-ESI-MS. To assess the rate of extraction, duplicate 100 mg samples of coffee leaf powder were transferred to 15 mL Falcon tubes and 10.0 mL of double distilled boiling water was added and maintained in a water bath at 100 °C for 1 to 30 min. The solutions were filtered and 1.0 mL was set aside for HPLC-ESI-MS. Finally, to assess the effect of temperature on efficiency of extraction, duplicate 100 mg samples of coffee leaf powder were transferred to 15 mL Falcon tubes and 5.0 mL of double distilled water at temperatures ranging from 35 to 100 °C was added and maintained in a heating block for 5 min. The solutions were filtered and 1.0 mL was set aside for HPLC-ESI-MS.
High-Performance Liquid Chromatography—UV
HPLC-UV analysis was conducted on an Agilent 1100 HPLC, coupled to an Agilent single quadrupole mass-selective detector (HP 1101; Agilent Technologies, Waldbronn, Germany). Chromatographic separation of methanol extracts (10 μL) and aqueous infusion (20 μL) was conducted using a C18, reverse-phase (5 μ), Gemini column (250 × 4 mm I.D.; Phenomenex, Aschaffenburg, Germany). The mobile phase consisted of 2 % acetic acid in water (solvent A) and acetonitrile (solvent B) with the following gradient: 95 % A for 10 min, to 90 % A in 1 min, to 60 % A in 9 min, to 80 % A in 10 min, to 60 % A in 10 min, to 0 % A in 5 min, and continuing at 0 % A until completion of the run. Detection of phenolic compounds was by means of UV absorbance (A) at 257, 278, 320, and 340 nm at room temperature.
High-Performance Liquid Chromatography Electrospray Ionization Mass Spectrometry
HPLC-ESI-MS analysis was conducted on an Agilent 1100 HPLC, coupled to an Agilent single quadrupole, mass-selective detector (HP 1101; Agilent Technologies, Waldbronn, Germany). Chromatographic separation of methanol extracts was conducted using a column of the same type, and dimensions as for analytical HPLC (Latek, Eppelheim, Germany). The mobile phase consisted of 2 % acetic acid in double distilled water (solvent A) and acetonitrile (solvent B) with the following gradient profile: initially 95 % A for 10 min; to 90 % A over 1 min; to 60 % A over 9 min; to 80 % A over 10 min; to 60 % A over 10 min, to 0 % A over 5 min and continuing at 0 % A until completion of the run. Detection of phenolic compounds was by means of UV absorbance (A) at 257, 278, 320, and 340 nm at room temperature. Mass spectra in negative-ion mode were generated under the following conditions: fragmentor voltage = 100 V, capillary voltage = 2500 V, nebulizer pressure = 30 psi, drying gas temperature = 350 °C, and mass range = 100–1500 D. Instrument control and data handling were performed with the HP Chemstation software on a PC. The amount of mangiferin and isomangiferin in the extracts was calculated from a standard curve of an authentic mangiferin standard in the range 0–422 μg/mL (0–1000 μM) at 257 nm (y = 24.084×).
Limits of Detection, Limits of Quantitation, and Repeatability
The estimated of limits of detection (LOD) and limits of quantitation (LOQ) were made using the method based on the regression parameters of the calibration curve (Huber 2003; Basilio et al. 2014). Intra- and inter RSD were made in five different concentrations (range, 25, 50, 125, 250, and 500 μM), with three replicates of each.
Statistics
Correlations between release of mangiferins by infusion, with regard to amount extracted and at various temperatures, were evaluated by linear regression using the Origin program (version 7.5).
Results and Discussion
Limits of Detection, Limits of Quantification, and Repeatability
The sensitivity of the developed method was assessed by determining the LOD and LOQ. The LOD for mangiferin and isomangiferin was 0.164 g/kg while the LOQ was 0.492 g/kg. These results are in agreement with the LOD and LOQ of mangiferin as reported in the literature (Jounert et al. 2003). Intra- and inter-RSD of five different concentrations (25, 50, 125, 250, and 500 μM) were <2 % for three replicates of each.
Identification and Quantitation of Mangiferins in Methanol Extracts of Coffee Leaves
The identification of mangiferin and isomangiferin in the methanol extracts (Fig. 2) and infusions (Fig. 3), which displayed typical UV spectra of xanthones, were determined from the mass spectra of the compounds in negative-ion mode. A pseudo-mole peak was detected at [M–H]− at m/z = 421 (exact mass = 422.085) in negative-ion mode, which was the base peak for both mangiferin and isomangiferin. Bombardment of the mole peaks at a fragmentor voltage of 100 V gave significant fragment ions at m/z 403, 331, 301, and 271 typical of C-glucosides. The proposed fragmentation is shown in Fig. 4, which is in agreement with the literature (Sun et al. 2009). An additional fragment peak at m/z 259 was indicative of the norathyriol xanthone core (Souza JRR et al. 2012). Quantitation was achieved against a standard curve of mangiferin (0–1000 μM) from the reverse-phase analytical chromatograms at 257 nm.
HPLC–ESI-MS chromatograms (negative-ion mode) of an aqueous infusion (100°C) of a commercial cultivar of Coffea arabica. Above: UV chromatogram (257 nm) under the conditions used for HPLC–ESI-MS. Below: The individual extracted ions at m/z = 421 [M–H]− of mangiferin (Peak 1) and isomangiferin (Peak 2)
Overall, the concentration (Tables 1 and 2) of mangiferin detected in the methanol extracts was in the range 0.48–4.17 g/kg whereas isomangiferin was in the range 0.16–0.80 g/kg representing 20 % or less in 8 (89 %) of the nine samples. Total mangiferins were in the range 0.67–4.97 g/kg. The range of total mangiferins, from both Brazil (0.67–4.97) and Costa Rica (0.85–4.01), was similar across the samples. Total mangiferin content tended to be considerably higher in Coffee leaves, obtained from trees growing in plantations under natural full-sun conditions, compared to other types of management such as organic treatment. Additionally, in one comparison with a species from Minas Gerais (Bom Sucesso), young leaves contained much higher concentrations (4.97 ± 0.11 vs 1.49 ± 0.19 g/kg) than old leaves.
Quantitation of Mangiferins in Coffee Leaf Tea Infusions
Infusion of varying amounts (100–1000 mg) of coffee leaf powder (Commercial variety) with boiling distilled water and maintenance at this temperature for 20 min revealed (Fig. 5) that release of mangiferins showed a linear dose response (r = 1.00; P = 0.0001). The amount released from 1 g was only 47 % lower than that after prolonged extraction with methanol.
The release of mangiferins by infusion with boiling water was instantaneous with 53 % extraction (compared to methanol extraction) after only 1 min, with no increase after 30 min (data not shown). Release of mangiferins from coffee leaf powder was strongly temperature dependent. At 35 °C, release of mangiferins was 59 % efficient compared to boiling water, rising to 88 % at 85 °C and to 100 % at 100 °C (Fig. 6). Therefore, infusion of Coffee tea leaves with boiling water (1.6 ± 0.06 g/kg) releases greater than 50 % of the available mangiferins present as compared to methanol (3.05 ± 0.16 g/kg) extraction.
The data shows that coffee leaves obtained from all nine cultivars of C. arabica from both Brazil (n = 4) and Costa Rica (n = 5) contain appreciable concentrations of mangiferins dominated by mangiferin at 80 % or over with regard to isomangiferin. The ranges, in both the Brazilian and Costa Rican cultivars at 0.67–4.97 and 0.85–4.01 g/kg are in only moderate agreement with the data reported for 7 out of 23 species from Africa as reported by Campa et al. (2012) which is the only other publication on the content of mangiferins in Coffee leaves.
Campa et al. (2012) presented their data in % dry weight, and the range reported in the positive species was 0.026–16.359 % equivalent to 0.26–163.6 g/kg. The higher values are in the range for those reported in young mango leaves (Barreto et al. 2008), which is surprising. Study of the data in Campa et al. (2012) reveals that the standard curve of mangiferin (in an unstated solvent) was generated at 320 nm in the range 10–100 mg/mL representing 23.70–236.97 mM which appears inconceivable, because at these concentrations in any solvent, mangiferin is not totally soluble and would saturate the UV source.
However, it cannot be discounted that the coffee leaves studied by Campa et al. (2012) represent wild species of the old World and that the major cultivated species used in the commercial production of coffee, namely C. arabica, in general, contain less mangiferin in their leaves. Nevertheless, the extremely limited availability of wild type Coffea and Cyclopia species renders their suitability as providers of a natural source of mangiferin, peripheral, when compared to the global availability of Coffea arabica leaves.
Conclusion
In conclusion, our data showing total mangiferins at concentrations in the range 0.67–4.97 g/kg (which are 53 % soluble by infusion) indicate that the leaves of cultivated Coffea species are a useful natural source of mangiferins and consumption of coffee leaf tea brews may contribute significantly to elevated intake of these potentially health-promoting phenolic compounds, the exact relevance of which needs to be determined by future bioavailability and epidemiologic studies.
References
Barreto JC, Trevisan MTS, Hull WE, Erben G, de Brito ES, Pfundstein B, Wüertele G, Spiegelhalder B, Owen RW (2008) Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mangiferaindica L.). J Agric Food Chem 56:5599–5610
Basilio FS, Bonfim MVJ, Almeida RJ, Abrantes SMP (2014) Intralaboratory validation of an analytical method for determining the migration of bis(2-ethylhexyl) adipate from packaging to fat foods. Accred Qual Assur 19:195–204
Campa C, Mondolot L, Rakotondravao A, Bidel LP, Gargadennec A, Couturon E, La FP, Rakotomalala JJ, Jay-Allemand C, Davis AP (2012) A survey of mangiferin and hydroxycinnamic acid ester accumulation in coffee (Coffea) leaves: biological implications and uses. Ann Bot 110(3):595–613
Carvalho AC, Guedes MM, de Souza AL, Trevisan MT, Lima AF, Santos FA, Rao VS (2007) Gastroprotective effect of mangiferin, a xanthonoid from Mangifera indica, against gastric injury induced by ethanol and indomethacin in rodents. Planta Med 73(13):1372–1376
De Beer D, Joubert E (2010) Development of HPLC method for Cyclopia subternata phenolic compound analysis and application to other Cyclopia spp. J Food Comp Anal 23:289–297
Garcia D, Escalante M, Delgado R, Ubeira FM, Leiro J (2003) Anthelminthic and antiallergic activities of Mangifera indica L. stem bark components Vimang and mangiferin. Phytother Res 17(10):1203–1208
Huber W (2003) Basic calculations about the limit of detection and its optimal determination. Accred Qual Assur 8(5):213–217
Jagetia GC, Baliga MS (2005) Radioprotection by mangiferin in DBAxC57BL mice: a preliminary study. Phytomedicine 12(3):209–215
Jounert E, Otto F, Grünner S, Weinreich B (2003) Reversed-phase HPLC determination of mangiferin, isomangiferin and hesperidin in Cyclopia and the effect of harvesting date on the phenolic composition of C. genistoides. Eur Food Res Technol 216(3):270–273
Matute Almau C, Sanchez Gomez MV, Campos Esparza R, Alberti Alfonso E, Gottlieb M, Ibarretxe Bilbao G,Delgado Garcia JM, Gruart i Masso A, Leal Campanario R (2007). Food products for treating and preventing neurodegenerative diseases and ageing symptoms, contain morin or mangiferin, Spanish patent, ES2277567-A1 WO2007077279-A1.
Morais TC, Lopes SC, Carvalho KMMB, Arruda BR, Sousa FTC, Trevisan MTS, Rao VSN, Santos FA (2012) Mangiferin, a natural xanthone accelerates gastrointestinal transit in mice involving cholinergic mechanism. World J Gastroent 18:3207–3214
Rajendran P, Ekambaram G, Sakthisekaran D (2008) Effect of mangiferin on benzo(a)pyrene induced lung carcinogenesis in experimental Swiss albino mice. Nat Prod Res 22(8):672–680
Rao VS, Carvalho AC, Trevisan MT, Andrade GM, Nobre-Junior HV, Moraes MO, Magalhaes HI, Morais TC, Santos FA (2012) Mangiferin ameliorates 6-hydroxydopamine induced cytotoxicity and oxidative stress in ketamine model of schizophrenia. Pharmacol Rep 64(4):848–856
Rasool M, Sabina EP, Mahinda PS, Clara-Gnanaselvi B (2012) Mangiferin, a natural polyphenol protects the hepatic damage in mice caused by CCl4 intoxication. Comp Clin Pathol 21:865–872
Rivera DG, Balmaseda IH, Leon AA, Hernandez BC, Montiel LM, Garrido GG, Cuzzocrea S, Hernandez RD (2006) Anti-allergic properties of Mangifera indica L. extract (Vimang) and contribution of its glucosyl xanthone mangiferin. J Pharm Pharmacol 58(3):385–392
Satish Rao BS, Sreedevi BMV, Nageshwar RB (2009) Cytoprotective and antigenotoxic potential of mangiferin, a glucosylxanthone against cadmium chloride induced toxicity in HepG2 cells. Food Chem Toxicol 47(3):592–600
Souza JRR , Feitosa JPA, Ricardo NMPS, Trevisan MTS, Frei E, Ulrich CM, Owen RW (2012) Cytotoxicity screen of mangiferin and its major metabolite norathyriol in human tumor cell lines, Available at: http://www.eposters.net/pdfs/cytotoxicity-screen-of-mangiferin-and-its-major-metabolite-norathyriol-in-human-tumor-cell-lines.pdf
Sun Y, Zhang X, Xue X, Zhang Y, Xiao H, Liang X (2009) Rapid identification of polyphenol C-glycosides from Swertia franchetiana by HPLC-ESI-MS-MS. Chromatogr Sci 47(3):190–196
Tang SY, Whiteman M, Peng ZF, Jenner A, Yong EL, Halliwell B (2004) Characterization of antioxidant and antiglycation properties and isolation of active ingredients from traditional Chinese medicines. Free Radic Biol Med 36(12):1575–1587
Vyas A, Syeda K, Ahmad A, Padhye S, Sarkar FH (2012) Perspectives on medicinal properties of mangiferin. Mini Rev Med Chem 12(5):412–425
Wada M (2007) Foodstuffs compounding agent for treating diabetes comprises glycoside having xanthone structure, Japanese patent, JP2007204462-A.
Wang Y, Ho C-T (2009) Polyphenolic chemistry of tea and coffee: a century of progress. J Agric Food Chem 57:8109–8114
Yoosook C, Bunyapraphatsara N, Boonyakiat Y, Kantasuk C (2000) Anti-herpes simplex virus activities of crude water extracts of Thai medicinal plants. Phytomedicine 6(6):411–419
Yoshimi N, Matsunaga K, Katayama M, Yamada Y, Kuno T, Qiao Z, Hara A, Yamahara J, Mori H (2001) The inhibitory effects of mangiferin, a naturally occurring glucosylxanthone, in bowel carcinogenesis of male F344 rats. Cancer Lett 163(2):163–170
Acknowledgments
This work was supported by Capes, CNPq, Funcap (Brazil), and DAAD (Germany)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Author Maria Teresa Salles Trevisan declares that she has no conflict of interest. Author Ricardo Farias Almeida declares that he has no conflict of interest. Author Gabriela Soto declares that she has no conflict of interest. Author Elias de Melo Virginio Filho declares that he has no conflict of interest. Author Cornelia M, Ulrich declares that she has no conflict of interest. Author Robert Wyn Owen declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable
Rights and permissions
About this article
Cite this article
Trevisan, M.T.S., Farias de Almeida, R., Soto, G. et al. Quantitation by HPLC-UV of Mangiferin and Isomangiferin in Coffee (Coffea arabica) Leaves from Brazil and Costa Rica After Solvent Extraction and Infusion. Food Anal. Methods 9, 2649–2655 (2016). https://doi.org/10.1007/s12161-016-0457-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0457-y